INTRODUCTION
Pancreatic endocrine tumors, also known as islet cell tumors, are rare neoplasms and occur in approximately 1 of 100 000 people, representing 1% to 10% of all pancreatic neoplasms[1-3]. Some pancreatic endocrine tumors release hormones into the blood stream that cause clinical syndromes, whereas others are non-syndromic and present as mass lesion[4,5]. The overall prevalence of functional pancreatic endocrine tumors is reported to be approximately 10/1 000 000. In contrast, the prevalence of pancreatic endocrine tumors reported by autopsy studies is higher (0.5% to 1.5%)[6]. Multi-detector row computed tomography (CT) plays an important role in the diagnosis and staging of both syndromic and non-syndromic pancreatic endocrine tumors. In general, syndromic pancreatic endocrine tumors are less than 3 cm in size. They are typically strongly enhanced and usually best seen on CT scans obtained during the arterial phase. Compared to syndromic pancreatic endocrine tumors, non-syndromic pancreatic endocrine tumors tend to be larger at presentation and are more likely to be cystic or necrotic[7]. In previous studies, endoscopic retrograde pancreatography (ERP) has been used to detect main pancreatic duct (MPD) stenosis and dilation of the upstream pancreatic duct caused by pancreatic endocrine tumors[8,9]. In addition, pancreatic endocrine tumors with serotonin production is suggested to be associated with prominent stromal fibrosis, which can extend to the MPD, causing ductal stenosis and upstream dilatation of the duct and/or upstream pancreatic atrophy[10,11]. We recently encountered 2 cases of small pancreatic endocrine tumors that revealed serotonin-positive by immnohistochemical staining causing obstruction of the MPD.
CASE REPORT
Case 1
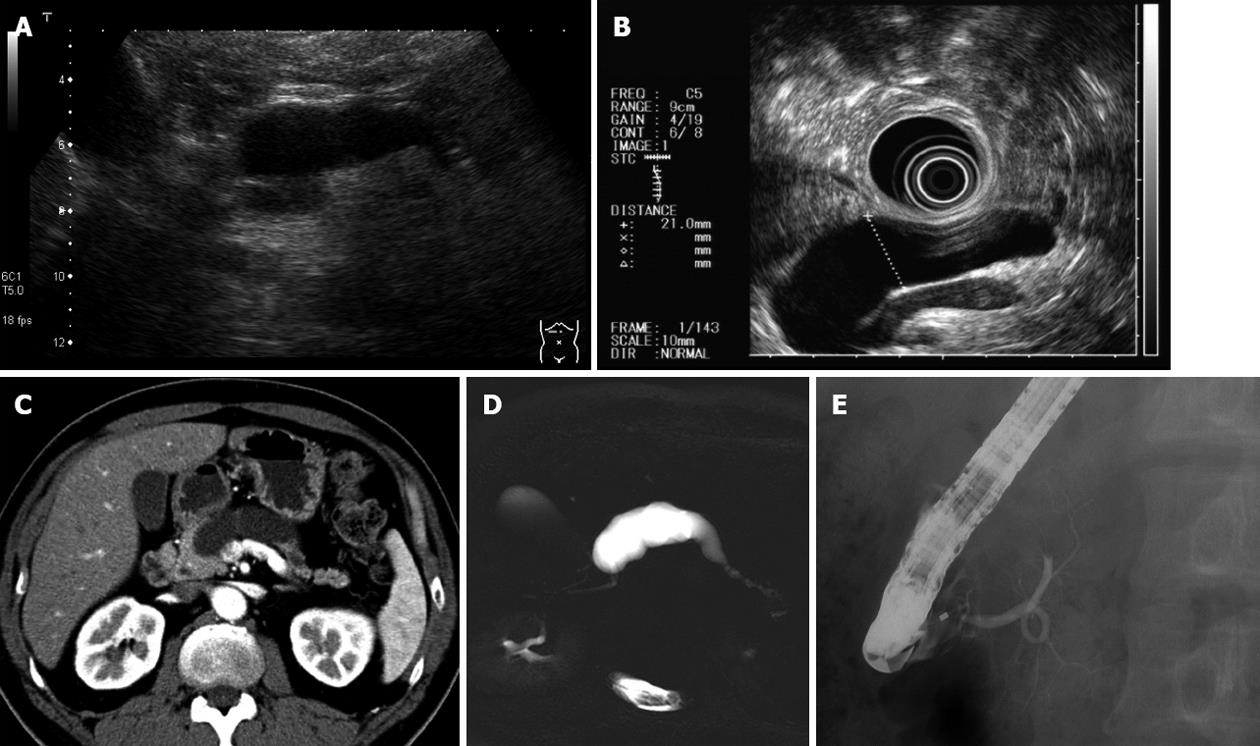
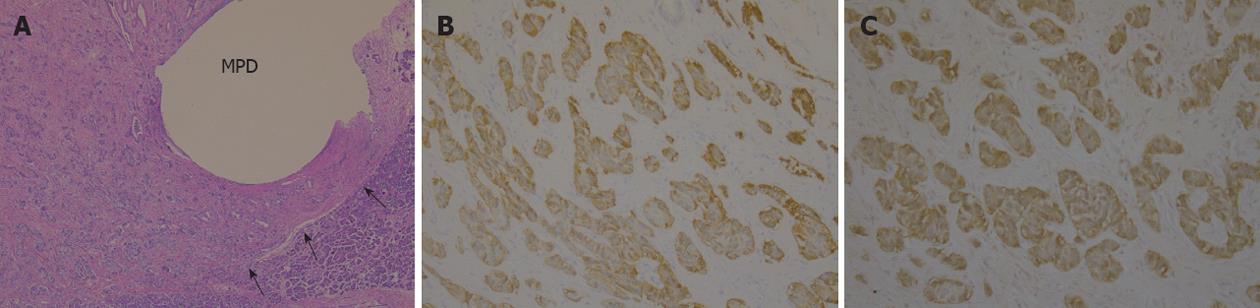
A 49-year-old asymptomatic man was referred to our institution because dilatation of the MPD was revealed by abdominal ultrasonography (US). However, no tumor was observed by endoscopic ultrasonography (EUS), CT, and magnetic resonance imaging (MRI). The diameter of the MPD was > 20 mm at the body and no dilation was noted at the head. Endoscopic retrograde cholangiopancreatography (ERCP) was performed; the MPD showed crab-like appearance (Figure 1). Cytology was benign. Although we could not confirm malignancy through cytology or imaging, pancreatic cancer was strongly suspected. Pancreaticoduodenectomy was performed, and pathological examination revealed a 5 mm × 3 mm tumor. Fibrosis was present around the MPD and seemed to cause stricture (Figure 2).
Figure 1 No tumor was detected by endoscopic ultrasonography, computed tomography and magnetic resonance imaging.
The diameter of the main pancreatic duct was > 20 mm at the body. A: Ultrasonography; B: Endoscopic ultrasonography; C: Computed tomography; D: Magnetic resonance cholangiopancreatography; E: Endoscopic retrograde pancreatography.
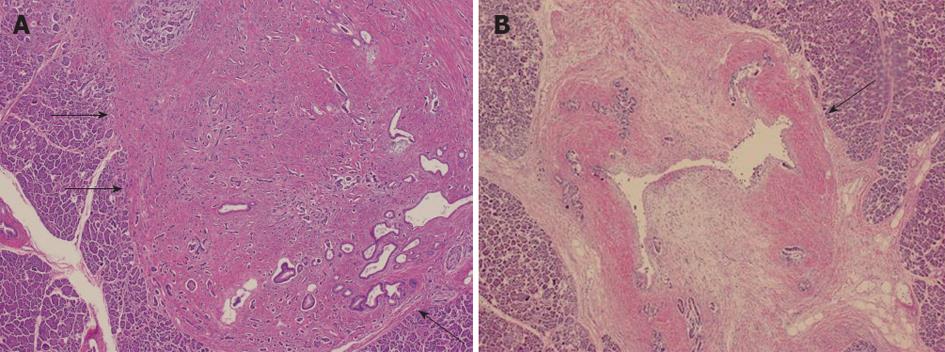
Figure 2 Pathological findings (hematoxylin/eosin staining).
A: A 5 mm × 3 mm tumor was detected (arrows); B: Fibrosis was present around the main pancreatic duct (arrow), and it seemed to cause stricture.
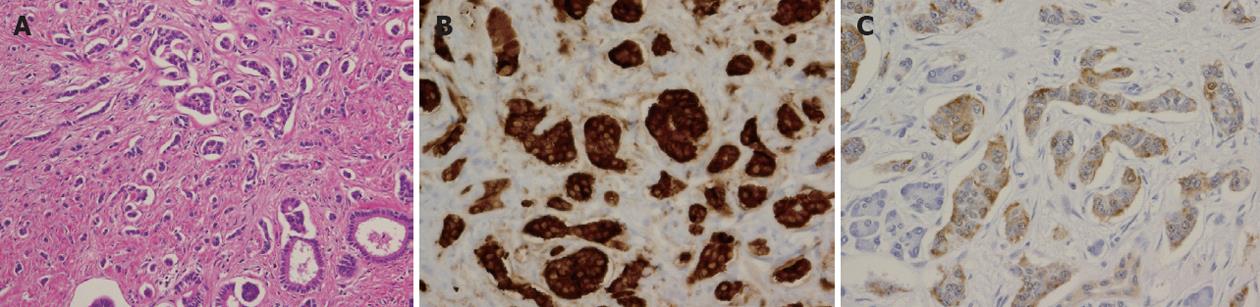
Immunohistochemical staining of tumor samples was positive for chromogranin A, synaptophysin, and serotonin (Figure 3). The MIB-1 (Ki-67) labeling index was less than 1%. We made the diagnosis of neuroendocrine tumor of grade 1 (NET G1).
Figure 3 Immunohistochemical staining was positive for chromogranin A, synaptophysin and serotonin.
A: Hematoxylin/eosin staining; B: Chromogranin A; C: Serotonin. Ki-67 labeling index was less than 1%.
Case 2
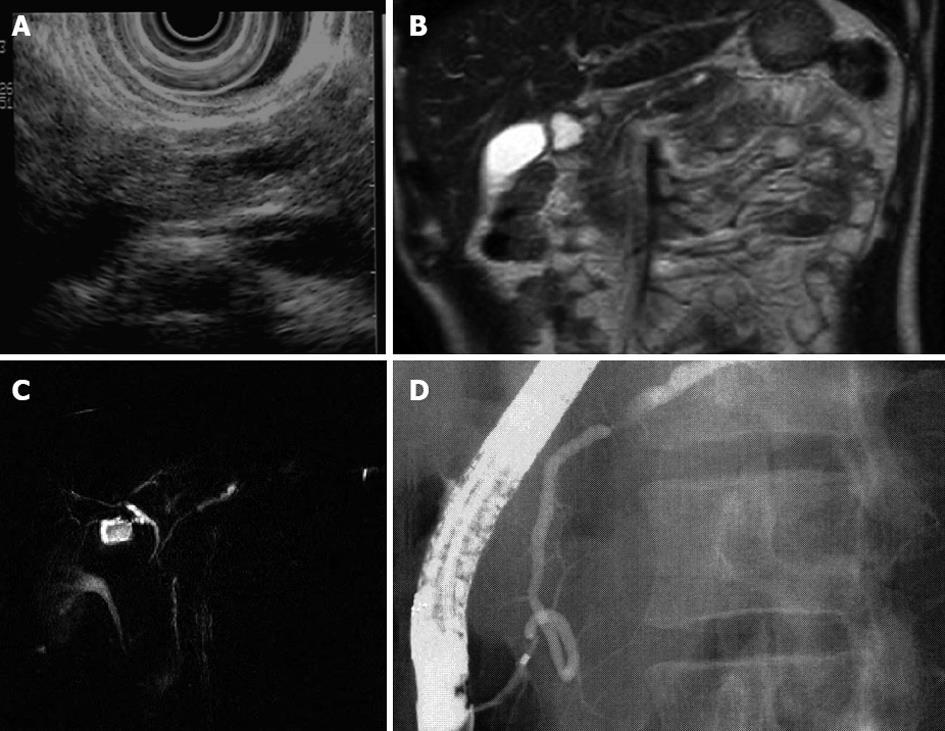
A 32-year-old man with no symptom had elevated serum amylase, and US demonstrated dilation of the MPD. No tumor but MPD stenosis and dilatation was revealed by MRI. EUS demonstrated presence of a small pancreatic tumor. ERCP revealed MPD stenosis and upstream dilatation (Figure 4). Cytology was benign.
Figure 4 Dilation of the main pancreatic duct.
Endoscopic ultrasonography was demonstrated presence of a small pancreatic tumor. A: Ultrasonography; B: Magnetic resonance imaging; C: Magnetic resonance cholangiopancreatography; D: Endoscopic retrograde pancreatography.
Pancreatectomy of middle part of pancreas was performed and pathological examination revealed an endocrine tumor sized 5 mm × 4 mm. Tumors with stromal fibrosis can cause stenosis of the pancreatic duct. Immunohistochemical staining of the tumor cells was positive for chromogranin A, synaptophysin, and serotonin (Figure 5). The Ki-67 labeling index was less than 1%, and we made the diagnosis of NET G1.
Figure 5 Pathological findings (hematoxylin and eosin staining) and immunohistochemical staining.
A: A 4 mm × 5 mm tumor was detected (arrows); B: Chromogranin A; C: Serotonin. MPD: Main pancreatic duct.
DISCUSSION
Pancreatic endocrine neoplasms that produce serotonin, including carcinoid tumors, account for only a small portion of pancreatic endocrine neoplasms. Compared with other well-differentiated endocrine neoplasms of the pancreas, pancreatic carcinoid tumors are associated with a higher rate of malignant behavior[12]. These pancreatic neoplasms may have the poor prognosis since they are usually found at an advanced stage, after distant metastases have occurred, and the patient has developed carcinoid syndrome. The problem is that the mass lesion is often asymptomatic and indistinct at early stages. In addition, Shi et al[11] reported that in serotonin-producing tumors, the neoplasm was subtle or unapparent on CT images; only marked dilatation of the upstream pancreatic duct or marked atrophy of the upstream pancreas was visible. Isolated reports of an association of pancreatic carcinoid tumor with dilatation of the pancreatic duct have been described before. Nagai et al[10] reported a case of pancreatic carcinoid tumor with obstructive pancreatitis. ERP revealed MPD stenosis and dilatation of the upstream pancreatic duct. They suggested that the pancreatic carcinoid tumor obstructing the pancreatic duct might have arisen from argentaffin cells located in the MPD. However, no clear relationship between pancreatic endocrine neoplasms and pancreatic duct stenosis has been described. Takaji et al[13] reported that 3 of 4 cases showed MPD dilatation upstream of the tumor. Our 2 cases were similar to these reports; the tumors were subtle on CT or MRI images and a markedly dilated MPD was noted, and they had no symptoms as carcinoid syndrome. We decided to perform the pancreatic resection because there was a possibility of pancreatic duct dilation was caused by a tumor. As a result, we could treat the pancreatic endocrine tumor in early stage. We observed that the serotonin immunoreactivity correlated with the degree of stromal fibrosis and that stromal fibrosis caused MPD stenosis. Carcinoid tumors of the midgut, in which serotonin is the predominant hormone secreted by neoplastic cells, are usually associated with extensive fibrosis[14]. In addition, serotonin has been shown to stimulate fibroblast mitosis in cell cultures[15]. Recently, serotonin has been shown to play a crucial role in the progression of liver fibrosis by enhancing the production of transforming growth factor βvia selective activation of the 5-HT2A serotonin receptors[16]. Shi et al[11] reported that a small portion of pancreatic endocrine neoplasms produces serotonin. Serotonin production may be associated with stromal fibrosis, and fibrosis, in turn, can cause stenosis of the pancreatic duct. In clinical practice, imaging findings of pancreatic duct stenosis that is out of proportion to the size of the causative strongly enhanced mass or that does not have an associated distinct mass are indicative of a pancreatic neoplasm. Recently, it was reported that many pancreatic NETs express high levels of somatostatin receptors, so somatostatin-receptor scintigraphy (OctreoScan) can be imaged with a radiolabeled form of the somatostatin analog octreotide. Somatostatin receptor scintigraphy has proven particularly effective for visualizing gastrinomas, glucagonomas, and nonfunctioning pancreatic tumors. However, in another study of 37 patients with a NET, MRI and CT were substantially superior to SRS for detection of liver metastases. The sensitivity of OctreoScans was found to be particularly poor (< 35 percent) in lesions smaller than 1.5 cm in diameter[17].
In conclusion, we report 2 cases of serotonin-positive pancreatic endocrine tumors that caused stricture of the MPD in spite of the small size of the tumor. It is necessary to consider that the MPD stenosis and upstream dilatation caused by the tiny pancreatic endocrine tumor as the differential diagnosis in addition to chronic pancreatitis and pancreatic cancer.
Peer reviewers: Run Yu, MD, PhD, Division of Endocrinology, Diabetes, and Metabolism, Cedars-Sinai Medical Center, 8700 Beverly Blvd, B-131, Los Angeles, CA 90048, United States; Juli Busquets, MD, PhD, Department of Surgery, Hospital Universitari de Bellvitge, C/Feixa Llarga s.n., 08907 Barcelona, Spain
S- Editor Gou SX L- Editor A E- Editor Li JY