Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6510
Revised: September 20, 2012
Accepted: September 28, 2012
Published online: November 28, 2012
Processing time: 145 Days and 22.3 Hours
We describe herein a 68-year-old woman who was diagnosed with a quite rare entity of intraductal papillary mucinous neoplasms (IPMNs) occurring simultaneously in the left lateral lobe of liver and the tail of pancreas. Abdominal computed tomography and magnetic resonance cholangiopancreatography showed a cystic dilatation of the pancreatic duct in the pancreatic tail, which suggested an IPMN, and multiple intrahepatic duct stones in the left lateral lobe. The patient underwent a laparoscopic left lateral hepatolobectomy and spleen-preserving distal pancreatectomy. Intra-operative finding of massive mucin in the dilated bile duct implied an intraductal mucinous tumor in the liver. The diagnosis of synchronous IPMNs in the liver and pancreas was confirmed by pathological examination. The patient was followed up for 6 mo without signs of recurrence. Although several cases of IPMN of liver without any pancreatic association have been reported, the simultaneous occurrence of IPMNs in the liver and pancreas is very rare. To the best of our knowledge, it is the first reported case treated by laparoscopic resection.
- Citation: Xu XW, Li RH, Zhou W, Wang J, Zhang RC, Chen K, Mou YP. Laparoscopic resection of synchronous intraductal papillary mucinous neoplasms: A case report. World J Gastroenterol 2012; 18(44): 6510-6514
- URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6510.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6510
Intraductal papillary mucinous neoplasm (IPMN) is rare and mostly occurs in the liver or pancreas. Synchronous occurrence of IPMNs in the liver and pancreas is even more unusual. It is only described in the form of case reports in the literature[1-5]. We present here a case of synchronous occurrence of IPMNs located respectively in the left lateral lobe of the liver and the tail of pancreas. These two lesions were resected by laparoscopic left lateral hepatolobectomy and spleen-preserving distal pancreatectomy. To the best of our knowledge, it is the first reported case treated by laparoscopic resection. We also summarized the features of five similar cases with detailed information reported in the English-language literature (Table 1).
| Source | Sex/age (yr) | IPMN-b | IPMN-p | Procedure | ||||||||
| Location | Size (cm2) | Histology | CMD | MC | Location | Size (cm2) | Histology | CMD | MC | |||
| Joo et al[1] | M/60 | Left lobe | 1.5 × 1.5 | Benign | Yes | Yes | Tail | 2.5 × 2.5 | Benign | Yes | Yes | LH + DP |
| Ishida et al[2] | M/67 | Caudate lobe | 4 × 3 | Benign | Yes | Yes | Uncinate process | 3.5 × 3 | Benign | Yes | Yes | LH + Caudate lobectomy +segmental resection of uncinate process |
| Yamaguchi et al[3] | M/69 | Left lateral lobe | 6.5 × 3.5 | Malignant | Yes | Yes | Head | 3 × 2.5 | Malignant | Yes | Yes | LLH + PPPD |
| Zalinski et al[4] | F/65 | Left lobe | 10 × 10 | Malignant | Unknown | Yes | Head | Unknown | High-grade dysplasia | Unknown | Yes | Unknown |
| Park et al[5] | M/67 | Left lateral lobe | Unknown | Benign | Yes | Yes | Tail | 2.5 × 1 | Benign | Yes | Yes | LLH + DP + splenectomy |
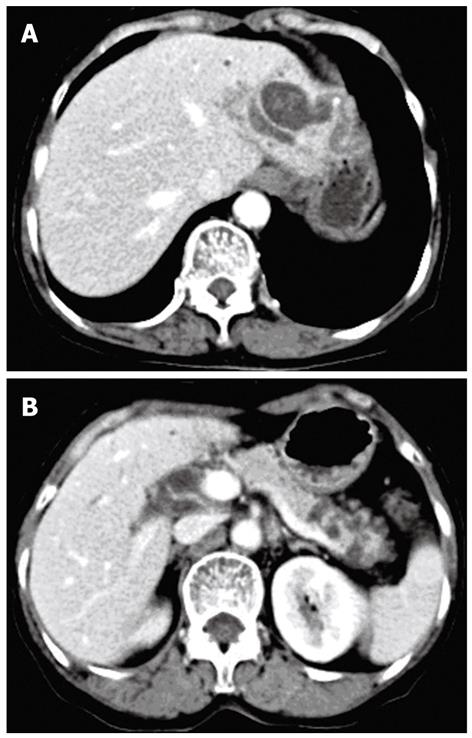
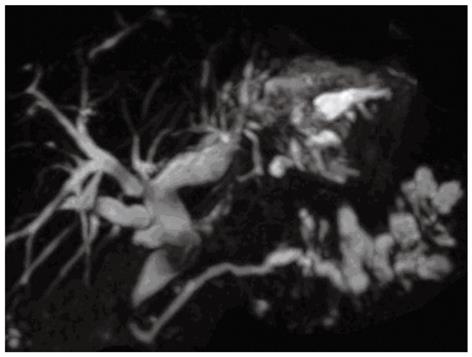
A 68-year-old woman was admitted to our department in Januray 2012 because of epigastric pain for half a month. She had no fever, no nausea or vomiting, no hematemesis or melena, and no weight loss. She underwent open cholecystectomy for gallbladder stones 25 years ago. Physical examination on admission showed no abnormalities. Blood biochemistry and tumor markers (carcinoembryonic antigen, alpha fetoprotein, carbohydrate antigen 19-9 carbohydrate antigen 153, carbohydrate antigen 125) were all within normal ranges. Computed tomography (CT) of the abdomen with intravenous contrast revealed cystic dilatation of left intrahepatic bile ducts accompanied with stones (Figure 1A) and multiple cystic lesions in the pancreatic tail (Figure 1B). Magnetic resonance cholangiopancreato-graphy revealed a diffuse dilated biliary tract with multiple filling defects in the left lateral lobe and segmental cystic dilatation of pancreatic duct in the pancreatic tail (Figure 2). According to the medical history and the imaging findings, left intrahepatic bile duct stones and pancreatic IPMN was first considered. Therefore, laparoscopic left lateral hepatolobectomy and spleen-preserving distal pancreatectomy were performed.
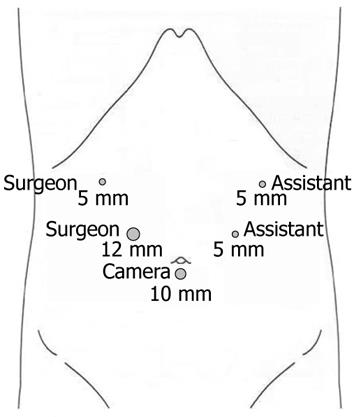
The patient was placed in supine position under general anesthesia. The operator and the second assistant who held the laparoscope stood on the right side of the patient and the first assistant stood on the left. Carbon dioxide pneumoperitoneum was established using a Veress needle to a pressure of 15 mmHg. One initial 10-mm trocar was placed for laparoscope below the umbilicus and another four trocars (one of 12 mm and three of 5 mm) were inserted into the left upper, left flank, right upper, and right flank quadrants, making the five trocars arrange in a V-shape (Figure 3). During laparoscopic exploration, severe adhesion in the right upper abdomen because of the former open cholecystectomy and the atrophy of left lateral lobe of the liver were found, but without abdominal metastasis.
After dissecting the adhesion, the gastrocolic omentum was divided for entrance to the lesser sac by ultrasonic coagulating shears (Harmonic Ace scalpel, Ethicon Endo-Surgery, Inc., Cincinnati, OH, United States). The stomach was reflected cephalad and the tumor was found in the tail of pancreas. According to the location of the tumor, the mobilization of the pancreas began at the upper border till the common hepatic artery and the proximal splenic artery were visualized. Then the pancreas was mobilized dorsally starting at the lower edge to visualize the splenic vein. Pancreas was transected 2 cm proximal to the right side of the tumor with an endoscopic linear staplers (Endocutter 60 staple, white cartridge; Ethicon, Endo-Surgery, Inc., Cincinnati, OH, United States) and the pancreatic tail was freely dissected from the splenic artery and vein by ligation of the small branches connected to the pancreas using small titanium vascular clips or ultrasonic coagulating shears.
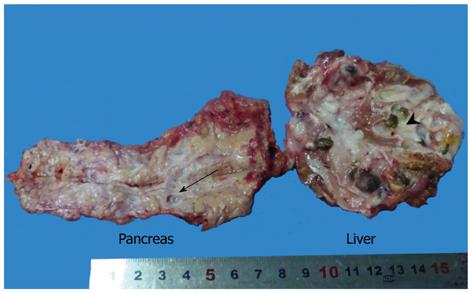
The left lateral lobe of the liver was mobilized by dissecting the round, falciform, left coronary and triangular ligaments. Liver parenchyma was also divided by ultrasonic coagulating shears 1 cm to the left side of the falciform ligament. The branches of left portal vein, hepatic artery and hepatic vein on the cut surface were ligated with hem-o-loks and divided. The dilated bile duct was cut and stones were found. However, it was surprising that a large amount of mucin outflowed from the bile duct, which implied the intraductal mucinous tumor. The common bile duct (CBD) was explored, because it was dilated to 2 cm. There was no stone but massive mucin. After inserting a No.24 T-tube, the CBD and bile duct in the cut surface were closed with a continuous suture of 3-0 Vicryl. The specimens were removed through the enlarged subumbilical port (Figure 4).
Intraoperative frozen section confirmed the mucinous tumors of the liver and pancreas and the resection margins were all negative. The operative time was 250 min and intraoperative bleeding was 150 mL. The postoperative course was uneventful, and the patient was discharged seven days later. She was followed up by abdominal CT six months later without signs of recurrence.
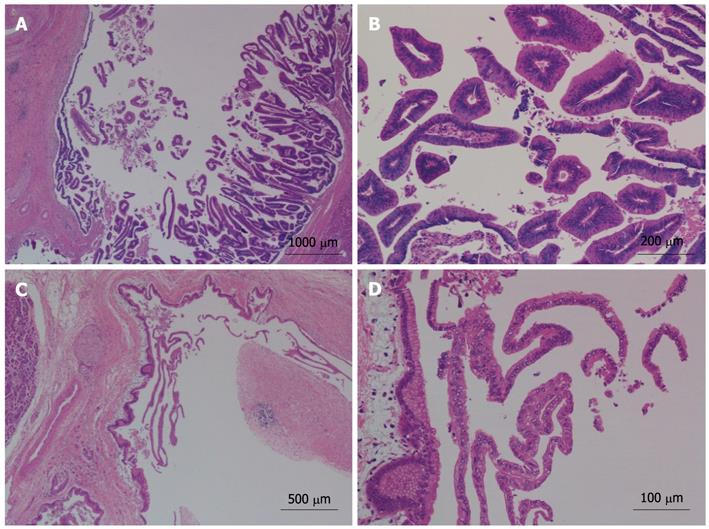
The gross finding was a 5 cm × 4 cm multiloculated cystic mass with stones in the left lateral lobe of liver, communicated with the dilated intrahepatic bile duct. Furthermore, multiple cystic lesions, ranging from 0.5 cm to 1.0 cm in diameter, were located in the pancreatic tail, in communication with the dilated pancreatic duct (Figure 4). All cystic lesions, in both the liver and pancreas, were filled with gelatinous transparent mucin. Microscopically, the cystic wall consisted of hyperplastic fibrous tissues lined by high columnar mucous epithelium demonstrating papillary growth, but no ovarian-like stroma was identified (Figure 5). There was no evidence of high-grade cellular dysplasia or stroma invasion suggesting malignancy in either specimen except for focal low-grade atypical hyperplasia of epithelium in the liver lesion. The immunohistochemical staining of the liver lesion showed cytokeratin 7 (CK7): +/-, CK20: +, cadual type homeobox transcription factor-2 (CDX-2): +, mucin core protein 1 (MUC1): -, mucin core protein 2 (MUC2): + and mucin core protein 5 (MUC5): +, while the pancreas lesion showed CK7: +, CK20: -, CDX-2: -, MUC1: +, MUC2: - and MUC5: +, which were subsequently categorized into pancreaticobiliary and intestinal subtypes of IPMN.
IPMN is a rare but well-established disease, mostly occurs in the biliary tract or pancreas (IPMN-b or IPMN-p). It manifests as a cystic lesion in the imaging findings which make it difficult to differentiate from other cystic tumors preoperatively, such as cystadenoma or cystadenocarcinoma. The clinicopathological characteristics of IPMN can be summarized as follows: (1) Communicating with bile duct or pancreatic duct; (2) Dilated bile duct or pancreatic duct because of mucin hypersecretion and accumulation; (3) Lack of ovarian-like stroma characteristic of mucinous cystic tumor; and (4) Papillary proliferation with delicate fibrovascular cores of ductal epithelium. The cystic lesions of the liver and pancreas in the present case possessed all the above characteristics, so a diagnosis of IPMN-b and IPMN-p was made. We believed that these two lesions were more likely to be the synchronous tumors arising in amenable ducts rather than metastasis, because each lesion was a definite benign tumor without lymph node involvement, and they were grossly and microscopically separate, without continuity between them. Besides, the IPMN-b and IPMN-p in the present case were categorized into different immunophenotypes, pancreaticobiliary and intestinal subtypes respectively, according to the results of immunohistochemical staining.
Although IPMN is frequently associated with malignancy of other organs, including the stomach, colon, esophagus and lung, the simultaneous occurrence of IPMN-b and IPMN-p is extremely rare[6,7]. To the best of our knowledge, only five such cases have been reported in the English-language literature and the present case is the first one treated by total laparoscopic resection[1-5]. Up till now, the origin of simultaneous occurrence of IPMN-b and IPMN-p is still unclear. However, similar clinicopathological findings supported the idea that both tumors might have the same pathogenesis. It was found that the multiple lesions of IPMN were consistent with the concept of field cancerization[8], proposed by slaughter[9], which has been invoked to explain the occurrence of multiple, independent and primary neoplasms[10]. Because the pancreas and the bile duct develop embryonically from the same primordium[11], the present case may also fit the concept of field cancerization.
IPMN-b and IPMN-p are slow-growing tumors with a lower malignant potential than ductal cell carcinoma and mucinous cystadenocarcinoma[12]. Regional lymph node metastasis was rarely found in previously reported cases and excellent prognosis can be expected after complete resection of tumors with negative margins, with a 5-year survival rate higher than 80%[13-15]. Therefore, an accurate preoperative or intraoperative diagnosis is very important in this disease to ensure the curative resection. In contrast to IPMN-p, IPMN-b is more difficult to be diagnosed because of lacking full recognition, especially when it is accompanied with stones. Missed diagnosis of IPMN-b also occurred in our case till massive mucin in bile duct was found during liver resection. Preoperative endoscopic retrograde cholangiopancreatography is more useful than routine CT and magnetic resonance imaging, for the diagnosis of lesions with the characteristic findings of a patulous ampulla, hypersecreted mucin and diffusely dilated biliary tract.
It has been reported that laparoscopic hepatectomy or pancreatectomy is safe, valid, and minimally invasive. However, there are few reports on laparoscopic resection of synchronous liver and pancreatic tumors except one case of laparoscopic resection of a pancreatic polypeptidoma with solitary liver metastasis[16]. In our case, two tumors were located separately in the left lateral lobe of liver and pancreatic tail, making the laparoscopic left lateral hepatolobectomy and distal pancreatectomy an optimal choice of treatment for the patient, since the complicated lymphadenectomy, hilar dissection and gastrointestinal reconstruction were not needed. The spleen was preserved, for there was no evidence of malignancy or splenic vessel invasion according to the imaging findings and intraoperative pathological diagnosis.
We wish to thank Yi-Hong Huang for proofreading all the pathological materials.
Peer reviewers: Benjamin M Yeh, MD, Assistant Chief, Department of Radiology and Biomedical Imaging, San Francisco Veterans Affairs Medical Center, UCSF, 505 Parnassus Ave, M372, Box 0628, San Francisco, CA 94143-0628, United States; Hon-Yi Shi, PhD, Associate Professor, Graduate Institute of Healthcare Administration, Kaohsiung Medical University, 100, Shih-Chuan 1st Road, San Ming District, Kaohsiung 807, Taiwan, China
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Joo YH, Kim MH, Lee SK, Seo DW, Yoo KS, Min YI, Chang JJ, Yu E. A case of mucin-hypersecreting intrahepatic bile duct tumor associated with pancreatic intraductal papillary mucinous tumor. Gastrointest Endosc. 2000;52:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Ishida M, Seki K, Honda K, Kimura T, Katayama K, Hirose K, Dojo M, Azuma T, Imamura Y, Hutchins RR. Intraductal mucinous tumors occurring simultaneously in the liver and pancreas. J Gastroenterol. 2002;37:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Yamaguchi Y, Abe N, Imase K, Mizuno H, Chinen K, Mori H, Sugiyama M, Atomi Y, Ishida H, Takahashi S. A case of mucin hypersecreting intraductal papillary carcinomas occurring simultaneously in liver and pancreas. Gastrointest Endosc. 2005;61:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Zalinski S, Paradis V, Valla D, Belghiti J. Intraductal papillary mucinous tumors of both biliary and pancreatic ducts. J Hepatol. 2007;46:978-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Park BH, Suh JH, Cha HJ, Kim YM, Choi HJ. Intraductal Papillary Mucinous Tumor Simultaneously Involving the Liver and Pancreas-A Case Report. Korean J Pathol. 2010;44:83-86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Riall TS, Stager VM, Nealon WH, Townsend CM, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803-13; discussion 813-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Tanaka M, Kobayashi K, Mizumoto K, Yamaguchi K. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2005;40:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 8. | Izawa T, Obara T, Tanno S, Mizukami Y, Yanagawa N, Kohgo Y. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001;92:1807-1817. [PubMed] |
| 9. | Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter's concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727-1730. [PubMed] |
| 11. | Kim HJ, Kim MH, Lee SK, Yoo KS, Park ET, Lim BC, Park HJ, Myung SJ, Seo DW, Min YI. Mucin-hypersecreting bile duct tumor characterized by a striking homology with an intraductal papillary mucinous tumor (IPMT) of the pancreas. Endoscopy. 2000;32:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Mukawa K, Kawa S, Aoki Y, Zhai Y, Nikaido T. Reduced expression of p53 and cyclin A in intraductal mucin-hypersecreting neoplasm of the pancreas compared with usual pancreatic ductal adenocarcinoma. Am J Gastroenterol. 1999;94:2263-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic analysis of surgically proven intraductal papillary mucinous neoplasms of the pancreas in SNUH: a 15-year experience at a single academic institution. Langenbecks Arch Surg. 2012;397:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Grützmann R, Post S, Saeger HD, Niedergethmann M. Intraductal papillary mucinous neoplasia (IPMN) of the pancreas: its diagnosis, treatment, and prognosis. Dtsch Arztebl Int. 2011;108:788-794. [PubMed] |
| 15. | Lee SS, Kim MH, Lee SK, Jang SJ, Song MH, Kim KP, Kim HJ, Seo DW, Song DE, Yu E. Clinicopathologic review of 58 patients with biliary papillomatosis. Cancer. 2004;100:783-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Chan WF, Lo CY, Lo CM, Fan ST. Laparoscopic resection of a pancreatic polypeptidoma with a solitary liver metastasis. Surg Endosc. 2004;18:554-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |