Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6452
Revised: October 8, 2012
Accepted: October 22, 2012
Published online: November 28, 2012
AIM: To investigate the effects of Saccharomyces boulardii (S. boulardii) in an experimental rat model of trinitrobenzene sulfonic acid (TNBS)-induced colitis.
METHODS: Thirty-two Wistar albino female rats were categorized into five groups. On the first day of the study, 50 mg TNBS was administered via a rectal catheter in order to induce colitis in all rats, except those in the control group. For 14 d, the rats were fed a standard diet, without the administration of any additional supplements to either the control or TNBS groups, in addition to 1 mg/kg per day S. boulardii to the S. boulardii group, 1 mg/kg per day methyl prednisolone (MP) to the MP group. The animals in the S. boulardii + MP group were coadministered these doses of S. boulardii and MP. During the study, weight loss, stool consistency, and the presence of obvious blood in the stool were evaluated, and the disease activity index (DAI) for colitis was recorded. The intestines were examined and colitis was macro- and microscopically scored. The serum and tissue levels of tumor necrosis factor-α (TNF-α) and nitric oxide (NO) were determined, and fungemia was evaluated in the blood samples.
RESULTS: The mean DAI scores for the MP and S. boulardii + MP groups was significantly lower than the TNBS group (3.69 ± 0.61 vs 4.46 ± 0.34, P = 0.018 and 3.77 ± 0.73 vs 4.46 ± 0.34, P = 0.025, respectively). While no significant differences between the TNBS and the S. boulardii or MP groups could be determined in terms of serum NO levels, the level of serum NO in the S. boulardii + MP group was significantly higher than in the TNBS and S. boulardii groups (8.12 ± 4.25 μmol/L vs 3.18 ± 1.19 μmol/L, P = 0.013; 8.12 ± 4.25 μmol/L vs 3.47 ± 1.66 μmol/L, P = 0.012, respectively). The tissue NO levels in the S. boulardii, MP and S. boulardii + MP groups were significantly lower than the TNBS group (16.62 ± 2.27 μmol/L vs 29.72 ± 6.10 μmol/L, P = 0.002; 14.66 ± 5.18 μmol/L vs 29.72 ± 6.10 μmol/L, P = 0.003; 11.95 ± 2.34 μmol/L vs 29.72 ± 6.10 μmol/L, P = 0.002, respectively). The tissue NO levels in the S. boulardii, MP and S. boulardii + MP groups were similar. The mean serum and tissue TNF-α levels were determined to be 12.97 ± 18.90 pg/mL and 21.75 ± 15.04 pg/mL in the control group, 18.25 ± 15.44 pg/mL and 25.27 ± 11.95 pg/mL in the TNBS group, 20.59 ± 16.15 pg/mL and 24.39 ± 13.06 pg/mL in the S. boulardii group, 9.05 ± 5.13 pg/mL and 24.46 ± 10.85 pg/mL in the MP group, and 13.95 ± 10.17 pg/mL and 24.26 ± 10.37 pg/mL in the S. boulardii + MP group. Significant differences in terms of the levels of serum and tissue TNF-α and the macroscopic and microscopic scores were not found between the groups. S. boulardii fungemia was not observed in any of the rats. However, Candida fungemia was detected in one rat (14%) in the TNBS group, two rats (28%) in the S. boulardii group, three rats (50%) in the MP group, and three rats (42%) in S. boulardii + MP group.
CONCLUSION: S. boulardii does not demonstrate considerable effects on the DAI, pathological scores, or cytokine levels but does decrease the tissue NO levels.
-
Citation: Soyturk M, Saygili SM, Baskin H, Sagol O, Yilmaz O, Saygili F, Akpinar H. Effectiveness of
Saccharomyces boulardii in a rat model of colitis. World J Gastroenterol 2012; 18(44): 6452-6460 - URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6452.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6452
It is currently speculated that inflammatory bowel disease (IBD) develops in genetically prone individuals as a result of defective immune responses against enteric bacterial flora antigens. Antibiotics and immunomodulatory therapies are believed to play an important role in the treatment of this disease[1].
Probiotics are live microorganisms that positively affect health when ingested. Saccharomyces boulardii (S. boulardii) is a thermophilic nonpathogenic yeast that is selectively used to treat antibiotic-associated and traveler’s diarrhea[2]. The main mechanisms of action of S. boulardii include antimicrobial activities, trophic effects upon the intestinal mucosa, and the modification of the host-signaling pathways that are involved in inflammatory and noninflammatory intestinal diseases. It has been shown that S. boulardii inhibits the production of proinflammatory cytokines by inhibiting the main regulators of inflammation, such as nuclear factor κB and mitogen-activated protein kinases, which play crucial roles in the pathogenesis of IBD[3,4]. S. boulardii is believed to effectively treat IBD because of its antimicrobial activities and its regulatory effects on enteric flora and the immune system[5]. In a recent study[6], it was shown that treating human colon epithelial cells with S. boulardii increases the expression of peroxisome proliferator-activated receptor-c and the secretion of inhibits interleukin-8 (IL-8). In the same study, it was demonstrated that S. boulardii decreases intestinal inflammation by reducing the mucosal expression of proinflammatory cytokines in rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis. This earlier study demonstrates that colonic inflammation can be reduced by S. boulardii through the regulation of inflammatory gene expression.
Nitric oxide (NO) is an important messenger that is involved in vein permeability and tissue damage. It is possible that in patients with active ulcerative colitis (UC) and Crohn’s disease (CD), the inducible activity of NO synthase is elevated in inflamed mucosal epithelial cells. Increased NO levels indicate inflammation and, in turn, the intensity of the disease. Variations in tissue NO levels are important indicators of progression and recovery from IBD. There is also evidence that S. boulardii inhibits inducible nitric oxide synthase[7].
In the current literature, there are studies suggesting that S. boulardii causes fungemia, particularly in immunosuppressed and intensive care patients[8]. However, controlled studies cannot be used to investigate the effectiveness of S. boulardii against IBD, although promising results have been reported in a few studies[9,10].
The primary aim of our present study was to investigate the effects of S. boulardii on colonic inflammation and the disease activity index (DAI) in a rat model of TNBS-induced colitis. The secondary purpose of our current analyses was to investigate the risk of fungemia resulting from treatment with S. boulardii alone or in combination with corticosteroids.
Approval was obtained from the animal ethics council of Dokuz Eylul University Medical Faculty (DEUTF). The DEUTF Hospital Experimental Research Laboratory provided 32 female Wistar albino rats weighing 200-250 g for use in this study which were divided into five groups. The control group included only four rats that were not treated with TNBS. All other groups consisted of seven rats (Table 1).
| Group | n | Application (day 1) | Application (days 1-14) |
| Control | 4 | Physiological serum | Physiological serum |
| TNBS | 7 | TNBS | No treatment |
| S. boulardii | 7 | TNBS | S. boulardii |
| MP | 7 | TNBS | MP |
| S. boulardii + MP | 7 | TNBS | S. boulardii + MP |
The rats were maintained in a room at a temperature of 23 ± 2 °C under a 12-h light/dark cycle at the DEUTF Experimental Animal Laboratory. Prior to and during the study, the rats were fed a standard diet (Yemta; Taris Ltd. Şti., Izmir, Turkey), and their weights were monitored daily. The rats were allowed water ad libitum.
After 24 h of fasting, 0.5 mL physiological serum was intracolonically administered to the rats in the control group via a cannula that was placed 8 cm proximal to the anus using a rectally inserted flexible polypropylene catheter. To induce colitis in the other groups, the rats were intracolonically treated with 0.5 mL of 100 mg/mL TNBS that was dissolved in 50% ethanol and administered via a cannula. Prior to catheter insertion, short-term sedation was provided via ether anesthesia. After TNBS administration, no rats developed perforation or exitus due to the formation of ulcerations in the colon.
As shown in the Table 1, 32 Wistar albino female rats were divided into five groups. The rats in the control group (n = 4) were not treated with TNBS after the intracolonic administration of physiological serum (via a cannula placed 8 cm proximal to the anus using a rectally inserted polypropylene catheter, similar to the administration of TNBS). After the administration of TNBS, the rats in the TNBS group (n = 7) were not treated. S. boulardii (Reflor; Biocodex laboratories, Gentilly, France) was prepared in its lyophilized form (282.5 mg/sachet with a biological activity of 5 × 109 viable cells) by the manufacturer. S. boulardii (1 mg/kg per day) was suspended in distilled water and added to the water supply of the rats in the S. boulardii group (n = 7) in the morning and evening starting on day 0. The rats in methyl prednisolone (MP) group (n = 7) were treated with MP (Prednol; Mustafa Nevzat, Istanbul, Turkey) at a dosage of 1 mg/kg per day, while the rats in the S. boulardii + MP group (n = 7) were treated with both S. boulardii and MP at the previously defined dosages using the previously discussed techniques.
TNBS-induced colitis was scored according to the DAI proposed by Murthy et al[11] (Table 2). Scoring was calculated according to body weight loss (as a percentage), differences in stool consistency, and the occurrence of rectal bleeding. Fecal occult blood testing (FOBT) of stool samples (Hemoccult II; Beckman Coulter Inc., Fullerton, CA, United States) was used to detect obscure bleeding.
| Score | Weight loss (%) | Stool consistency | Rectal bleeding |
| 0 | - | Normal | - |
| 1 | 1-5 | Loose stool | Occult blood in stool |
| 2 | 5-10 | Loose stool | Occult blood in stool |
| 3 | 10-20 | Loose stool | Occult blood in stool |
| 4 | > 20 | Watery stool | Obvious blood in stool |
After 14 d, blood was drawn from the abdominal aorta under ether anesthesia following 24 h of fasting, and then the rats were sacrificed due to hypovolemia. Decapitation was performed after tissue samples were collected for pathological examination. The abdominal cavity was opened via a midline incision, and the whole small and large intestines were harvested from the pylorus to the rectum. The intestinal lumen was washed with physiological serum containing phosphate buffer (PBS), and the intestinal materials collected from the opened lumen was fixed in formaldehyde. A pathologist who was blind to the groups conducted the pathological examinations of the intestinal samples.
The scoring method defined by Wallace et al[12] was used to evaluate damage due to colonic inflammation. Fixed intestinal tissue samples were microscopically examined (5 × magnification) and scored from 0-10 according to various inflammation markers, such as the diameters of any developing ulcers, thickening of the intestinal wall, and hyperemia. While intestinal tissues without any evidence of lesions were scored as 0, intestinal tissues with serious ulcerations were scored as 10. Subsequently, histological sections, including the peripheral normal mucosa, were prepared from gross ulcerative lesions. Approximately 1-cm sections were obtained from the intestines and transported on ice to the Department of Clinical Microbiology (DEUTF, Izmir, Turkey) for homogenization before fixation in formaldehyde. The tissues were fixed in formaldehyde, embedded in paraffin, and stained with hematoxylin and eosin. For the microscopic evaluation, we employed the defined scoring system described by Ameho et al[13].
Prior to sacrifice, blood samples were drawn from the vena cava under ether anesthesia, and the serum was separated by centrifugation and stored at -70 °C until use. Homogenization of the intestinal tissues was performed in accordance with current methods[14]. The intestinal tissues were first homogenized in an ice-cold buffer [0.1 mol/L potassium phosphate (pH 7.5) and 20 mmol/L ethylene diamine tetra acetic acid (EDTA), 1:10 w/v] using a mechanical homogenizer (Potter B. Braun; Gemini, Apeldoorn, The Netherlands) and then in an ultrasonic homogenizer on ice. The resulting lysates were centrifuged at 14 000 rpm for 10 min followed by an additional spin at 14 000 rpm for 20 min. The proteins were purified using zinc sulfate (300 g/L) at a 1:20 ratio, and the final sample concentration of 15 g/L was obtained via centrifugation. The final products were centrifuged at 4 °C for 20 min at 2000 rpm, and 100-μL samples were subsequently prepared for evaluation of cytokine and NO levels.
The 100-μL intestinal tissue lysates and serum samples were mixed with an equal volume of 100 μL of Griess reagent (Ingredient A: 0.1% naphthalene diamine dihydrochloride at a final concentration of 5 mmol/L; Ingredient B: 1% sulfanilamide at a final concentration of 5 mmol/L in orthophosphoric acid) in a 96-well microtiter plate (Maxisorb Immunoplate; NUNC, Roskilde Denmark). After incubation for 10 min at room temperature, the absorbance at 540 nm was measured using a microplate reader (Reader Model 230S; Organon Teknika, Boxtel, The Netherlands). For each measurement, 2-fold increases in sodium nitrite in PBS, from 0-128 mmol/L, were used to generate a standard curve[15].
Serum and tissue tumor necrosis factor (TNF)-α levels were measured using a commercially available rat-specific enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (Invitrogen; Life Technologies, Camarillo, CA, United States).
Blood samples were cultured in Petri dishes containing the following culture media: Sabouraud dextrose agar (BD Difco; Becton, Dickinson, NJ, United States) supplemented with 100 mg/L chloramphenicol and selective CHROMagar Candida (CHROMagar Candida; CHROMagar, Paris, France). Following incubation at 30 °C for 72 h, the yeast colonies were visually quantified, if they existed. A specimen from each colony variant was placed in a tube containing Sabouraud dextrose agar for storage and identification. Identification was based on the results of filament production tests; the production of the germinative tube, ascospores, urease, and phenoloxidase; and zymogram, auxanogram, and growth rates at different incubation temperatures, as recommended by Boekhout et al[16,17].
Statistical analyses were conducted using SPSS software for windows 11.0 (SPSS, Chicago, IL). Data are expressed as the mean ± SD. Quantitative data, such as the macroscopic and microscopic scores and NO and TNF-α levels, were compared between groups using the Kruskal-Wallis and Mann-Whitney U tests. Differences between the mean were considered statistically significant when P < 0.05.
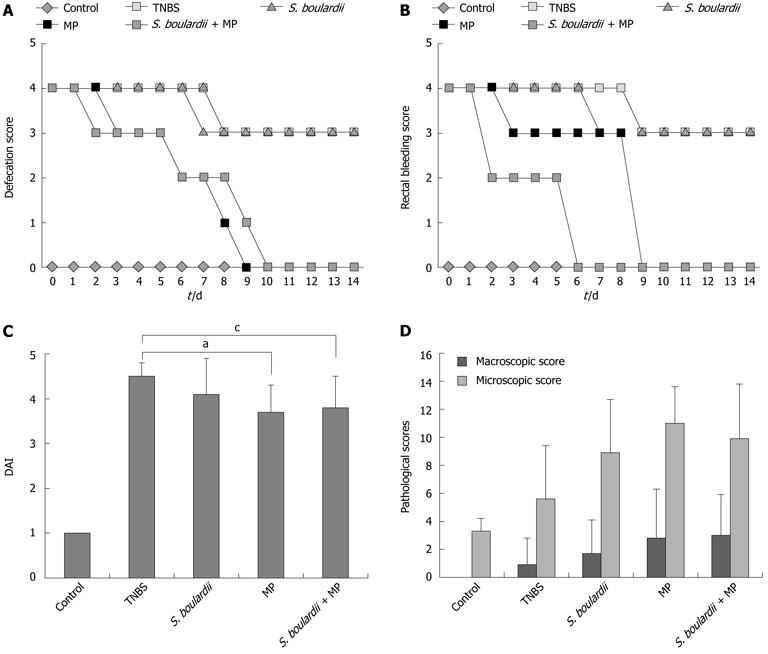
We did not observe diarrhea or bloody stool in the four rats in the control group during the course of this study but these effects were observed in 27 rats starting on day 1, plus one rat on day 2, following the administration of TNBS. In the TNBS and S. boulardii groups, a loose stool was noted on day 7 and never returned to normal and diarrhea persisted for a total of 14 d over the course of this study. In the MP group, a loose stool was observed on day 3 and had returned to normal by day 9. However, in the S. boulardii + MP group, the stool returned to normal by day 10 after becoming loose on day 2. The MP and S. boulardii + MP groups demonstrated 9 and 10 d of diarrhea, respectively (Figure 1A).
While defecation in the TNBS and S. boulardii groups macroscopically continued for an average of 9 and 7 d, respectively, in the following days the FOBT results were positive until day 14. In the MP group, bloody defecation was observed for an average of three days, while for the following six days the FOBT results were positive even without clearly noting bloody defecation. In addition, the blood in the stool was, on average, not observed until day 9. In the S. boulardii + MP group, bloody stool was observed for an average of two days, and the FOBT results were positive for seven days; however, there was no traceable blood in the stool samples after day 9 (Figure 1B).
The rats in the control group gained an average of 4.3 g in weight, those in the TNBS and S. boulardii groups gained an average of 11.9 and 2.4 g, respectively, and the rats in the MP and S. boulardii + MP groups lost 3 and 5.9 g, respectively. No significant differences in body weight changes could be determined between the S. boulardii, MP, and S. boulardii + MP groups. There were significant differences in body weight changes for the MP and S. boulardii + MP groups compared with the TNBS group (P = 0.01 and 0.02, respectively) (Table 3).
Based on the scoring system that was previously suggested by Murthy et al[11], DAI scores were 1 ± 0 for the control group, 4.46 ± 0.34 for the TNBS group, 4.07 ± 0.77 for the S. boulardii group, 3.69 ± 0.61 for the MP group, and 3.77 ± 0.73 for the S. boulardii + MP group. The DAI scores of the MP and S. boulardii + MP groups were significantly lower compared with the TNBS group (P = 0.018 and 0.025, respectively). Regarding the other groups, no significant differences in DAI could be determined (Figure 1C).
Macroscopic ulceration was not observed in the control group, although inflammation was observed at the microscopic level. Upon macroscopic examination, a 14-mm ulcer in the intestinal tissue was observed in one rat in the TNBS group, while 3, 2, and 3 larger ulcers (> 2 cm) were observed in the S. boulardii, MP, and S. boulardii + MP groups, respectively. After macroscopic and microscopic scoring, of all the groups that were induced to form colitis, the lowest score was observed in the TNBS group. When the treated groups were examined, the S. boulardii group demonstrated the lowest scores, although significant differences were not found between the MP and S. boulardii + MP groups (Figure 1D).
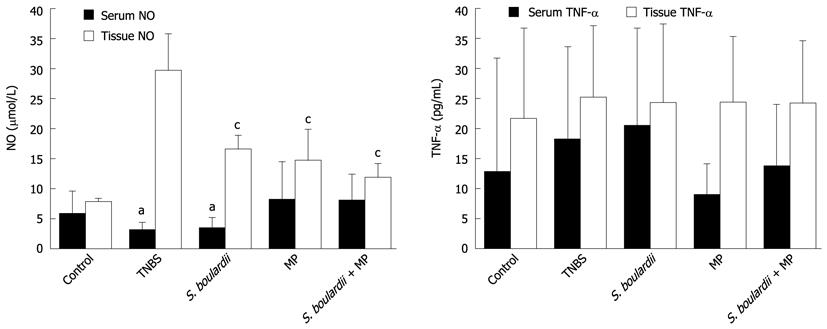
The mean serum NO levels in the control, TNBS, S. boulardii, MP, and S. boulardii + MP groups were determined to be 5.92 ± 3.65 μmol/L, 3.18 ± 1.19 μmol/L, 3.47 ± 1.66 μmol/L, 8.22 ± 6.28 μmol/L, and 8.12 ± 4.25 μmol/L, respectively. The lowest serum NO levels were observed in the TNBS and S. boulardii groups. The serum NO levels in S. boulardii + MP group were higher than those in the TNBS and S. boulardii groups (P = 0.013 and 0.012, respectively).
The mean tissue NO levels in the control, TNBS, S. boulardii, MP, and S. boulardii + MP groups were determined to be 7.95 ± 0.50 μmol/L, 29.72 ± 6.10 μmol/L, 16.62 ± 2.27 μmol/L, 14.66 ± 5.18 μmol/L, and 11.95 ± 2.34 μmol/L, respectively. The highest tissue NO level was observed in the TNBS group. The tissue NO levels in the S. boulardii, MP, and S. boulardii + MP groups were significantly lower in comparison with the TNBS group (P = 0.002, 0.003 and 0.002, respectively). However, the tissue NO levels in the S. boulardii, MP, and S. boulardii + MP groups were similar (Figure 2A).
The mean serum TNF-α levels were determined to be 12.97 ± 18.90 pg/mL in the control group, 18.25 ± 15.44 pg/mL in TNBS group, 20.59 ± 16.15 pg/mL in S. boulardii group, 9.05 ± 5.13 pg/mL in MP group, and 13.95 ± 10.17 pg/mL in S. boulardii + MP group. The mean tissue TNF-α levels were determined to be 21.75 ± 15.04 pg/mL in the control group, 25.27 ± 11.95 pg/mL in TNBS group, 24.39 ± 13.06 pg/mL in S. boulardii group, 24.46 ± 10.85 pg/mL in MP group, and 24.26 ± 10.37 pg/mL in S. boulardii + MP group. The serum and tissue TNF-α levels were comparable between groups (Figure 2B).
Fungemia was not observed in the control group but was detected in 1 of 7 rats (14%) in the TNBS group, 2 of 7 rats (28%) in the S. boulardii group, 3 of 6 rats (50%) in the MP group (1 rat in the MP group was excluded because sufficient blood samples could not be obtained), and 3 of 7 rats (42%) in the S. boulardii + MP group. The fungemia was determined to be Candida fungi other than C. albicans using an identification process as explained in materials and methods section. S. boulardii fungemia was not identified in any of the rats.
It is thought that IBD develops in genetically prone individuals as a result of defective immune responses against the antigens of enteric bacterial flora. Antibiotics and immunomodulatory therapies are believed to be important for the treatment of these diseases[1]. S. boulardii is a probiotic yeast, and it is known that probiotics inhibit pathogenic invasion and also demonstrate regulatory effects on the enteric flora and immune system[2-5]. Although there are several published studies on probiotic bacterial therapies in experimental animals with induced IBD, there are only a few studies on S. boulardii. In this study, the effectiveness of S. boulardii, DAI, macro- and microscopic pathological ulcer scores, proinflammatory cytokine levels (i.e., serum and tissue levels of TNF-α), and the serum and tissue levels of lipid peroxidation products (i.e., NO) were evaluated.
In our study, the DAI in the groups that were administered individual MP or MP with S. boulardii were evaluated at significantly lower levels compared with the TNBS group. Meaningful differences in the DAI scores of the S. boulardii group were not determined, although the DAI score of the S. boulardii group was lower than that of the TNBS group. As expected, these results show that the treatment of colitis using MP effectively improves the symptoms that are observed in rat models of induced colitis. However, the DAI scores were comparable between the MP and S. boulardii + MP groups, suggesting that the application of MP in combination with S. boulardii might not be more effective than the individual application of MP. In a recent study[18], Lactobacillus and Bifidobacterium were used to prevent colitis in rats using dextran sodium sulfate, and DAI scores in the probiotic-treated group were lower than the control group. However there are no other reported studies that have investigated the effects of S. boulardii on DAI in an experimental model of colitis.
There are a few clinical studies on the application of S. boulardii for the treatment of UC and CD patients. Guslandi et al[9] conducted a study using 32 CD patients who were medically treated and had been in remission for 3 mo. For 6 mo, a group was administered only mesalamine (3 g/d) and another group was administered mesalamine (2 g/d) and S. boulardii (1 g/d). After 6 mo, 10 patients in the mesalamine-treated group and 15 patients from the mesalamine + S. boulardii-treated group were still in remission (P < 0.04). The same researchers conducted another pilot study[10] on 25 patients with UC of mild/moderate intensity who could not be treated with corticosteroids. After 3 mo of mesalamine (3 g/d) treatment, the patients were additionally supplemented with S. boulardii (250 mg administered 3 × per day) for 4 wk. At the end of treatment, 17 patients (68%) were in remission and had demonstrated an obvious reduction in their clinical activity scores (P < 0.05). However, in both studies, S. boulardii was not used alone and a control group was not evaluated.
In our study, macroscopic and microscopic pathological scoring was performed to evaluate the effectiveness of S. boulardii against colonic inflammation. Statistically significant differences were not observed between the treated and untreated groups that were induced to form colitis. In addition, no improvement was noted in the colon mucosa following the administration of individual applications of MP and S. boulardii or the dual application of MP and S. boulardii. However, the use of probiotics, such as Lactobacillus and VSL#3 (contains one strain of streptococcus thermophilus, three strains of bifidobacterium and four strains of lactobacillus), for the treatment TNBS-induced colitis in rat models have demonstrated significant improvements in macroscopic and microscopic scores[19,20]. Surprisingly, in our study, the macroscopic scores in the S. boulardii, MP, and S. boulardii + MP groups were higher, although they were not significantly different when compared with the TNBS group. In the MP and S. boulardii + MP groups, the macroscopic and microscopic pathology scores were higher than in the TNBS group, although these groups also demonstrated significantly lower DAI scores. Thus, these results suggest that the clinical responses are not consistent with the histopathological results. The higher macroscopic and microscopic pathology scores in the treatment groups compared with the TNBS group can be explained by delayed effects in ulcer improvement due to fungal colonization in the gastrointestinal (GI) tract. In our study, non-Candida albicans fungemia was detected at considerable frequencies in the treated groups. In a study[21] on inhibiting Candida translocation in the GI tract using probiotics, a group of patients with UC and rats with acetic acid-induced stomach ulcers were included. It was shown that Candida colonies formed in these groups, which was accompanied by the delayed recovery of stomach ulcers and the persistence of both of gastric ulcers and UC symptoms. An increase in cytokine expression, especially TNF-α and IL-1 levels, was detected in the rats that were inoculated with Candida.
IBD is an immunosuppressive disease caused by a defective intestinal mucosal barrier that can be brought on by applied treatments. Thus, during the course of the disease, insidious infections, such as cytomegalovirus and C. albicans, can develop. There are reported cases of the development of fungemia caused by Candida species, such as C. parapsilosis, C. albicans, and Saccharomyces cerevisiae, in UC patients. S. boulardii fungemia was reported in a 33-year-old male patient who was diagnosed with IBD, underwent intestinal surgery, and was in the intensive care unit[22]. In our study, fungemia due to S. boulardii did not develop in any of the groups. Accordingly, in the colitis rat model, an increase in the risk of developing S. boulardii fungemia was not determined upon the application of S. boulardii alone or in conjunction with MP. In this study, while fungemia was not observed in the control group, non-C. albicans was observed in the TNBS, S. boulardii, MP, S. boulardii + MP groups with frequencies of 14%, 28%, 50%, and 48%, respectively. This result is not consistent with previously published reports on the inhibition of Candida translocation in the gastrointestinal tract due to the use of probiotics in immunosuppressed rats[21,23].
In this study, the serum NO level in the group treated with S. boulardii + MP was high compared with the TNBS and S. boulardii groups. In addition, serum NO levels were comparable between the other groups. However, tissue NO levels in all 3 treatment groups were statistically and significantly lower in comparison with the TNBS group. As observed, the serum and tissue NO levels were inconsistent. However, it is known that the serum NO level is affected by systemic events, and tissue NO levels are more reliable. As a result, based on the results of the TNBS group, tissue NO levels are found to be low in all treatment groups. These results suggest that use of S. boulardii and MP alone or in combination can reduce the intensity of inflammation and damage to the colitis mucosa. It was also revealed that the addition of S. boulardii to MP treatment does not yield a synergistic effect because the tissue NO levels of the MP and S. boulardii + MP groups were similar. In another study[7], S. boulardii treatment reportedly affected NO levels in a rat diarrhea model that was induced by castor oil. In that study, S. boulardii was a successful diarrhea treatment that inhibited inducible NO synthase activity. In addition, other probiotics, especially Lactobacillus that has been used in induced colitis models, have been reported to reduce the tissue NO level by inhibiting inducible NO synthase activities[24].
TNF-α is produced by CD4 + T lymphocytes that are assembled around inflamed mucosa. TNF-α is a strong chemokine that functions in pathological inflammatory signal transduction by directing the migration of neutrophils to inflamed mucosa. Therefore, serum and tissue TNF-α levels are mainly used to evaluate the intensity of inflammation. In many studies conducted using Lactobacillus in TNBS-induced colitis models, the tissue TNF-α levels in the groups that were administered Lactobacillus were significantly reduced compared with the control group[25,26]. It has been demonstrated that the Lactobacillus species used in those studies reduces the number of CD4 + T cells in inflamed mucosa, thus reducing TNF-α production. In addition to these effects, Lactobacillus increases the production of anti-inflammatory IL-10 by shifting the T helper1 (Th1) cellular immune response towards Th2 and Th3. By changing the TNF-α/IL-10 ratio, the intensity of inflammation can be reduced[26]. In a recent study[6], it was demonstrated that S. boulardii decreases intestinal inflammation by reducing the mucosal expression of proinflammatory cytokines in rats with TNBS-induced colitis. In our study, the serum and tissue TNF-α levels were similar in all groups. These results can be explained by non-Candida albicans fungemia, which can cause an increase in cytokine expression[21]. While there are an insufficient number of studies conducted on S. boulardii, S. boulardii is believed to be involved in anti-inflammatory effects by affecting various inflammatory mechanisms[5-7].
In conclusion, this study establishes that S. boulardii does not improve DAI or colonic inflammation in rats with TNBS-induced colitis and does not reduce serum or tissue TNF-α levels. The only significant effect of S. boulardii is reducing tissue NO levels. S. boulardii-based fungemia was not detected in any of the rats included in this study.
It is thought that inflammatory bowel disease (IBD) develops in genetically prone individuals as a result of a defective immune response against the antigens of enteric bacterial flora. Antibiotics and immunomodulatory therapies play an important role in the treatment of these diseases. Saccharomyces boulardii (S. boulardii) is a probiotic yeast, and it is known that probiotics inhibit pathogenic invasion and demonstrate regulatory effects on the enteric flora and immune system. Although there are several published studies on the use of probiotic bacterial therapy in experimental animals with induced IBD, there are a few studies on the involvement of S. boulardii.
The present study shows that S. boulardii is a probiotic agent that demonstrates no effects on the disease activity index (DAI), serum and tissue tumor necrosis factor-α (TNF-α) levels, or pathologic findings in a rat model of trinitrobenzene sulfonic acid (TNBS)-induced colitis. However S. boulardii may reduce tissue nitric oxide (NO) levels, which is an important messenger involved in vein permeability and tissue damage. S. boulardii-based fungemia was not detected.
There are a few clinical studies on the efficacy of S. boulardii for treating IBD. Based on the findings of these published studies, S. boulardii appears to be promising. In a recent study, it was shown that treating human colon epithelial cells with S. boulardii increases the expression of peroxisome proliferator-activated receptor-c and inhibits the secretion of IL-8. In the same study, it was demonstrated that S. boulardii decreases intestinal inflammation by reducing the mucosal expression of proinflammatory cytokines in rats with TNBS-induced colitis. However the effects of S. boulardii on DAI and NO were not evaluated. The present study was conducted to investigate the effects of S. boulardii on DAI, pathological scores, TNF-α, and NO. Additionally, the risk of fungemia, which could result from treatment with S. boulardii alone or in combination with corticosteroids, was also evaluated.
The present study shows that S. boulardii does not improve DAI or colonic inflammation in rats with TNBS-induced colitis or reduce TNF-α levels. These results suggest that S. boulardii may not be an effective treatment for patients with IBD. In contrast, the limited number of studies conducted on this issue have reported some promising results. Therefore, further studies are needed in order to draw a firm conclusion.
Crohn’s disease and ulcerative colitis, both of which are referred to as IBD, are chronic inflammatory disorders of the gastrointestinal tract that have characteristic clinical, pathological, endoscopic, and radiological features. TNBS-induced colitis is well-established in various animal models of mucosal inflammation that have been used for over 2 decades for the study of IBD pathogenesis and in preclinical studies. Probiotics are live microorganisms that positively affect health when ingested. S. boulardii is a live yeast that is extensively used as a probiotic.
This study examine the impact of S. boulardii on TNBS colitis. This is an excellent experimental study that evaluated the effects of S. boulardii on clinical activity scores, TNF-α levels, serum and tissue NO levels, and macroscopic and microscopic pathological scores in a rat model of TNBS-induced colitis.
Peer reviewer: Alan C Moss, MD, FACG, Assistant Professor of Medicine, Director of Translational Research, Center for Inflammatory Bowel Disease, Beth Israel Deaconess Medical Center, Harvard Medical School, Rose 1/East, 330 Brookline Ave, Boston, MA 02215, United States
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Farrell RJ, LaMont JT. Microbial factors in inflammatory bowel disease. Gastroenterol Clin North Am. 2002;31:41-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Surawicz CM, Elmer GW, Speelman P, McFarland LV, Chinn J, van Belle G. Prevention of antibiotic-associated diarrhea by Saccharomyces boulardii: a prospective study. Gastroenterology. 1989;96:981-988. [PubMed] |
| 3. | Coskun M, Olsen J, Seidelin JB, Nielsen OH. MAP kinases in inflammatory bowel disease. Clin Chim Acta. 2011;412:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Sougioultzis S, Simeonidis S, Bhaskar KR, Chen X, Anton PM, Keates S, Pothoulakis C, Kelly CP. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-kappaB-mediated IL-8 gene expression. Biochem Biophys Res Commun. 2006;343:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Buts JP, De Keyser N. Effects of Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci. 2006;51:1485-1492. [PubMed] |
| 6. | Lee SK, Kim YW, Chi SG, Joo YS, Kim HJ. The effect of Saccharomyces boulardii on human colon cells and inflammation in rats with trinitrobenzene sulfonic acid-induced colitis. Dig Dis Sci. 2009;54:255-263. [PubMed] |
| 7. | Girard P, Pansart Y, Lorette I, Gillardin JM. Dose-response relationship and mechanism of action of Saccharomyces boulardii in castor oil-induced diarrhea in rats. Dig Dis Sci. 2003;48:770-774. [PubMed] |
| 8. | Lherm T, Monet C, Nougière B, Soulier M, Larbi D, Le Gall C, Caen D, Malbrunot C. Seven cases of fungemia with Saccharomyces boulardii in critically ill patients. Intensive Care Med. 2002;28:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 148] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Guslandi M, Mezzi G, Sorghi M, Testoni PA. Saccharomyces boulardii in maintenance treatment of Crohn's disease. Dig Dis Sci. 2000;45:1462-1464. [PubMed] |
| 10. | Guslandi M, Giollo P, Testoni PA. A pilot trial of Saccharomyces boulardii in ulcerative colitis. Eur J Gastroenterol Hepatol. 2003;15:697-698. [PubMed] |
| 11. | Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 448] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 12. | Wallace JL, MacNaughton WK, Morris GP, Beck PL. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology. 1989;96:29-36. [PubMed] |
| 13. | Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 196] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Kumral A, Baskin H, Duman N, Yilmaz O, Tatli M, Ozer E, Gökmen N, Genc S, Ozkan H. Erythropoietin protects against necrotizing enterocolitis of newborn rats by the inhibiting nitric oxide formation. Biol Neonate. 2003;84:325-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Baskin H, Ellermann-Eriksen S, Lovmand J, Mogensen SC. Herpes simplex virus type 2 synergizes with interferon-gamma in the induction of nitric oxide production in mouse macrophages through autocrine secretion of tumour necrosis factor-alpha. J Gen Virol. 1997;78:195-203. [PubMed] |
| 16. | Boekhout T, Nakase T. Bullera Derx. The Yeasts: A Taxonomic Study. 4th ed. Amsterdam: Elsevier Science 1998; 731-741. |
| 17. | Silva JO, Franceschini SA, Lavrador MA, Candido RC. Performance of selective and differential media in the primary isolation of yeasts from different biological samples. Mycopathologia. 2004;157:29-36. [PubMed] |
| 18. | Osman N, Adawi D, Ahrne S, Jeppsson B, Molin G. Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium. Dig Dis Sci. 2004;49:320-327. [PubMed] |
| 19. | Foligné B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci. 2006;51:390-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Pavan S, Desreumaux P, Mercenier A. Use of mouse models to evaluate the persistence, safety, and immune modulation capacities of lactic acid bacteria. Clin Diagn Lab Immunol. 2003;10:696-701. [PubMed] |
| 21. | Zwolińska-Wcisło M, Brzozowski T, Mach T, Budak A, Trojanowska D, Konturek PC, Pajdo R, Drozdowicz D, Kwiecień S. Are probiotics effective in the treatment of fungal colonization of the gastrointestinal tract? Experimental and clinical studies. J Physiol Pharmacol. 2006;57 Suppl 9:35-49. [PubMed] |
| 22. | Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks? Am J Clin Nutr. 2006;83:1256-164; quiz 1256-164;. [PubMed] |
| 23. | Algin C, Sahin A, Kiraz N, Sahintürk V, Ihtiyar E. Effectiveness of bombesin and Saccharomyces boulardii against the translocation of Candida albicans in the digestive tract in immunosuppressed rats. Surg Today. 2005;35:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Adrio JL, Olivares M, Xaus J, Zarzuelo A, Galvez J. Lactobacillus fermentum, a probiotic capable to release glutathione, prevents colonic inflammation in the TNBS model of rat colitis. Int J Colorectal Dis. 2006;21:737-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Guarner F, Malagelada JR. Role of bacteria in experimental colitis. Best Pract Res Clin Gastroenterol. 2003;17:793-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 66] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Peran L, Camuesco D, Comalada M, Nieto A, Concha A, Diaz-Ropero MP, Olivares M, Xaus J, Zarzuelo A, Galvez J. Preventative effects of a probiotic, Lactobacillus salivarius ssp. salivarius, in the TNBS model of rat colitis. World J Gastroenterol. 2005;11:5185-5192. [PubMed] |