Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6504
Revised: September 26, 2012
Accepted: September 29, 2012
Published online: November 28, 2012
Progressive familial intrahepatic cholestasis type 1 is a rare disease that is characterized by low serum γ-glutamyltransferase levels due to mutation in ATP8B1. We present a 23-year-old male who experienced persistent marked pruritus for eighteen years and recurrent jaundice for thirteen years, in addition to cholestasis that eventually became fatal. Genetic sequencing studies of the entire coding (exon) sequences of ATP8B1 and ABCB11 uncovered a novel heterozygous missense 3035G>T mutation (S1012I) and a synonymous 696T>C mutation in ATP8B1. The patient’s progression was associated with not only impaired familial intrahepatic cholestasis 1 (FIC1) function but also impaired bile salt export pump expression due to the impaired FIC1 function. Our findings show that patients with intermittent cholestasis can develop progressive liver disease even after several decades and require regular follow up.
-
Citation: Deng BC, Lv S, Cui W, Zhao R, Lu X, Wu J, Liu P. Novel
ATP8B1 mutation in an adult male with progressive familial intrahepatic cholestasis. World J Gastroenterol 2012; 18(44): 6504-6509 - URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6504.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6504
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of severe autosomal recessive liver disorders of childhood that result in cholestasis that can progress to end-stage in the first or second decade of life[1,2]. PFIC-1 and PFIC-2 are characterized by low serum γ-glutamy-ltransferase (GGT) levels. Patients with PFIC-1 have mutations in ATP8B1 (encoding FIC1), which maps to chromosome 18q21-22, and patients with PFIC-2 have mutations in ABCB11 (encoding bile salt export pump, BSEP), which maps to chromosome 2q24. PFIC-3 is characterized by high serum GGT levels and is caused by genetic mutations in ABCB4 (encoding multidrug resistant protein 3)[3].
We report an unusual patient who presented with childhood-onset progressive intrahepatic cholestasis with low serum GGT levels and died in his third decade of life. Genetic sequencing analysis revealed a novel heterozygous 3035G>T mutation (S1012I) and a synonymous 696T>C mutation in ATP8B1.
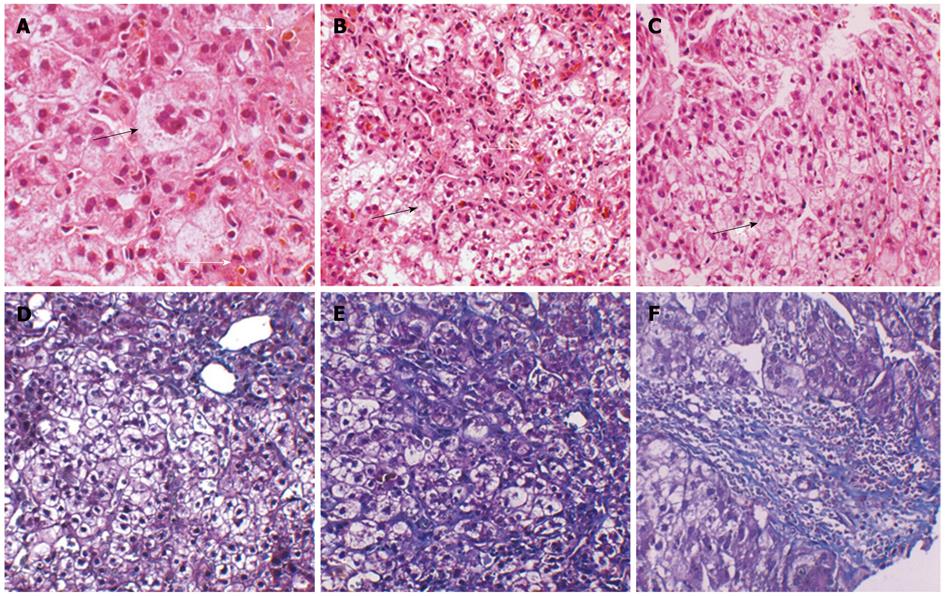
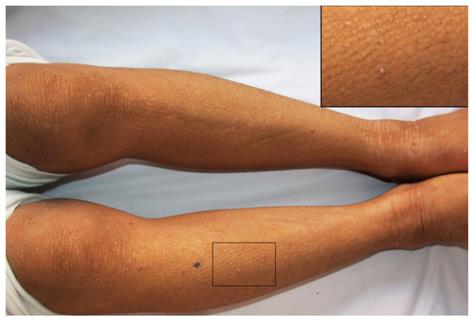
A 23-year-old male presenting with an 18-year history of persistent pruritus and a 13-year history of recurrent jaundice was admitted to the department of infectious disease. He had gradually developed severe pruritus with pale yellow stools and dark urine 2 mo before admission. He had been admitted to hospital 13 years previously for pruritus, hyperbilirubinemia and mildly elevated alanine aminotransferase levels (Table 1). Skin pathology conducted at that time showed hyperkeratosis of epidermal cells, papilloma hyperplasia, acanthosis, and a slight increase of basal pigmentation (results not shown). The liver biopsy revealed intrahepatic cholestasis with ballooning degeneration of hepatocytes, no significant fibrosis, and multinucleated giant cells (Figure 1). Jaundice recurred four times during the subsequent 13 years. Serum biochemistry was returned to normal levels by treatment with a liver protectant. There was no family history of liver disease. On physical examination, the patient was 150 cm tall and 55 kg in weight. The skin and sclera were markedly icteric. The skin was rough and thickened (Figure 2). Palmar erythema and mild splenomegaly were noted. The rest of the examination was unremarkable.
| Variables | Reference range | Age 10 yr | On admission | 2 wk | 1 mo | 2 mo |
| Bilirubin, total (μmol/L) | 3.4-20.5 | 213.5 | 250.1 | 303.7 | 180.5 | 420.1 |
| BiLirubin, direct (μmoL/L) | 0.0-6.8 | 158.6 | 188.6 | 213.1 | 115.6 | 286.7 |
| GGT (U/L) | 10-47 | 8 | 7 | 31 | 26 | NA |
| ALP (U/L) | 40-129 | 237 | 345 | 322 | 300 | NA |
| AST (U/L) | 8-40 | 256 | 202 | 222 | 156 | 466 |
| ALT (U/L) | 5-40 | 375 | 196 | 183 | 130 | 148 |
| ALbumin (g/L) | 40-50 | NA | 35 | 33.3 | NA | 24.7 |
| PT (s) | 11.5-13.5 | NA | 14.9 | NA | NA | 28.5 |
| PTA (%) | 80-120 | NA | 80.6 | NA | NA | 24.7 |
| BiLe acids (μmoL/L) | 0-10 | NA | 296 | 239 | 218 | NA |
| PLasma ammonia (μmoL/L) | 0-23 | NA | 15 | NA | NA | 61.5 |
| CHE (U/L) | NA | NA | 1519 | 1720 | NA | NA |
Liver function tests showed conjugated hyperbilirubinemia, elevated serum aminotransferase levels, low serum GGT values, increased bile acids levels and hypoalbuminemia (Table 1). The patient was negative for markers of hepatitis virus. Antibodies to cytomegalovirus and Epstein-Barr virus were negative. Ceruloplasmin and serum copper were normal, and no Kayser-Fleischer ring was observed upon examination by an experienced ophthalmologist. A qualitative urinary porphyrin test was negative. Autoimmune antibodies including antimitochondrial antibody, antinuclear antibodies, antineutrophil cytoplasmic antibody were all negative, and serum α-1-antitrypsin concentration, thyroid function and other laboratory investigation results were normal.
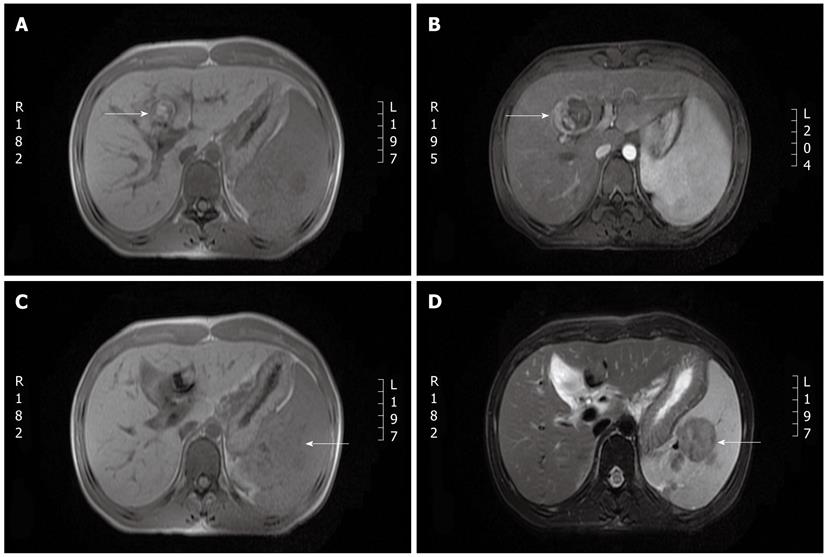
A lesion in segment 4 of the liver, gallbladder stones, splenomegaly and an oval-shaped signal intensity in the spleen were observed upon magnetic resonance imaging (MRI) (Figure 3). No dilatation of the bile duct was observed by magnetic resonance cholangiopancreatography. A repeat biopsy was performed.
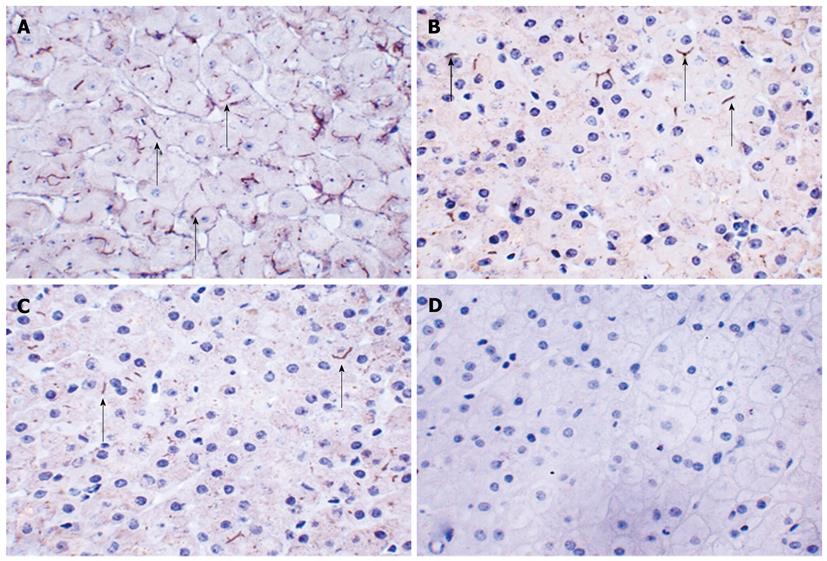
The liver biopsy revealed cholestasis, inflammation and fibrosis at the portal area. The mass consisted of normal liver tissue with normal liver architecture and mild fibrosis (data not shown). The fibrosis stained with hematoxylin and eosin and Masson’s trichrome at portal area in the 23-year-old liver tissue was more severe than that in the liver tissue sample taken from the same patient at 10 years of age. Atypical hyperplasia was not observed in the mass, and CD10, CD34 and α fetoprotein (AFP) immunochemistry staining were unremarkable (data not shown). BSEP expression was present only focally and was assessed as both faint and patchy in the liver biopsy tissue (Figure 4).
Ursodeoxycholic acid (UDCA) and vitamin supplements were prescribed. Serum total bilirubin was persistently elevated, and liver transplantation and partial external biliary diversion were advised. The patient declined surgery to resolve the cholestasis and was discharged. The patient was followed up but died of liver failure and hepatic encephalopathy two months later.
Genetic studies were performed with the informed consent of the patient, and the study was approved by the Ethics Committee of China Medical University. Genomic DNA was extracted from peripheral blood leucocytes. ABCB11 and ATP8B1 were analyzed by direct sequencing of polymerase chain reaction (PCR) products. PCR primers were designed to amplify the 27 exons of the entire coding sequence of the ATP8B1 gene and the 28 exons of the ABCB11 gene, followed by direct sequencing using a Genetic Analyzer 3730 instrument (Applied Biosystems). A synonymous 696T>C mutation and a novel heterozygous missense 3035G>T mutation (S1012I) were discovered in exons 7 and 24 of the ATP8B1 gene. We obtained blood samples from the patient’s parents and sisters, all of which were negative for the ATP8B1 and ABCB11 mutations. The mutations were absent in 100 control chromosomes (evaluated by direct sequencing). This missense mutation has not been reported in any other benign recurrent intrahepatic cholestasis (BRIC)-1 or PFIC-1 patient.
In this study, we present a patient with a novel mutation in ATP8B1, who experienced the onset of persistent sever pruritus at 5 years old and the onset of progressive intrahepatic cholestasis at 10 years old, eventually dying of liver failure at age 23. Common chronic cholestatic diseases such as primary biliary cirrhosis, sclerosing cholangitis and idiopathic ductopenia were excluded by laboratory, radiographic and histological examinations. Based on the absence of the missense mutation (S1012I) in 100 control chromosomes, the novel mutation S1012I is believed to be the disease-causing mutation. ATP8B1 is also associated with BRIC1 and intrahepatic cholestasis of pregnancy, which are characterized by intermittent cholestasis without liver scarring[4]. These clinical and histopathological features are generally considered diagnostic for PFIC-1 rather than BRIC type 1. The differences in clinical outcome and disease duration may be dictated by the variations of the structure and/or function of the FIC1 protein due to the mutation in ATP8B1[5]. Although the novel mutation 3035G>T is predicted to impair FIC1 function, the effects of the missense 3035G>T mutation and the synonymous 696T>C mutation on the FIC1 protein are unknown.
Though the function of FIC1 protein is not yet known, it is thought to mediate the inward translocation of phosphatidylserine from the exoplasmic to the cytoplasmic leaflet of the plasma membrane[5]. Immunohistochemistry for FIC1 protein was not performed due to the current lack of a commercially available specific antibody to detect the absence/alteration of the FIC1 protein. Impaired FIC1 function may lead to a loss of lipid asymmetry in the canalicular membranes of hepatocytes, resulting in BSEP dysfunction; decreased expression of BSEP (encoded by ABCB11) has been observed in livers from PFIC-1 patients, and this decrease is associated with the accumulation of bile salts in hepatocytes[6,7]. It is also reported that a marked decrease in the activity of farnesoid X receptor, which can positively regulate the expression of BSEP and affects canalicular membrane composition to impair ABCB11 activity, can be induced by a loss of FIC1 expression[8,9]. Immunohistochemistry for BSEP in this patient revealed that BSEP expression was present only focally and was assessed as both faint and patchy in the liver biopsy sample. Thus, the chronic cholestasis in this patient was associated with not only impaired FIC1 protein function but also impaired BSEP expression. No mutation in ABCB11 was detected, which suggested that impaired expression of BSEP in the patient was most likely induced by impaired FIC1 protein function.
PFIC-1 patients may exhibit extrahepatic features such as persistent short stature, diarrhea, pancreatitis and sensorineural hearing loss[10], which were not present in this patient. Here, we show that a patient with intermittent cholestasis can develop progressive liver disease even after several decades. However, this does not imply that all patients with BRIC will progress to PFIC. Although van Ooteghem et al[11]reported that 4 of 63 patients with intermittent cholestasis developed progressive liver disease after several decades, only 2 of the 4 patients were diagnosed genetically, in assays that revealed the same splice site mutation resulting in skipping of exon 24. Here, we have uncovered a novel missense mutation in ATP8B1 associated with progressive liver disease in a patient with intermittent cholestasis. A possible phenotypic continuum between BRIC-2 and PFIC-2 in a patient with mild disease, who experienced a complete remission of liver fibrosis following treatment with UDCA, was reported[12]. The patient we presented did not respond to treatment with UDCA and died of liver failure, in contrast to the previously described patient with phenotypic continuum.
PFIC may induce hepatic malignancies, which can be coincident with hepatoblastoma[13]. CD10, CD34 and AFP immunochemistry staining was performed to distinguish a well-differentiated hepatocellular carcinoma from normal and cirrhotic liver tissue or benign liver nodules were performed; the results were negative[14,15]. The liver mass in this patient was found to be a benign lesion with normal liver architecture.
The current medical therapy for PFIC-1 is either non-existent or useful for only a limited duration, and some agents, including ursodeoxycholic acid, provide mainly symptomatic relief. Mutation-specific drugs may become a new tool for PFIC-1 therapy in the near future. Most patients with progressive cholestasis eventually require surgical intervention[10]. Liver transplantation should be considered in patients who develop cirrhosis or progressive liver disease despite treatment.
In conclusion, we report a rare case that presented with onset persistent sever pruritus progressing to PFIC-1 due to a novel heterozygous ATP8B1 mutation. Both impaired FIC1 function and impaired BSEP expression induced by the impaired FIC1 function were associated with this progression. This case shows that patients with intermittent cholestasis can develop progressive liver disease, even after several decades, and should be followed up regularly. Further studies are necessary to investigate the impact of the 3035G>T mutation on functional defects in FIC1 and BSEP.
Peer reviewer: Dr. Seyed Mohsen Dehghani, MD, Associate Professor, Department of Pediatrics, Nemazee Hospital, Shiraz University of Medical Sciences, 71937-11 Shiraz, Iran
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Cavestro GM, Frulloni L, Cerati E, Ribeiro LA, Corrente V, Sianesi M, Franzè A, Di Mario F. Progressive familial intrahepatic cholestasis. Acta Biomed. 2002;73:53-56. [PubMed] |
| 2. | Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, Dahan K, Childs S, Ling V. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 766] [Cited by in RCA: 673] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | Chen HL, Liu YJ, Su YN, Wang NY, Wu SH, Ni YH, Hsu HY, Wu TC, Chang MH. Diagnosis of BSEP/ABCB11 mutations in Asian patients with cholestasis using denaturing high performance liquid chromatography. J Pediatr. 2008;153:825-832. [PubMed] [DOI] [Full Text] |
| 4. | Klomp LW, Vargas JC, van Mil SW, Pawlikowska L, Strautnieks SS, van Eijk MJ, Juijn JA, Pabón-Peña C, Smith LB, DeYoung JA. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Folmer DE, van der Mark VA, Ho-Mok KS, Oude Elferink RP, Paulusma CC. Differential effects of progressive familial intrahepatic cholestasis type 1 and benign recurrent intrahepatic cholestasis type 1 mutations on canalicular localization of ATP8B1. Hepatology. 2009;50:1597-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Alvarez L, Jara P, Sánchez-Sabaté E, Hierro L, Larrauri J, Díaz MC, Camarena C, De la Vega A, Frauca E, López-Collazo E. Reduced hepatic expression of farnesoid X receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum Mol Genet. 2004;13:2451-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Davit-Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, Bernard O, Jacquemin E. ATP8B1 and ABCB11 analysis in 62 children with normal gamma-glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 8. | Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, Strautnieks SS, Thompson RJ, Magid MS, Gordon R. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756-764. [PubMed] |
| 9. | Paulusma CC, de Waart DR, Kunne C, Mok KS, Elferink RP. Activity of the bile salt export pump (ABCB11) is critically dependent on canalicular membrane cholesterol content. J Biol Chem. 2009;284:9947-9954. [PubMed] |
| 10. | Stapelbroek JM, van Erpecum KJ, Klomp LW, Houwen RH. Liver disease associated with canalicular transport defects: current and future therapies. J Hepatol. 2010;52:258-271. [PubMed] |
| 11. | van Ooteghem NA, Klomp LW, van Berge-Henegouwen GP, Houwen RH. Benign recurrent intrahepatic cholestasis progressing to progressive familial intrahepatic cholestasis: low GGT cholestasis is a clinical continuum. J Hepatol. 2002;36:439-443. [PubMed] |
| 12. | Lam CW, Cheung KM, Tsui MS, Yan MS, Lee CY, Tong SF. A patient with novel ABCB11 gene mutations with phenotypic transition between BRIC2 and PFIC2. J Hepatol. 2006;44:240-242. [PubMed] |
| 13. | Richter A, Grabhorn E, Schulz A, Schaefer HJ, Burdelski M, Ganschow R. Hepatoblastoma in a child with progressive familial intrahepatic cholestasis. Pediatr Transplant. 2005;9:805-808. [PubMed] |
| 14. | Chu PG, Ishizawa S, Wu E, Weiss LM. Hepatocyte antigen as a marker of hepatocellular carcinoma: an immunohistochemical comparison to carcinoembryonic antigen, CD10, and alpha-fetoprotein. Am J Surg Pathol. 2002;26:978-988. [PubMed] |
| 15. | Coston WM, Loera S, Lau SK, Ishizawa S, Jiang Z, Wu CL, Yen Y, Weiss LM, Chu PG. Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol. 2008;32:433-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |