Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6468
Revised: July 6, 2012
Accepted: July 18, 2012
Published online: November 28, 2012
AIM: To evaluate the utility of magnified narrow-band imaging (NBI) endoscopy for diagnosing and treating minute pharyngeal neoplasia.
METHODS: Magnified NBI gastrointestinal examinations were performed by the first author. A magnification hood was attached to the tip of the endoscope for quick focusing. Most of the examinations were performed under sedation. Magnified NBI examinations were performed for all of the pharyngeal lesions that had noticeable brownish areas under unmagnified NBI observation, and an intrapapillary capillary loop (IPCL) classification was made. A total of 93 consecutive pharyngeal lesions were diagnosed as IPCL type IV and were suspected to represent dysplasia. Sixty-two lesions of approximately 1 mm in diameter were biopsied in the clinic, and 17 lesions with larger diameters were resected by endoscopic submucosal dissection (ESD) at the Hiroshima University Hospital. In addition to the histological diagnoses, the lesion diameters were microscopically measured in 45 of the 62 biopsies. Thirty-four of the 62 biopsied patients received endoscopic follow up.
RESULTS: Minute pharyngeal lesions were diagnosed in 93 of approximately 3000 patients receiving magnified NBI examinations at the clinic. Of the 93 patients with IPCL type IV lesions, 80 were men, and 13 were women. Fifty-six were drinkers, and 57 were smokers. Two had esophageal cancer. Twenty-one lesions were located on the posterior hypopharyngeal wall, and 72 lesions were located on the posterior oropharyngeal wall. All 93 lesions were flat and showed similar findings in the magnified and unmagnified NBI examinations. Although almost all of the IPCL type IV lesions showed faint redness when examined under white light, it was difficult to diagnose the lesions using only this technique because the contrast was weaker than that achieved in the NBI examinations. Of the 93 lesions, only 3 had diameters greater than 2.1 mm. Sixty-two lesions of approximately 1 mm were biopsied in the clinic, whereas 17 larger lesions were treated by ESD at the Hiroshima University Hospital. Of the 79 pharyngeal lesions that were biopsied or resected by ESD, 5 were histologically diagnosed as high-grade dysplasia, 39 were diagnosed as low-grade dysplasia, and 39 were determined to be non-dysplastic lesions. There were no cancerous lesions. Histologically, abnormal cell size variations and increased nuclear size were observed in all of the high-grade dysplasia lesions, while the incidence of these findings in the low-grade dysplasia lesions was low. Of the 62 biopsied lesions, 45 were microscopically measurable. The measured diameters ranged from 0.1 to 2.0 mm. The dysplasia ratios increased with the diameters. A follow-up endoscopic examination of the 34 biopsied patients found the rate of complete resection by biopsy to be 79%. The largest lesion in which complete resection was expected was a low-grade dysplasia of 1.9 mm in diameter.
CONCLUSION: Minute pharyngeal lesions suspected to be dysplasia that are identified by NBI magnifying endoscopy should be biopsied to determine the diagnosis and further treatment.
- Citation: Kumamoto T, Sentani K, Oka S, Tanaka S, Yasui W. Clinicopathological features of minute pharyngeal lesions diagnosed by narrow-band imaging endoscopy and biopsy. World J Gastroenterol 2012; 18(44): 6468-6474
- URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6468.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6468
Magnified narrow-band imaging (NBI) endoscopy is reportedly useful for early pharyngeal[1-5] and esophageal cancer diagnosis[6-10]. Magnified NBI is useful both for detecting suspicious changes and for further diagnostic purposes, such as determining whether a lesion is suspected of being dysplasia or cancer[11,12]. We routinely examined upper gastrointestinal tracts using magnified NBI for one and a half years. When the unmagnified NBI detected a brownish area in the pharynx, a magnified NBI examination was used to aid the intrapapillary capillary loop (IPCL) classification[12-14]. If IPCL type IV or V was observed, dysplasia or cancer was suspected, and treatment was considered. Although endoscopic submucosal dissection (ESD) leads to complete resection, ESD is an in-patient procedure that requires intubation anesthesia. For smaller (approximately 1 mm) lesions, therefore, our first choice is to biopsy the lesion for histological diagnosis while performing a complete resection. There have been no previous reports on the utility of biopsying minute pharyngeal lesions. This article reports our histological diagnosis, treatment, and follow-up findings from minute pharyngeal dysplasia biopsies.
Most patients over 30 years old who require gastrointestinal endoscopy at the clinic are examined by magnified NBI. From August 2008 to March 2010, a total of 93 consecutive patients with IPCL type IV pharyngeal lesions, as determined by magnified NBI, were enrolled in this study (Table 1). The patients included 80 men aged 39-87 years (mean age, 66.9 years) and 13 women aged 43-84 years (mean age, 67.1 years). Drinking and smoking habits were assessed for 86 patients. Lesions of approximately 1 mm in diameter were found in 62 patients and were biopsied at the clinic, and the 17 patients with larger lesions were endoscopically resected by ESD at the Hiroshima University Hospital. Detailed histological assessments were performed for 62 of the biopsies. Thirty-four of the 62 biopsied patients received follow up endoscopy.
| Characteristics | No. |
| Age, yr (range) | 67 (39-87) |
| Sex, men/women | 80/13 |
| Alcohol consumption | |
| Yes | 56 (54 men, 2 women) |
| No | 30 (22 men, 8 women ) |
| Smoker | |
| Yes | 57 (56 men, 1 woman) |
| No | 29 (20 men, 9 women) |
| Esophageal cancer history | |
| Yes | 2 |
| No | 91 |
| Other cancer history | |
| Yes | 9 (8 gastric cancer, 1 colon cancer) |
| No | 84 |
| Location | |
| Hypopharynx | 21 (17 men, 4 women) |
| Oropharynx | 72 (63 men, 9 women) |
| Biopsy cases | 62 |
| High-grade dysplasia | 3 |
| Low-grade dysplasia | 25 |
| Non-dysplasia | 34 |
| ESD cases | 17 |
| High-grade dysplasia | 2 |
| Low-grade dysplasia | 14 |
| Non-dysplasia | 1 |
The following instruments were used in this study: a magnifying endoscope that was capable of × 80 magnification (GIF H260Z; Olympus Optical Co. Ltd, Tokyo, Japan), a standard videoendoscopy system (EVIS LUCERA; Olympus), and an NBI system (Olympus).
All of the endoscopic examinations were performed by the first author (Kumamoto T). A magnification hood (MB-46, Olympus) was attached to the tip of the endoscope. Intravenous access and pulse oximetry monitoring were established prior to the examination. Most of the examinations were performed under intravenous pethidine hydrochloride (17.5-70 mg) and midazolam (0.5-4 mg) sedation. The pharynx was mainly observed by NBI from the beginning of the examination. The pharynx was examined in the following order: uvula, posterior oropharyngeal wall, epiglottis, posterior hypopharyngeal wall, and pyriform sinus. The pharynx observation time was approximately 1 min. Magnified NBI was used for all pharynx lesions with noticeable brownish areas in the NBI examination, and an IPCL classification was performed. The IPCL classification followed the criteria of Dr. Inoue H[12,13]. According to these criteria, a lesion must meet three of the following four characteristics to be classified as IPCL type IV: dilatation, tortuous running, caliber changes, and different shapes in each IPCL[12-14]. In the 93 IPCL type IV lesions, smaller lesions of approximately 1 mm in diameter were biopsied with disposable biopsy forceps (FB-210K, Olympus). To avoid post-biopsy bleeding, all anticoagulants were discontinued from 3 d before to 3 d after the biopsy. The biopsy patients remained in the clinic for 2-3 h, including a 1 h post-sedation recovery time. Annual magnified NBI examinations were recommended to all of the biopsy patients, and 34 of 62 biopsy patients received follow up. To reduce inter-observer variation, the results of the NBI and magnified NBI examinations were independently evaluated by 2 endoscopy examiners (Kumamoto T and Oka S). When the evaluations differed, a consensus decision was achieved by reviewing the magnified NBI images.
The biopsy specimens were extended and fixed to a styrene foam plate by fine acupuncture needles. All of the specimens were fixed in 10% formalin and embedded in paraffin wax. The tissue specimens were cut into 3-μm thick sections, and all of the sections received routine pathological diagnoses. The pathological parameters of each lesion were independently evaluated by two pathologists (Sentani K, Yasui W) and used for further analyses. The dysplasia diagnoses followed the criteria proposed by the World Health Organization[15]. In this study, dysplasia was classified as low-grade (mild or moderate dysplasia) or high-grade (severe dysplasia). These criteria were based on the architectural and cytological abnormalities. In addition to these abnormalities, IPCL changes were considered[16]. As shown in Table 2, the histological diagnoses were based on the IPCL changes and on architectural and cytological atypia. The IPCL changes were defined as upward extension, dilatation and branching, and diameter expansion. Architectural atypia was determined by a proliferative cell distribution and the tumor front, and cytological atypia was assessed by cell size, nuclear arrangement and nuclear size. The lesion diameters were measured under light microscopy using the built-in measurement system of the light microscope, which measured to an accuracy of 0.1 mm.
| Non-D | LGD | HGD | |
| Number | 34 | 25 | 3 |
| IPCL | |||
| Upward extension | 26 (76) | 24 (96) | 3 (100) |
| Dilatation and branching | 5 (15) | 21 (84) | 3 (100) |
| Diameter expansion | 0 (0) | 8 (25) | 1 (33) |
| Architectural atypia | |||
| Proliferative cell distribution | |||
| ≥ 2/3 | 0 (0) | 0 (0) | 3 (100) |
| < 2/3 | 34 (100) | 25 (100) | 0 (0) |
| Tumor front | 0 (0) | 23 (92) | 3 (100) |
| Cytological atypia | |||
| Abnormal variation in cell size | 0 (0) | 2 (8) | 3 (100) |
| Abnormal nuclear arrangement | 0 (0) | 23 (92) | 3 (100) |
| Increased nuclear size | |||
| High | 0 (0) | 0 (0) | 3 (100) |
| Absent or low | 34 (100) | 25 (100) | 0 (0) |
Minute pharyngeal lesions were diagnosed in 93 of approximately 3000 patients who were examined by magnified NBI. The clinicopathological characteristics of the patients are shown in Table 1. Of the 93 patients, 80 were men, and 13 were women. Fifty-six patients were drinkers, 57 were smokers, and 2 had esophageal cancer. Other cancers included gastric cancer in 8 patients and colon cancer in 1 patient. Twenty-one lesions were located on the posterior hypopharyngeal wall, and 72 lesions were found on the posterior oropharyngeal wall.
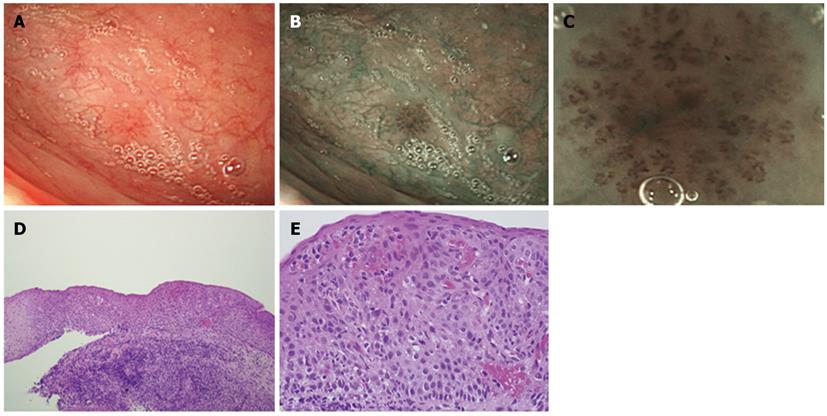
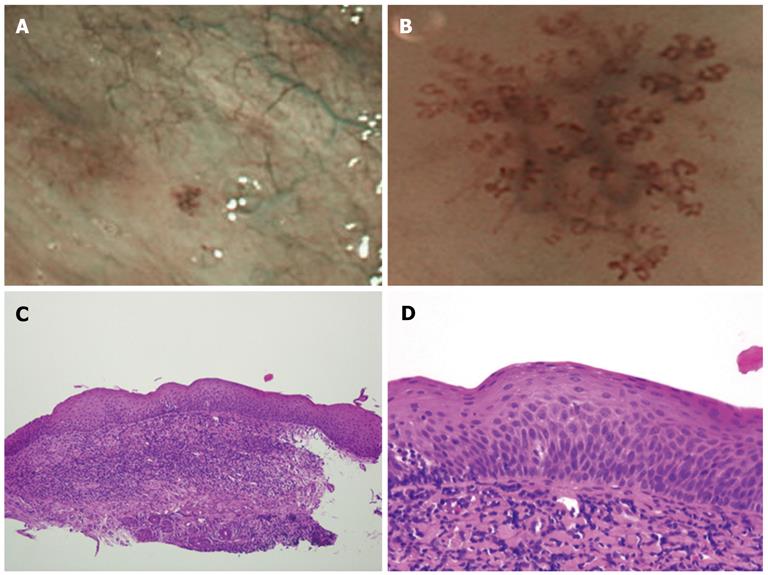
All 93 lesions were flat and showed similar findings in the magnified and unmagnified NBI examinations (Figures 1B, C; 2A, B). Most of the IPCL type IV lesions were identifiable immediately after the magnified NBI diagnosis by a faint redness under white light (Figure 1A). However, it was difficult to diagnose IPCL type IV lesions by this characteristic before an NBI examination because the contrast is weaker than that of NBI (Figure 1A, B). Of the 93 lesions, only 3 were greater than 2.1 mm in diameter, and the remaining lesions were less than 2.0 mm in diameter. Sixty-two lesions of approximately 1 mm in diameter were biopsied for histological diagnoses at the clinic, and 17 larger lesions were treated by ESD at the Hiroshima University Hospital. There were no complications, such as bleeding or pharyngeal pain, during or after the ESD.
The histological diagnoses of the 79 lesions resected by biopsy or ESD included 5 cases of high-grade dysplasia, 39 cases of low-grade dysplasia, and 35 non-dysplastic lesions. The 35 non-dysplastic cases consisted of 19 inflamed (pharyngitis) and 16 normal lesions. A lesion diagnosed as high-grade dysplasia is shown in Figure 1, and an example of low-grade dysplasia is shown in Figure 2. There were no cancerous lesions. The histological features of each type of dysplasia are shown in Table 2. In high-grade dysplasia, the polarity of the nucleus was lost, and the nuclear density was markedly increased throughout the intraepithelial layer, although the superficial portion of the epithelium was mature (Figure 1E). The microvascular irregularities were more severe than those seen in low-grade dysplasia. Basal cell palisading was observed in the low-grade dysplasia lesions; however, proliferative cells with enlarged nuclei that proliferated in a lamellar pattern were limited to the lower two-thirds of the epithelial layer (Figure 2D). IPCL abnormalities such as upward extension, dilatation, and diameter expansion were clearly recognized. In the high-grade dysplasia lesions, abnormal variations in cell size and increased nuclear size were observed in all of the lesions, whereas the incidence of these findings in the low-grade dysplasia lesions was low (Figures 1E, 2D). In the non-dysplastic lesions, such as inflamed squamous epithelium, intercellular edema and intraepithelial inflammatory cells were recognizable.
Of the 62 biopsied lesions, 45 were measurable (Table 3). The measured diameters were 0.1-2.0 mm (average, 1.12 mm); 30 lesions were 0.1-1.0 mm in diameter, and 15 lesions were 1.1-2.0 mm in diameter. The distribution of the lesion diameters and the neoplasia ratios are shown in Table 3. The dysplasia ratios (low-grade or high-grade) were 0% less than 0.2 mm, 20%-40% from 0.3 to 0.8 mm, 71% from 0.9 to 1.0 mm, 89% from 1.1 to 1.2 mm, and 100% from 1.3 to 2.0 mm. The ratio increased as the diameter increased. The diameters of the 3 high-grade lesions were 1.0, 1.1, and 1.3 mm.
| Diameter (mm) | n | Non-D | LGD | HGD |
| 0.1-0.2 | 3 | 3 (100) | 0 (0) | 0 (0) |
| 0.3-0.4 | 5 | 3 (60) | 2 (40) | 0 (0) |
| 0.5-0.6 | 5 | 4 (80) | 1 (20) | 0 (0) |
| 0.7-0.8 | 10 | 6 (60) | 4 (40) | 0 (0) |
| 0.9-1.0 | 7 | 2 (29) | 4 (57) | 1 (14) |
| 1.1-1.2 | 9 | 1 (11) | 7 (78) | 1 (11) |
| 1.3-1.4 | 2 | 0 (0) | 1 (50) | 1 (50) |
| 1.5-1.8 | 1 | 0 (0) | 1 (100) | 0 (0) |
| 1.9-2.0 | 3 | 0 (0) | 3 (100) | 0 (0) |
Twenty-seven of the 34 biopsy patients who received endoscopic follow up (79%) had no lesions at the biopsy site in their NBI examinations, and complete resection from the biopsy was expected in these patients. The interval from the first diagnosis to the follow-up ranged from 4-22 mo (mean, 12.5 mo). The diameters of the lesions in the 3 incomplete resection cases were 0.7 mm, 1.1 mm, and 1.1 mm. Sixteen of the 34 cases were of high- or low-grade dysplasia, and 13 of these 16 cases (81%) had no lesions at the biopsy site. Complete resection from the biopsy was expected in these cases. The largest lesion for which complete resection was expected was 1.9 mm in diameter and was classified as low-grade dysplasia.
Magnified NBI endoscopy is preferable for diagnosing minute pharyngeal lesions[17-21]. The current practice at our clinic is to observe the pharynx and esophagus by NBI from the beginning of the examination. A distal magnification attachment on the endoscope tip is effective for quick focusing. The typical time for a magnified NBI examination from the pharynx to the duodenum was 10-15 min. The routine pharynx examination time was approximately 1 min.
During the period from September 2008 to March 2010, 93 patients with minute pharyngeal IPCL type IV lesions of approximately 1 mm in diameter were diagnosed at our clinic by magnified NBI endoscopy. During this period, we performed approximately 3000 routine examinations of the upper gastrointestinal tract using magnified NBI endoscopy. Thus, the frequency of such lesions was approximately 3%. Although it is well known that male drinkers and smokers aged over 50 are at high risk for pharyngeal carcinoma[22], we also observed several cases of low-grade dysplasia in women, and the youngest case was a 39-year-old man. Therefore, we routinely examine almost all men and women over 30 with magnified NBI endoscopy. In the present study, 2 patients had esophageal cancer[23-26], 8 had gastric cancer, and one had colon cancer. A history of other cancers may also be considered a risk factor.
All 93 lesions were intraepithelial flat lesions. Because the magnified NBI endoscopy findings were similar and showed no clear differences among high-grade dysplasia, low-grade dysplasia and non-dysplastic lesions, the 93 lesions appeared to have similar characteristics. If the lesions had been followed for a longer period, transitions from low-grade to high-grade dysplasia might have occurred. There is controversy over whether such lesions should be followed, biopsied, or treated. Because the histological diagnosis for 3 biopsied cases and 2 ESD cases was high-grade dysplasia, at least 5 lesions (all of which were in men) were considered to be precancerous. Although there were no cancerous lesions in this series[18], routinely using magnified NBI endoscopy seemed effective for diagnosing pharyngeal precancerous lesions early and showed that IPCL type IV lesions should be biopsied or resected by ESD. Because esophageal IPCL type IV lesions are thought to represent high-grade dysplasia, treatment is recommended[13]. However, treating smaller esophageal or pharyngeal IPCL type IV lesions (approximately 1 mm in diameter) is still controversial. Whether they should be treated or followed remains to be determined. Because the smaller pharyngeal IPCL type IV lesions in our study contained some precancerous lesions, we recommend biopsy and treatment over follow up.
A survey of the diameters of the biopsied lesions indicated an increase in the dysplasia ratio as the diameter increased, and all lesions over 1.3 mm were found to be dysplastic. Our clinic sees typical outpatients with varied gastrointestinal symptoms or abnormal gastric X-ray findings, and the diameter distribution we observed appears to be close to the natural distribution of such lesions. The diameter of the 3 high-grade lesions ranged from 1.0 to 1.3 mm; therefore, it would appear that lesions greater than 1.0 mm in diameter should be resected by biopsy or ESD. Furthermore, because low-grade lesions as small as 0.3 mm in diameter were observed, it would be better to resect all lesions less than 1.0 mm in diameter. The diameter distribution appears to show the natural progression from low-grade to high-grade dysplasia.
A follow-up study of the biopsied cases revealed that the resection rate by biopsy alone was 79%. The diameter of the largest resected lesion was 1.9 mm. Although annual endoscopic follow-up must be continued, biopsy may lead to complete resection in some cases and can be performed as a first-line therapy. Single-use disposable biopsy forceps were used to obtain a biopsy specimen as large and as deep as possible for complete resection. For the biopsy, the plane of the opened biopsy forceps should be horizontal, which can be achieved by rotating the handle. Precise, lesion-centered biopsies of 1 mm lesions are difficult[13] and require high levels of concentration and cooperation between the examiner and the assisting medical staff. Recently, we have biopsied these lesions prior to inserting the endoscope into the esophagus because if we try to biopsy during the final withdrawal stage, the observations may be disturbed by secreted mucus and minute bleeding. Biopsying the pharynx appeared to be safe providing all anticoagulant drugs were discontinued from 3 d before to 3 d after biopsy. We experienced no complications (such as bleeding) after the biopsies.
Compared to the esophagus, the pharynx is sensitive to being touched by an endoscope. Therefore, almost all of the magnified NBI magnifying examinations were performed under sedation. The hypopharynx is particularly sensitive. Without sedation, the pharyngeal reflex causes responsive artificial bleeding, particularly when touched by the distal attachment during examination, and NBI observation becomes difficult due to the brownish color change in the entire endoscopic field. In our experience, magnified NBI endoscopy can be routinely performed with the patient under sedation. However, because deep sedation may cause respiratory depression, sedation should be used cautiously with an intravenous drip, and with oxygen and the counteracting effect of flumazenil readily available. In high-risk patients, such as those with respiratory or heart disease, for safety, we removed the distal attachment on the scope tip or changed the scope to a smaller diameter non-magnifying scope (GIF H260, Olympus).
In conclusion, magnified NBI endoscopy appears to be preferable for diagnosing pharyngeal neoplasia. Biopsy was useful for the diagnosis and treatment of minute pharyngeal neoplasia.
It has been difficult to diagnose an early-stage pharyngeal carcinoma. Narrow-band imaging (NBI) has enabled more accurate diagnosis and increased the detection rate of superficial pharyngeal carcinomas. Magnified NBI endoscopy is quite effective for diagnosing early-stage pharyngeal and esophageal carcinoma, that is, carcinomas in the squamous cell regions. The intrapapillary capillary loop (IPCL) classification for magnified NBI endoscopy is applicable only to the squamous cell regions, that is, the pharynx and the esophagus. Magnified NBI endoscopy for the stomach and the colon is evaluated by different diagnostic classifications.
Magnified NBI endoscopy made it possible to diagnose minute pharyngeal lesions with diameters of approximately 1 mm. It includes two steps. First, using NBI endoscopy without magnification, the authors detected suspicious changes as brownish areas. Second, using NBI magnifying of the brownish areas, the authors were able to diagnose whether the changes were suspicious of dysplasia or cancer or not according to the IPCL classification. IPCL type IV or V shows the possibility of dysplasia or cancer, whereas IPCL type I neglects the possibility.
For larger pharyngeal IPCL type IV lesions over 10 mm diameter, endoscopic submucosal dissection (ESD) is recommended. However, for smaller lesions of approximately 1 mm diameter, it remains to be determined whether they should be resected by ESD or followed up. Studies on the biopsy of such lesions are few. The authors concluded that biopsy can be the first-line procedure for such lesions not only for diagnosis but also for treatment.
The NBI system and magnifying endoscope are necessary to begin magnified NBI endoscopy. As the pharynx is sensitive, magnified NBI endoscopy of the pharynx should be performed under sedation. For safety, sedation should be carried out cautiously. As several biopsied minute pharyngeal lesions were high grade dysplasia, minute pharyngeal lesions should be biopsied for diagnosis. Furthermore, follow-up study of the biopsied lesions showed that even complete resection was expected by biopsy.
NBI: NBI is a new image-enhanced optical technology that uses narrow band NBI filters; IPCL: IPCL is the microvascular tumor vessel classification system used for NBI magnifying endoscopy.
Recently it has been reported that NBI can be useful in the early detection of superficial pharyngeal cancer. To determine the criteria of endoscopic treatment in patients with pharyngeal cancer and dysplasia, the resected specimen and follow up data are required. It is a good idea to investigate the usefulness of NBI magnifying endoscopy in the pharynx because biopsy in the pharynx is difficult.
Peer reviewers: Hoon Jai Chun, MD, PhD, AGAF, Professor, Department of Internal Medicine, Institute of Digestive Disease and Nutrition, Korea University College of Medicine, 126-1, Anam-dong 5-ga, Seongbuk-gu, Seoul 136-705, South Korea; Hiroki Nakamura, MD, Department of Gastroenterology and Hepatology, 1-1-1, Minami Kogushi, Ube, Yamaguchi 755-8505, Japan
S- Editor Lv S L- Editor Webster JR E- Editor Zhang DN
| 1. | Nonaka S, Saito Y. Endoscopic diagnosis of pharyngeal carcinoma by NBI. Endoscopy. 2008;40:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Ugumori T, Muto M, Hayashi R, Hayashi T, Kishimoto S. Prospective study of early detection of pharyngeal superficial carcinoma with the narrowband imaging laryngoscope. Head Neck. 2009;31:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 4. | Watanabe A, Tsujie H, Taniguchi M, Hosokawa M, Fujita M, Sasaki S. Laryngoscopic detection of pharyngeal carcinoma in situ with narrowband imaging. Laryngoscope. 2006;116:650-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 5. | Matsuba H, Katada C, Masaki T, Nakayama M, Okamoto T, Hanaoka N, Tanabe S, Koizumi W, Okamoto M, Muto M. Diagnosis of the extent of advanced oropharyngeal and hypopharyngeal cancers by narrow band imaging with magnifying endoscopy. Laryngoscope. 2011;121:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288-295. [PubMed] |
| 7. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 525] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 8. | Muto M, Hironaka S, Nakane M, Boku N, Ohtsu A, Yoshida S. Association of multiple Lugol-voiding lesions with synchronous and metachronous esophageal squamous cell carcinoma in patients with head and neck cancer. Gastrointest Endosc. 2002;56:517-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Muto M, Takahashi M, Ohtsu A, Ebihara S, Yoshida S, Esumi H. Risk of multiple squamous cell carcinomas both in the esophagus and the head and neck region. Carcinogenesis. 2005;26:1008-1012. [PubMed] |
| 10. | Muto M, Katada C, Sano Y, Yoshida S. Narrow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin Gastroenterol Hepatol. 2005;3:S16-S20. [PubMed] |
| 11. | Muto M, Ugumori T, Sano Y, Ohtsu A. Yoshida S. Narrow-band imaging combined with magnified endoscopy for cancer at the head and neck region. Dig Endosc. 2005;17:S23-S24. |
| 12. | Inoue H. Magnification endoscopy in the esophagus and stomach. Dig Endosc. 2001;13:S40-S41. |
| 13. | Inoue H, Kaga M, Sato Y, Sugaya S, Kudo S. Magnifying endoscopic diagnosis of tissue atypia and cancer invasion depth in the area of pharyngo-esophageal squamous epithelium by NBI enhanced magnification image: IPCL pattern classification. Advanced Digestive Endoscopy: Comprehensive Atlas of High Resolution Endoscopy and Narrowband Imaging. Oxford: Wiley-Blackwell 2007; 49-66. |
| 14. | Kumagai Y, Inoue H, Nagai K, Kawano T, Iwai T. Magnifying endoscopy, stereoscopic microscopy, and the microvascular architecture of superficial esophageal carcinoma. Endoscopy. 2002;34:369-375. [PubMed] |
| 15. | Barnes L, Eveson JW, Reichart P, Sidransky D (Eds): World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. Lyon: IARC Press 2005; . |
| 16. | Fujii S, Yamazaki M, Muto M, Ochiai A. Microvascular irregularities are associated with composition of squamous epithelial lesions and correlate with subepithelial invasion of superficial-type pharyngeal squamous cell carcinoma. Histopathology. 2010;56:510-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Yoshimura N, Goda K, Tajiri H, Yoshida Y, Kato T, Seino Y, Ikegami M, Urashima M. Diagnostic utility of narrow-band imaging endoscopy for pharyngeal superficial carcinoma. World J Gastroenterol. 2011;17:4999-5006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Tanaka S, Morita Y, Fujita T, Yokozaki H, Obata D, Fujiwara S, Wakahara C, Masuda A, Sugimoto M, Sanuki T. Clinicopathological characteristics of abnormal micro-lesions at the oro-hypopharynx detected by a magnifying narrow band imaging system. Dig Endosc. 2012;24:100-109. [DOI] [Full Text] |
| 19. | Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Watanabe A, Kawabori S, Kato M, Sugiyama T, Asaka M. Head and neck cancer arising after endoscopic mucosal resection for squamous cell carcinoma of the esophagus. Endoscopy. 2003;35:322-326. [PubMed] |
| 20. | Katada C, Nakayama M, Tanabe S, Koizumi W, Masaki T, Takeda M, Okamoto M, Saigenji K. Narrow band imaging for detecting metachronous superficial oropharyngeal and hypopharyngeal squamous cell carcinomas after chemoradiotherapy for head and neck cancers. Laryngoscope. 2008;118:1787-1790. [PubMed] |
| 21. | Katada C, Tanabe S, Koizumi W, Higuchi K, Sasaki T, Azuma M, Katada N, Masaki T, Nakayama M, Okamoto M. Narrow band imaging for detecting superficial squamous cell carcinoma of the head and neck in patients with esophageal squamous cell carcinoma. Endoscopy. 2010;42:185-190. [PubMed] |
| 22. | Yokoyama A, Kato H, Yokoyama T, Tsujinaka T, Muto M, Omori T, Haneda T, Kumagai Y, Igaki H, Yokoyama M. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and glutathione S-transferase M1 and drinking, smoking, and diet in Japanese men with esophageal squamous cell carcinoma. Carcinogenesis. 2002;23:1851-1859. [PubMed] |
| 23. | Morita M, Kuwano H, Ohno S, Sugimachi K, Seo Y, Tomoda H, Furusawa M, Nakashima T. Multiple occurrence of carcinoma in the upper aerodigestive tract associated with esophageal cancer: reference to smoking, drinking and family history. Int J Cancer. 1994;58:207-210. [PubMed] |
| 24. | Nonaka S, Saito Y, Oda I, Kozu T, Saito D. Narrow-band imaging endoscopy with magnification is useful for detecting metachronous superficial pharyngeal cancer in patients with esophageal squamous cell carcinoma. J Gastroenterol Hepatol. 2010;25:264-269. [PubMed] |
| 25. | Matsubara T, Yamada K, Nakagawa A. Risk of second primary malignancy after esophagectomy for squamous cell carcinoma of the thoracic esophagus. J Clin Oncol. 2003;21:4336-4341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Piazza C, Cocco D, De Benedetto L, Bon FD, Nicolai P, Peretti G. Role of narrow-band imaging and high-definition television in the surveillance of head and neck squamous cell cancer after chemo- and/or radiotherapy. Eur Arch Otorhinolaryngol. 2010;267:1423-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |