Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6447
Revised: August 13, 2012
Accepted: August 16, 2012
Published online: November 28, 2012
AIM: To determine the incidence of human epidermal growth factor receptor 2 (HER2) over expression in oesophageal cancers.
METHODS: A retrospective study, of one hundred consecutive cases of endoscopic histological samples of oesophageal cancers from a single British cancer network were included. Cancer cases were diagnosed between April 2007 and June 2010. HER2 over expression was assessed using immunohistochemistry, those that scored “0” and “+1” were considered “negative” for HER2; those that scored “+3” were considered “Positive”. Cases that were scored “+2” on immunohistochemistry further went on to have HER2 gene analysis using the Ventana HER brightfield dual-colour in situ hybridisations (HER B DISH) assay and either came back to be positive or negative for HER2 over expression. Overall survival was measured from date of histological diagnosis until date of death. 93% of the cases were followed up till five years or death, and all were followed up till two years. Cases of gastro-oesophageal junctional tumours were excluded.
RESULTS: The median age of our sample was 66 years (range: 38-91 years). Eighty one were male and 19 female. Ninety-one of the cases were adenocarcinoma of the oesophagus and the rest were cases of squamous cell carcinoma. The anatomical distribution of the tumours was; upper oesophagus 2, middle oesophagus 11, and 87 were in the lower oesophagus. Operative resection was completed in 15 cases; seven cases had attempted surgical resections, i.e., open and close, 33 patients received definitive chemo-radiation and 52 had palliative treatment. Twenty-five of the cancers showed evidence of HER2 over expression, all were adenocarcinomas. Of the 25 cases that showed evidence of HER2 over expression, 21 (84%) were located in the lower third of the oesophagus. On staging, 24 out of the 25 HER2 positive cases were at stage 3 or more (13 at stage 3 and 11 at stage 4), For HER2 negative cases 37 were at stage 3 and 32 were staged as stage 4. Seventeen out of twenty five cases (68%) with HER2 over expression received palliative therapy, in comparison to thirty five out of seventy five (46.7%) in tumours not expressing HER2. No significant difference in overall survival was demonstrated between patients whose cancers showed evidence of HER2 over expression and those who did not; median overall survival for HER2 positive tumours was 15 mo (95%CI, 11-19 mo) compared to 13 mo (95%CI, 9-17 mo) for HER2 negative ones. Two years cumulative survival for cases with HER2 over expression was 33.7% compared to 31.6% in cases without HER2 over expression (P = 0.576). Only cancer’s stage significantly affected overall survival on both univariant and multivariable analysis (P = 0.034 and P = 0.009 respectively). None of the patients included in this study received Trastuzumab.
CONCLUSION: Twenty-seven point five percent of oesophageal adenocarcinomas showed evidence of HER2 over expression. Routine testing for human HER2 in oesophageal adenocarcinomas can have significant implication on treatments offered to patients that may potentially affect their prognosis.
- Citation: Al-Momani H, Barnes R, El-Hadi A, Shah R, Lewis WG, Edwards P. Human epidermal growth factor receptor-2 in oesophageal cancers: An observational study. World J Gastroenterol 2012; 18(44): 6447-6451
- URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6447.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6447
Gastro-oesophageal (GO) cancer is the fifth most common malignancy (and fourth most common cause of cancer death) in the United Kingdom, affecting around 13 500 people each year[1]. Despite all recent advances in the diagnosis and management of these tumours their prognosis remains poor. Worldwide, gastric cancer is the second most common cause of cancer death[2]; Oesophageal cancer has an overall five year survival of only 8%, and in view of the relatively high rate of chemotherapy resistance in oesophageal adenocarcinomas[3,4], additional treatment options are desperately needed.
Human epidermal growth factor receptor 2 (HER2) is the second member of the cell membrane surface bound receptor tyrosine kinase family; this family is made up of four glycoproteins[5]. Trastuzumab (Herceptin®) is a humanized monoclonal antibody targeting HER2[6]; more evidence is mounting to support its use in upper gastrointestinal tract malignancies.
The ToGA trial studied the benefits of adding Trastuzumab to chemotherapy in patients with inoperable locally advanced, recurrent or metastatic adenocarcinoma of the stomach or GO junction[7]. Addition of Trastuzumab to chemotherapy significantly improved overall survival. Previous studies to the ToGA trial demonstrated over expression of HER2 in gastric cancers[8,9]. However, compared to gastric cancer, comprehensive data about HER2 expression in oesophageal adenocarcinomas are relatively scarce[10].
In view of these facts, the HER2 status for oesophageal tumours was studied in a consecutive series of one hundred oesophageal cancer biopsy specimens obtained at endoscopy between April 2007 and June 2010 aiming to determine the incidence of HER2 in oesophageal cancer. Secondary end points were to assess how HER2 over expression reflects on patients’ overall survival, tumours’ location and what treatments patients received.
HER2 assays were performed by an independent, validated and audited laboratory. A Ventana pathway HER2/neu 4B5 assay was utilised. Immunohistochemistry (IHC) scoring criteria were those described by Hofmann et al[11]. Samples were scored as 0, +1, +2 and +3. 0 and +1 are considered negative +3 is positive. Patients with +2 results underwent HER2 gene analysis using the Ventana HER [brightfield dual-colour in situ hybridisations (HER B DISH)] assay and either shown to be positive or negative for HER2 over expression.
One hundred consecutive cases of oesophageal cancer were identified between April 2007 and June 2010 from a prospectively maintained British regional upper gastrointestinal tract cancer network database. Biopsies obtained at index endoscopy were analysed for HER2 status. All patients were followed up for two years or until death, 93% were followed up till five years or death. Death certification was obtained from the Office for National Statistics. Cases of GO junctional tumours were excluded.
Overall survival was measured from date of histological diagnosis until date of death. Methods appropriate for parametric data were used. Cumulative survival was calculated according to the life-table method of Kaplan and Meier, and differences in survival between groups of patients were analyzed with the log rank test. Correlation was assessed using the Pearson χ2 test; P < 0.05 was considered a statistically significance value. Data analysis was performed using SPSS version 18.0 (Chicago, United States).
The median age of our sample was 66 ± 10.51 years (range: 38-91 years). Eighty-one were male and 19 female. The anatomical distribution of the tumours was; upper oesophagus 2, middle oesophagus 11, and 87 were in the lower oesophagus. Ninety-one of the cases were adenocarcinoma of the oesophagus and the rest were cases of squamous cell carcinoma. Operative resection was completed in 15 cases; eleven cases had an Ivor-Lewis oesophagectomy, three cases had Transhiatal oesophagectomy and one case underwent a three stage oesophagectomy. Seven cases had attempted surgical resections, i.e., open and close. In cases not suitable for surgery, 33 patients received definitive chemo-radiation and 52 had palliative treatment.
In this group of one hundred patients, 25 cases showed evidence of HER2 over expression. On IHC, 28 cases were scored as “0”, 38 cases were scored as “+1” and 18 cases were scored as “+3”. Of the 16 cases that were scored as “+2” and further underwent analysis using bright field DISH, seven were found positive. All tumours expressing HER2 were adenocarcinomas. None of the oesophageal squamous cell carcinoma cases showed evidence of HER2 over expression.
Of the 25 cases that showed evidence of HER2 over expression, 21 (84%) were located in the lower third of the oesophagus, four (16%) were in the middle third and none were in the upper third.
On staging, 24 out of the 25 HER2 positive cases were at stage 3 or more (13 at stage 3 and 11 at stage 4); one case was a stage 2 and none were a stage 1. For HER2 negative cases 37 and 32 were at stage 3 or 4 respectively, four cases were a stage 2 and two were a stage 1.
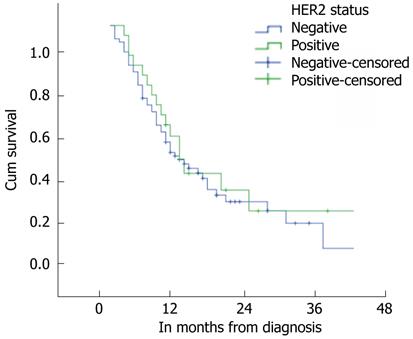
No significant difference in overall survival was demonstrated between patients whose cancers showed evidence of HER2 over expression and those who did not: Median overall survival for HER2 positive tumours was 15 mo (95%CI, 11-19 mo) compared to 13 mo (95%CI, 9-17 mo) for HER2 negative ones. This difference was not significant, both on univariant analysis (P = 0.576) and multivariable analysis (P = 0.419) that included patient’s age, cancer stage, cancer location and HER2 status. On the other hand, cancers’ stage significantly affected overall survival on both univariant and multivariable analysis (P = 0.034 and P = 0.009 respectively). Two years cumulative survival for cases with HER2 over expression was 33.7% compared to 31.6% in cases without HER2 over expression (P = 0.576). Figure 1 demonstrates Kaplan-Meier survival curves for the two groups.
Only two of the fifteen cases who had disease amenable to surgical resection showed HER2 over expression (P = 0.208). Both cases underwent Ivor-Lewis oesophagectomy. Definitive chemo-radiation was given to 33 patients, only six (18.2%) of them expressed HER2. Seventeen out of twenty five cases (68%) with HER2 over expression received palliative therapy, in comparison to thirty five out of seventy five (46.7%) in tumours not expressing HER2 (P = 0.064). None of the patients included in this study received Trastuzumab.
In our study population, 27.5% of oesophageal adenocarcinomas cases in a British regional cancer network demonstrated evidence of HER2 over expression, this rate is in line with previously published series from other countries[12-14], and is as high, if not higher, than that found in other types of cancer.
In this study, HER2 positive oesophageal adenocarcinomas accounted for a small portion of cases which were suitable to have surgery or definitive chemo-radiation, 13.3% and 18.2% respectively; on the other hand, nearly two thirds of tumours expressing HER2 received palliative treatment (68%). These results may indicate a more aggressive behaviour for oesophageal adenocarcinomas expressing HER2, an observation expressed by other authors[15].
In this series, HER2 overexpression did not affect overall survival; a recent series by Langer et al[10] suggested otherwise, with tumours overexpressing HER2 having a shorter survival. Similar discrepancy has been described before and the literature holds conflicting data about the prognostic significance of HER2 overexpression[16-19]. In our sample overall survival was measured from the time of histological diagnosis until time of death. This is inherently inaccurate in that it takes no account of the patient’s onset and duration of symptoms nor the biological behaviour of the individual tumour. In Langer et al[20] series, unlike ours, they used two different tissue microarrays for the assessment of HER2 status; with cores from different regions in the microarrays being assessed false-negative results due to intratumoral heterogeneity[21,22] may have been eliminated. Moreover, for the determination of HER2 status, they used different in situ hybridisation methods, i.e., fluorescence in situ hybridisation, 3D fluorescence in situ hybridisation or bright field double in situ hybridisation, which eliminates false-negative results even further.
In this series none of the nine cases of oesophageal squamous cell carcinoma expressed HER2, there is, however, a growing body of evidence showing HER2 to be abnormally expressed in oesophageal squamous cell carcinomas and is associated with poor prognosis. Reported rates of HER2 overexpression in oesophageal squamous cell carcinoma can be as high as that found in oesophageal adenocarcinomas; researchers had described 26%-64% of oesophageal squamous cell carcinomas to test positive to HER2[23,24]. HER2 over expression has been reported to be associated with extra mucosal tumour invasion and poor response to chemo radiation in oesophageal squamous cell carcinomas[25,26].
Traditional phase III studies to ascertain the potential role of Trastuzumab in the treatment of oesophageal adenocarcinomas has proven difficult. A power calculation based on our results (α = 0.05; 80% power) suggests a retrospective study of 180 patients would be required to clarify the situation. Having said that, a recent Phase I/II study by Safran et al[27] exploring the use of Trastuzumab alongside chemo-radiotherapy in the treatment of oesophageal adenocarcinoma had initially aimed to recruit 25 patients; however, and after 42 mo, they only managed to enrol 19. Safran et al[27] work had concluded that adding Trastuzumab to chemo-radiotherapy regimens is safe and not associated with increased toxicity.
In the United Kingdom, The standard of care for patients with HER2 positive breast cancer is treatment with Trastuzumab[28,29]. Also, the National Institute of Clinical Excellence guideline recommends Trastuzumab in combination with cisplatin and capecitabine or 5-fluorouracil in patients with metastatic adenocarcinoma of the stomach or GO junction expressing HER2. There is, however, no British guideline about the use Trastuzumab with HER2 positive oesophageal adenocarcinoma.
So, to summarise, this study demonstrates that in this British population of patients with oesophageal adenocarcinoma, 27.5% of them are HER2 positive. This is at least as high as that found in breast[30] and probably higher than that found in gastric or GO junctional tumours. HER2 status in patients with oesophageal adenocarcinoma should be routinely assayed and patients should be offered treatment with Trastuzumab if their tumours showed evidence of HER2 over expression. New guidelines should be implemented in the United Kingdom in line with those issued in other countries to offer patients with oesophageal cancers a treatment that can potentially prolong their survival. More work is needed to look at HER2 status in patients with squamous carcinoma of the oesophagus.
The authors would like to thank Dr. Howell S and Mr. Gable C for their help in preparing the histological specimens used in this study. We also would like to thank Ms. Winston K for her hard work liaising with the laboratory throughout the study period.
Over the last 20 years there has been a steady increase in the annual incidence of gastro-oesophageal (GO) cancers in the Western hemisphere. With that came a lot of research that brought developments to various aspects to these cancers diagnosis and treatment; never the less, these cancers continue to have a poor prognosis and new treatment modalities are desperately needed.
Molecular targeted therapy offers a new frontier and hope to patients with GO cancers, especially since these cancers had shown resistance to conventional treatment modalities; i.e., chemotherapy and radiotherapy.
Molecular targeted therapy is becoming the standard of care for patients with suitable cancers of the oesophagus in many countries. In the United Kingdom, however, there are still no guidelines to recommend such therapy to British patients. This study demonstrates that the incidence of the target molecule for these new drugs in cases of oesophageal cancer in the United Kingdom is as high as that reported in other countries. British patients whose cancers express this molecule should be offered these new drugs.
Adding molecular targeted therapy to conventional treatments in patients with cancers of the oesophagus can potentially prolong their survival and improve their prognosis.
The authors describe the results of human epidermal growth factor receptor 2 (HER2) assessment in esophageal cancers. They report that 27% of esophageal adenocarcinomas overexpressed HER2. The study is relatively straightforward and confirms existing knowledge on the subject.
Peer reviewer: Julian Abrams, MD, MS, Assistant Professor of Clinical Medicine, Division of Digestive and Liver Diseases, Columbia University Medical Center, 622 W 168th Street, PH 20-303, New York, NY 10032, United States
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011;60:1449-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 415] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2646] [Article Influence: 139.3] [Reference Citation Analysis (0)] |
| 3. | Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4899] [Cited by in RCA: 4609] [Article Influence: 242.6] [Reference Citation Analysis (0)] |
| 4. | Langer R, Ott K, Feith M, Lordick F, Siewert JR, Becker K. Prognostic significance of histopathological tumor regression after neoadjuvant chemotherapy in esophageal adenocarcinomas. Mod Pathol. 2009;22:1555-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4920] [Cited by in RCA: 5111] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 6. | Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1728] [Cited by in RCA: 1925] [Article Influence: 106.9] [Reference Citation Analysis (0)] |
| 7. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5326] [Article Influence: 355.1] [Reference Citation Analysis (3)] |
| 8. | Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol. 2008;19:1523-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 872] [Article Influence: 51.3] [Reference Citation Analysis (2)] |
| 9. | Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 525] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Langer R, Rauser S, Feith M, Nährig JM, Feuchtinger A, Friess H, Höfler H, Walch A. Assessment of ErbB2 (Her2) in oesophageal adenocarcinomas: summary of a revised immunohistochemical evaluation system, bright field double in situ hybridisation and fluorescence in situ hybridisation. Mod Pathol. 2011;24:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 868] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 12. | al-Kasspooles M, Moore JH, Orringer MB, Beer DG. Amplification and over-expression of the EGFR and erbB-2 genes in human esophageal adenocarcinomas. Int J Cancer. 1993;54:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Jankowski J, Coghill G, Hopwood D, Wormsley KG. Oncogenes and onco-suppressor gene in adenocarcinoma of the oesophagus. Gut. 1992;33:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Duhaylongsod FG, Gottfried MR, Iglehart JD, Vaughn AL, Wolfe WG. The significance of c-erb B-2 and p53 immunoreactivity in patients with adenocarcinoma of the esophagus. Ann Surg. 1995;221:677-83; discussion 683-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Brien TP, Odze RD, Sheehan CE, McKenna BJ, Ross JS. HER-2/neu gene amplification by FISH predicts poor survival in Barrett's esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:35-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 101] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Hardwick RH, Barham CP, Ozua P, Newcomb PV, Savage P, Powell R, Rahamin J, Alderson D. Immunohistochemical detection of p53 and c-erbB-2 in oesophageal carcinoma; no correlation with prognosis. Eur J Surg Oncol. 1997;23:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Langer R, Von Rahden BH, Nahrig J, Von Weyhern C, Reiter R, Feith M, Stein HJ, Siewert JR, Höfler H, Sarbia M. Prognostic significance of expression patterns of c-erbB-2, p53, p16INK4A, p27KIP1, cyclin D1 and epidermal growth factor receptor in oesophageal adenocarcinoma: a tissue microarray study. J Clin Pathol. 2006;59:631-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Reichelt U, Duesedau P, Tsourlakis MCh, Quaas A, Link BC, Schurr PG, Kaifi JT, Gros SJ, Yekebas EF, Marx A. Frequent homogeneous HER-2 amplification in primary and metastatic adenocarcinoma of the esophagus. Mod Pathol. 2007;20:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Walch A, Bink K, Gais P, Stangl S, Hutzler P, Aubele M, Mueller J, Höfler H, Werner M. Evaluation of c-erbB-2 overexpression and Her-2/neu gene copy number heterogeneity in Barrett's adenocarcinoma. Anal Cell Pathol. 2000;20:25-32. [PubMed] |
| 20. | Langer R, Specht K, Becker K, Ewald P, Bekesch M, Sarbia M, Busch R, Feith M, Stein HJ, Siewert JR. Association of pretherapeutic expression of chemotherapy-related genes with response to neoadjuvant chemotherapy in Barrett carcinoma. Clin Cancer Res. 2005;11:7462-7469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Owonikoko T, Rees M, Gabbert HE, Sarbia M. Intratumoral genetic heterogeneity in Barrett adenocarcinoma. Am J Clin Pathol. 2002;117:558-566. [PubMed] |
| 22. | Walch A, Bink K, Hutzler P, Höfler H, Werner M. HER-2/neu gene amplification by FISH predicts poor survival in Barrett's esophagus-associated adenocarcinoma. Hum Pathol. 2000;31:1332-1334. [PubMed] |
| 23. | Akamatsu M, Matsumoto T, Oka K, Yamasaki S, Sonoue H, Kajiyama Y, Tsurumaru M, Sasai K. c-erbB-2 oncoprotein expression related to chemoradioresistance in esophageal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2003;57:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Mimura K, Kono K, Hanawa M, Mitsui F, Sugai H, Miyagawa N, Ooi A, Fujii H. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2005;92:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 25. | Miyazono F, Metzger R, Warnecke-Eberz U, Baldus SE, Brabender J, Bollschweiler E, Doerfler W, Mueller RP, Dienes HP, Aikou T. Quantitative c-erbB-2 but not c-erbB-1 mRNA expression is a promising marker to predict minor histopathologic response to neoadjuvant radiochemotherapy in oesophageal cancer. Br J Cancer. 2004;91:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Tanaka S, Mori M, Akiyoshi T, Tanaka Y, Mafune K, Wands JR, Sugimachi K. Coexpression of Grb7 with epidermal growth factor receptor or Her2/erbB2 in human advanced esophageal carcinoma. Cancer Res. 1997;57:28-31. [PubMed] |
| 27. | Safran H, Dipetrillo T, Akerman P, Ng T, Evans D, Steinhoff M, Benton D, Purviance J, Goldstein L, Tantravahi U. Phase I/II study of trastuzumab, paclitaxel, cisplatin and radiation for locally advanced, HER2 overexpressing, esophageal adenocarcinoma. Int J Radiat Oncol Biol Phys. 2007;67:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8139] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 29. | Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1060] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 30. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8336] [Cited by in RCA: 8534] [Article Influence: 224.6] [Reference Citation Analysis (0)] |