INTRODUCTION
The kidney and the liver play the major role in the elimination of drugs. No common rules exist for the preference of kidney or liver in the elimination of drugs. While for some compounds one of these two routes is the dominant excretory pathway, for certain other substances both the kidneys and the liver play an important and overlapping role as excretory organs[1,2].
The relationship between renal and hepatic excretion of xenobiotics depends on the integrity of the elimination mechanisms. Impairment of liver or kidney function can be followed by compensation via the alternative elimination route, changing the relationship between renal and hepatic excretion of drugs[1-3].
Since 1990, several membrane transport proteins have been cloned and characterized[4-6]. Organic anion transporters (OATs) are located in the barrier epithelia of diverse organs, where they mediate the absorption and excretion of a wide range of metabolites, signaling molecules, and xenobiotics. The presence of overlapping substrate specificities among the different OAT isoforms suggests a possible role in remote signaling. Substrates excreted through one OAT isoform in one organ are taken up by another OAT isoform located in a different organ, thereby mediating communication between different organ systems. Ahn et al[7,8] have developed a “remote sensing and signaling hypothesis”. They suggested how the regulation of solute carrier 22 subfamily members (including those of the organic cation, organic carnitine, and unknown substrate transporter subfamilies) can be better understood by considering the organism’s broader need to communicate between epithelia and other tissues by simultaneous regulation of transport of metabolites, signaling molecules, drugs and toxins.
On a mechanistic basis, cholestasis usually is divided into “extrahepatic” and “intrahepatic” forms[9-11]. The first refers to obstruction of large bile ducts outside the liver, for instance due to gallstones, while the causes of intrahepatic cholestasis lie within the liver. The main clinical manifestations and characteristics of “intrahepatic” and “extrahepatic” cholestasis are similar[11,12]. It is possible to separate the two kinds of cholestasis by means of a careful evaluation of clinical, serologic, and histologic features. The mechanisms underlying the development of cholestasis in patients can be extremely varied. Intrahepatic cholestasis has multiple causes. Among these, familial cholestasis, drug-induced cholestasis, and many processes that affect bile formation/excretion and finally produce cholestasis, could be cited. Obstruction of the biliary tree due to gallstones or hepatic or biliary tumours causes stagnation of bile flow. Actually, patients with gallstones or tumours in the common biliary tract generally suffer from extrahepatic cholestasis. A well-established model of cholestasis in rodents is obtained by performing double ligation and division of the common bile duct (BDL). In animal models, a decrease in bile flow is associated in general with elevated cholestatic serum markers, altered liver histology and changes in liver functions related to sinusoidal and canalicular transport. Indeed, prolonged cholestasis alters the liver function due to an impaired uptake, changed biotransformation and secretion of compounds[12]. In fact, cholestasis has been shown to alter the transport of the bile salts and of other organic anions[9,11,13]. Moreover, altered absorption, distribution and elimination of drugs have been described in this pathology[4].
In obstructive jaundice, adaptive mechanisms may permit urinary excretion of those potentially toxic compounds that could not be eliminated by the liver because biliary transport is impaired[4,10,11]. The modulation in the expression of renal OATs constitutes a compensative mechanism to overcome the hepatic dysfunction in the elimination of organic anions.
The expression and function of several transporters in kidneys and livers from rats with obstructive cholestasis will be reviewed. These transport proteins are as follows: (1) multidrug resistance-associated protein 2 (MRP2); (2) organic anion transporting polypeptide 1 (OATP1); (3) OAT3; (4) bilitranslocase (BTL); (5) bromosulfophthalein (BSP)/bilirubin binding protein (BBBP); (6) OAT1; and (7) sodium dependent bile salt transporter (ASBT).
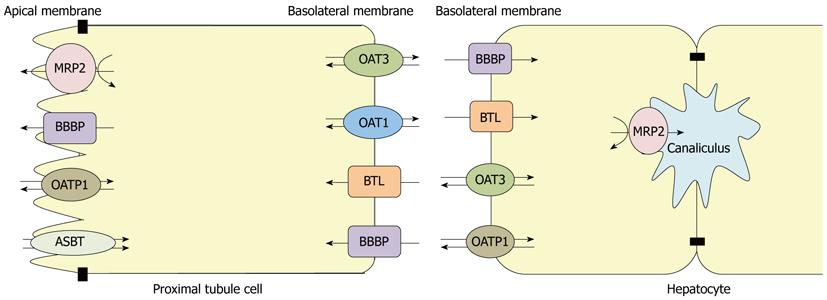
MRP2, OATP1, OAT3, BTL and BBBP are expressed in kidney and liver, while OAT1 and ASBT are not expressed in liver. Their membrane localization is shown in Figure 1.
Figure 1 Membrane localization of organic anion transporters in hepatocytes and proximal renal tubule cells from rat.
MRP2: Multidrug resistance-associated protein 2; OATP1: Organic anion transporting polypeptide 1; OAT: Organic anion transporter; BTL: Bilitranslocase; BBBP: Bilirubin binding protein; ASBT: Sodium dependent bile salt transporter.
MRP2
Several members of MRP are expressed in liver and kidney tissues[10,14]. They are known to function as ATP-dependent pumps for a broad range of organic anionic substrates. MRP2 is an efflux transporter that mediates the transfer or translocation of a wide variety of potentially toxic endogenous and exogenous compounds, including bilirubin, drugs, and carcinogens, in the form of amphiphilic anionic conjugates by an ATP-dependent mechanism[10,14]. This efflux pump is also involved in BSP-conjugated derivatives and p-aminohippurate (PAH) transport[15-18]. It has been localized on the apical membranes of hepatocytes, renal proximal tubular cells and jejunal cells, and is implicated in the excretion of different organic anions[19].
During cholestasis, the canalicular conjugate export pump MRP2 exhibits a reduced expression that may contribute to impaired hepatic excretion of bile acids and other biliary constituents such as conjugated bilirubin[20,21]. Thereafter, in obstructive jaundice, adaptive mechanisms may permit the liver to adapt to the higher burden of biliary constituents in part by altering the expression of hepatobiliary transporters. In fact, in order to limit hepatocellular accumulation of toxic compounds, upregulation of hepatocellular basolateral efflux pumps have been described[10,13,22,23]. Afterwards, alternative elimination routes, such as via urine, might provide the only pathway to excrete these compounds from the body.
Under conditions of deficient hepatobiliary secretory function, an induction of renal proximal tubular MRP2 expression has been reported. Lee et al[13] demonstrated an upregulation in MRP2 protein expression in kidneys as early as one day after BDL. Moreover, renal MRP2 function has been studied by PAH clearance in a rat model of obstructive jaundice by Tanaka et al[24]. They described a significant increase in PAH clearance 24 h after BDL that was associated with high levels of renal MRP2 expression. In this study, in contrast to the liver where MRP2 protein and mRNA expression were downregulated after BDL, the protein and mRNA expression of MRP2 were significantly increased in the kidneys 24 h after BDL. They proposed that the increased renal MRP2 expression might be an alternative pathway for accelerating excretion of bilirubin conjugates during obstructive cholestasis. In order to gain insight into the mechanism by which BDL affects renal and hepatic MRP2 expression they also evaluated the effects of synthetic conjugated bilirubin, sulfate-conjugated bile acid, human bile, and unconjugated bilirubin on human MRP2 expression in an in vitro system using different cell lines. They described that mRNA expression of MRP2 increased in human renal proximal tubular epithelial cells after treatment with conjugated bilirubin, sulfate-conjugated bile acid or human bile. On the other hand, mRNA expression of MRP2 did not change in a human hepatoma cell line (HepG2 cells) compared with controls after the same treatments. These findings suggest that these substrates might regulate renal MRP2 expression by a cell-specific mechanism.
Downregulation of MRP2 in both liver and intestine from rats undergoing extrahepatic cholestasis might be explained by the increased levels of the inflammatory cytokine interleukin-1β (IL-1β) observed in these animals[20,21]. These increased levels of IL-1β would lead to decreased binding of RARα:RXRα nuclear receptor dimer to the promoter region of MRP2 in BDL rats, which in turn downregulates MRP2 expression in the liver. In contrast, BDL rats exhibited upregulation of expression and function of MRP2 in the kidney, which likely results from preserved levels of RARα:RXRα dimer in this tissue.
Villanueva et al[25] have reported an increased renal tubular conversion of 1-chloro-2,4-dinitrobenzene to its glutathione conjugate dinitrophenyl-S-glutathione (DNP-SG), followed by subsequent MRP2-mediated secretion into urine that partially compensates for altered liver function in experimental obstructive cholestasis.
At this point, it is noteworthy to mention that we have found increased BSP and PAH renal excretion in our experimental model of BDL in rats[26-28], which in turn might be also explained by the upregulation of renal MRP2 expression[29].
Finally, obstructive cholestasis leads to an upregulation of the apical transporter MRP2 in the kidneys that would be involved in the increased secretion into urine of organic anions, including BSP, PAH and DNP-SG. The current data demonstrate the relevance of renal elimination as an alternative pathway, particularly under conditions of impaired hepatobiliary secretory function, as occurs in obstructive cholestasis, for excreting those toxic compounds that the liver could not eliminate by itself from the body. Together, all these findings provide further evidence that transporters are regulated in cholestatic liver disease in a manner that facilitates extrahepatic mechanisms for organic anions excretion.
OATP1
OATP are members of a group of multispecific transporters with a wide spectrum of amphipathic substrates, including endogenous compounds and clinically important drugs[30]. OATP1, the first member of this group of transporters identified, was isolated from rat liver and shown to mediate Na+-independent saturable transport of BSP[31,32]. Nowadays, the sequences of almost 160 different members of OATP superfamily in over 25 animal species can be found in GenBank[4].
Several members of this family of polypeptides are expressed in liver and kidneys, among others organs. In hepatic tissue, OATP1 is found at the basolateral membrane of hepatocytes where it plays an important role in the Na+-independent uptake of a wide variety of compounds, including bilirubin, BSP and conjugated and unconjugated bile acids[33]. In contrast, in the kidneys, OATP1 is located at the apical membrane of the proximal tubules, specifically in the S3 segment, and has been suggested to play a role in the secretion/reabsorption of selected anionic substrates, although its renal physiological function remains unclear[4,30,32,34]. It could participate in the reabsorption of organic compounds that are filtered, like estradiol-17β-glucuronide, or in the secretion process of some organic compounds that were taken up into tubular cells across the basolateral membrane[34,35]. It is interesting to emphasize that renal secretion of BSP, a characteristic substrate for OATP1 transport, is negligible in non-pathological conditions.
Regulation of OATP1 expression and function occurs at both transcriptional and post-transcriptional levels, being tissue specific. Higher OATP1 renal expression in males than in females has been demonstrated in rodents probably as a result of testosterone stimulation and estrogen inhibition. On the other hand, hepatic OATP1 expression is not influenced by sex hormones[10,34,36,37]. Phosphorylation of this transporter, in serine residues, by extracellular ATP produces its functional down regulation[38,39]. In addition, protein kinase C (PKC) activation leads to a decreased transport of estrone-3-sulfate in OATP1 expressing X. laevis oocytes[33,40].
The consequences of cholestasis, produced by BDL, are hepatic and systemic accumulation of potentially toxic biliary compounds, which are associated with progressive liver damage and jaundice[41]. OATP1 hepatic mRNA and protein expression are downregulated in this pathology, with a less pronounced decrease in mRNA expression. This could be due to OATP1 protein having a longer half-life than its mRNA, but the occurrence of a post-transcriptional mechanism cannot be dismissed[33,42]. It has been proposed that the observed OATP1 downregulation could be due, at least in part, to a toxic effect of bile constituents retained in the hepatocyte. It was also proposed that this phenomenon might be explained as a protective feedback mechanism to prevent uptake of possible hepatotoxic bile constituents[42]. In this model, the hepatic mRNA expression levels of cytochrome P450, transporters and conjugation enzymes were found altered. It has been suggested that increased bilirubin and fatty acid levels produced in cholestasis could modify the mRNA and protein levels of the constitutive androstane receptor and peroxisome proliferator-activated receptor α in the nucleus, altering the mRNA expression of several transporters, like OATP1[43].
In obstructive cholestasis, several proinflammatory cytokines are released in the organism, such as tumor necrosis factor α (TNF-α) and IL-1β. Cytokine-inactivation studies have demonstrated the existence of a TNF-α-dependent signaling pathway that mediates the downregulation of the OATP1 gene, at mRNA and protein levels[33].
Geier et al[33] also found unaltered kinetic parameters in Na+-independent bile salts (cholate and taurocholate) uptake in the presence of decreased OATP1 expression in extrahepatic cholestasis. In addition, they found that other sinusoidal OATPs, OATP2 and OATP4, have preserved expression in cholestatic hepatocytes, suggesting that, in this pathology, Na+-independent bile salts uptake is mediated by OATP2 and OATP4 rather than OATP1, to compensate for its downregulation. This may be related to continuous elimination of other organic anions which also play an important role in determining cholestatic liver injury.
We have shown that BDL rats have a higher OATP1 protein renal expression at apical membranes despite no change in OATP1 abundance in kidney homogenates[27]. These results could suggest altered OATP1 trafficking, as a result of an increased recruitment of preformed transporters into the membranes where they are functional, or impairment in the internalization of membrane transporters. These results indicate that the kidneys are able to adapt rapidly to obstructive cholestasis, since BSP renal elimination has been shown to increase almost 30-fold during the first day after induction of BDL. Renal adaptation to cholestasis could be due to an upregulation of the transport capacity of OATP1 in the proximal tubules. This increase in OATP1 protein expression at the apical membrane of renal cells may be a compensatory mechanism for reducing injury to hepatocytes or renal epithelia from cytotoxic substances that may accumulate in rats with obstructive cholestasis.
It is also important to emphasize that differential processing and trafficking of the OATP1 transporter in liver and kidney may have important functional and regulatory consequences. A complex series of hormonal changes in kidneys are induced in extrahepatic cholestasis[44], which could affect OATP1 regulation. Several systemic and local factors are produced at the same time during cholestasis, and the role of such factors in the regulation of channels and transporters in renal cells is still unknown. Most likely, the accumulation of bilirubin, bile acids and other potential toxics existing in this cholestatic model may affect transcriptional regulation (e.g., fetal transcription factor, pregnene X receptor) and post-transcriptional regulatory mechanisms[45].
In conclusion, the presented data shows that extrahepatic cholestasis induced by BDL in the rat produces renal OATP1 protein units redistribution into apical membranes from renal cells[27] and a diminished OATP1 expression in the liver[33,42]. Moreover, this probable adaptation to hepatic injury, particularly in elimination of biliary components, could possibly explain, at least to some extent, the huge increase in BSP renal excretion observed in this experimental model.
OAT3
Human and rat OAT3 is expressed primarily in the kidneys and to a lesser extent in the brain. In rats, OAT3 is also found in the liver[46]. In the kidneys, immunohistochemistry studies showed that OAT3 protein is localized at the basolateral membrane of proximal tubule cells[47,48]. Rat OAT3 was also observed in cortical and medullary thick ascending limb of Henle’s loop, connecting tubules, and cortical and medullary collecting ducts[49]. OAT3 was characterized as an organic anion/dicarboxylate exchanger[50,51]. This protein has the ability to transport PAH, estrone sulfate (ES), ochratoxin A, estradiol glucuronide, benzylpenicillin, cimetidine, glutarate and taurocholate. Although the selectivity of OAT3 overlaps that of OAT1, the affinity for the different substrates could permit discrimination between both transporters. Several studies demonstrated that ES is a specific substrate for mammalian OAT3 with moderately high affinity, whereas OAT1 interacts little with ES[52-55]. Accordingly, ES is frequently used as a test substrate for OAT3 activity. Sweet et al[56] reported reduced uptake of PAH, ES, and taurocholate in renal cortical slices and nearly complete inhibition of transport of the fluorescent organic anion fluorescein in intact choroid plexus in OAT3 knockout mice.
The effects of obstructive cholestasis on the cortical renal expression of OAT3 and the consequences of these effects on the pharmacokinetics and renal excretion of PAH and furosemide (FS) were described by Brandoni et al[26,57] employing male Wistar rats. Results showed that rats at 21 h after BDL exhibit an enhanced systemic and renal clearance of both PAH and FS. At variance with OAT1 expression (as described below), Western blotting studies showed that OAT3 protein expression was increased significantly only in homogenates and not in basolateral membranes from renal cortex in BDL rats. These results were confirmed by immunohistochemical techniques. The upregulation observed for both OAT1 and OAT3 in this model of acute jaundice, could explain, at least in part, the increased systemic and renal elimination of PAH and FS. In addition, the production of various cytokines and growth factors that may affect gene transcription is associated with extrahepatic cholestasis[58].
The function and expression of OAT1 and OAT3 were also studied by Brandoni et al[59] after 3 d of biliary obstruction. It was well documented that serum bile acids and bilirubin levels reach a peak after 3 d of BDL[13,24,60]. A significantly lower renal clearance of PAH was observed after this period of time in rats with obstructive jaundice. In contrast to OAT1, OAT3 was increased both in homogenates and basolateral membranes from renal tissue after 3 d of BDL. As well as OAT1, both human and rat OAT3 transport PAH with relatively high affinity (87 μmol/L and 65 μmol/L respectively)[6,47,61,62]. On the other hand, it was described, using in vivo and in vitro techniques, that OAT3 but not OAT1 has as substrates ES, cholate, and taurocholate[6,47,56,61-63]. Furthermore, whereas OAT1 is limited to proximal tubules, OAT3 is expressed in different parts of the nephron[48]. In extrahepatic cholestasis, the high plasma levels of bile acids compete with PAH for OAT3 transport, therefore, upregulation of OAT3 does not compensate for the downregulation of OAT1 in PAH transport[59,64]. The expression of a number of genes implicated in the transport of bile salts is regulated by bile acids[65,66]. In this sense, and as another example of substrate-specific regulation, it is possible that OAT3 overexpression would be regulated by high bile acid levels, while OAT1 expression would be downregulated.
Urinary excretion is the principal route of excretion of bile acids in obstructive jaundice. It was found that OAT3 mediated the renal secretion of bile acids such as cholic acid, glycocholic acid and taurocholic acid, which are mainly increased during cholestasis[55,67].The pharmacokinetic profile of its substrates could also be affected by cholestasis[67]. Eisai hyperbilirubinemic rats (EHBR) are mutant rats lacking MRP2. Serum and urinary concentrations of total bile acids in EHBR rats are higher than those in wild-type Sprague-Dawley rats. It was described that OAT3 protein in renal plasma membranes was overexpressed in EHBR rats whereas OAT1 expression was unchanged. In addition, rat and human OAT3 transport activities are notably inhibited by diverse bile acids such as chenodeoxycholic acid and cholic acid but OAT1 is not. Cefotiam is also a specific substrate for OAT3. Some authors found that cefotiam clearance was reduced in EHBR despite upregulation of OAT3. This may be due to the inhibition of cefotiam transport via OAT3 by elevated serum bile acids. In summary, these results suggested that renal OAT3, but not OAT1, plays a critical role in the adaptative responses to the renal handling of bile acids in cholestasis.
Northern blot analyses demonstrated that OAT3 mRNA is expressed in rat liver, although such expression is not detected in mouse liver[56,68]. Nevertheless, Buist et al[69], using branched DNA analysis, reported hepatic OAT3 mRNA expression in mice. In addition, gender differences in OAT3 mRNA expression in liver tissue were shown in both mice and rats[56,69,70]. It was described also that the hepatic isoform of OAT3 may be the major contributor OAT in the basolateral uptake of organic anions in rats[71]. Human OAT3 messenger is not expressed in liver.
We have observed in liver plasma membranes from rats with acute obstructive jaundice of 21 h, no differences in OAT3 protein expression when compared with sham rats[72].
All these results might suggest the involvement of different regulatory mechanisms for OATs in obstructive cholestasis. To minimize liver injury, adaptive or protective responses to damage in the hepatobiliary transport of organic anions would appear, or reduced uptake into hepatocytes or enhanced efflux into the circulation. The observed changes could respond to increased intracellular levels of compounds that are normally excreted in the bile duct or to alterations in the levels of cytokines and other mediators in the liver, as previously described[13,73,74]..
To summarize, OAT3 could play a considerable pathophysiological role in protecting tissues from cholestatic damage by stimulating the renal secretion of bile acids. Indeed, ursodeoxycholic acid (UDCA) has been introduced as cholestatic liver disease therapy[75]. It is possible that increased serum bile acids and/or administration of UDCA could influence the tubular secretion of anionic drugs via OAT3 as was evidenced for cefotiam. Therefore, more attention should be paid to prevent the occurrence of drug interactions or drug-induced toxicity.
BTL
BTL is a bile pigment transporter that was originally isolated from rat livers[18,76]. It is also expressed in kidney and stomach. BTL mediates the electrogenic hepatic uptake of cholephilic organic anions, such as BSP and thymol blue, the tetrapyrrole bilirubin, and flavonoids (the anthocyanin malvidin 3-glucoside and the flavonol quercetin)[18]. BTL has been demonstrated to be involved in the renal transport of BSP, bilirubin and anthocyanins[77,78]. In this way, BTL contributes to the hepatic and renal excretion of exogenous organic anions (such as BSP), endogenous metabolites (such as bilirubin) and anthocyanins (flavonoid-based pigments present in fruits and vegetables used in the human diet, which have been reported to be positively implicated in human health[18,78]).
The protein expression and the functional activity of BTL was evaluated in rats with an early phase of acute extrahepatic cholestasis (21 h post BDL), by Western blotting and by measuring BSP electrogenic uptake, respectively, in liver plasma membrane and in renal basolateral membrane vesicles[28]. No modifications were detected in BTL protein expression and in its activity in liver plasma membrane vesicles from BDL rats. In contrast, extrahepatic cholestasis caused a marked increase in renal BSP uptake, which was due to an increase in Vmax (capacity). The difference in Vmax suggests that a higher number of functional carrier units exists in renal basolateral membrane vesicles from BDL rats, which is in agreement with the higher expression of BTL detected in renal basolateral membranes. BDL rats have a higher renal expression of BTL at the basolateral membranes despite no change in kidney homogenates. This suggests an impairment in BTL trafficking.
These results suggest that the complex series of hormonal changes induced in kidneys by extrahepatic cholestasis[44] might influence the regulation of BTL. The characteristic accumulation of bile acids, bilirubin, and other potential toxins in cholestasis may affect transcriptional and posttranscriptional regulatory mechanisms[33,79] of BTL in kidneys. In this connection, as has been mentioned earlier, bilirubin, sulfate-conjugated bile acid and human bile upregulate the expression of MRP2 in renal tubular cells but not in liver cells[24].
In summary, the higher expression and function of BTL observed in renal basolateral membranes from rats with obstructive cholestasis may also contribute to the marked increase in BSP renal excretion described in this experimental model. This would be another compensatory mechanism to overcome the hepatic impairment in the excretion of organic anions.
BSP/BBBP
The transporter protein BBBP has been isolated from rat liver and described as an organic anion carrier protein involved in the well-known sodium-independent hepatic uptake of BSP and bilirubin[80-83]. BBBP exhibits electroneutral transport as indicated by experiments in liver plasma membrane vesicles. In kidneys, BBBP has been localized in both apical and basolateral membrane domains, being implicated in PAH tubular transport[84].
In rats with obstructive cholestasis, we have found an increased expression of BBBP in homogenates and in basolateral membranes from kidney cortex with no change in its apical membrane expression[29,85,86]. These results might suggest an increase in the synthesis or a decrease in the degradation of this membrane transporter, while the trafficked protein would be preferably directed to the basolateral domain as no changes have been observed in the protein expression of BBBP in the apical membrane. As a consequence of hepatic function impairment, alterations in the renal elimination of organic anions were observed. Connected to this, we have also demonstrated a significant increase in renal excretion of two different organic anions in rats with extrahepatic cholestasis, BSP, an organic anion mainly excreted by the liver and PAH, an organic anion mainly excreted by the kidney. These observations could be explained, at least in part, by the higher basolateral membrane expression of BBBP described in this experimental model of acute cholestasis[29,85,86].
As has already been mentioned, BBBP is a protein isolated from the sinusoidal membranes of rat liver. In liver plasma membranes from rats with extrahepatic cholestasis of 21 h, we have demonstrated no difference in BBBP abundance compared to sham animals[85,86]. Modifications in hepatic excretion of organic anions have been demonstrated in rats with extrahepatic cholestasis[9,10,13]. However, BBBP might not be involved in this observation since no change in its abundance was found in this experimental model in rats.
To this extent, it is noteworthy to remark on the relevant role of this kind of transporter, such as BBBP, in renal elimination of those organic anions excreted mainly by the liver, in the presence of obstructive cholestasis. In fact the increased renal abundance of the organic anion carrier BBBP, observed in rats with obstructive jaundice, might improve renal capacity to eliminate distinct negatively charged compounds, especially those that could not be removed by the liver.
OAT1
OAT1 is a key organic anion/α-ketoglutarate exchanger[48,87]. OAT1 is expressed predominantly in the kidneys, and weakly in the brain, and no expression has been described in the liver[52,53,55,64]. This protein has been immunolocalized at the basolateral surface of the proximal tubule. OAT1 mediates the transport of many compounds (dicarboxylates, nucleotides, prostaglandins, antivirals, loop and thiazide diuretics, β-lactam antibiotics, non-steroidal anti-inflammatory drugs, including the prototypical substrate of the classical pathway, PAH)[53,55]. Eraly et al[63] have generated a colony of OAT1-knockout mice, permitting the elucidation of the role of OAT1 in the context of other potentially functional transporters. They found that the knockout mice manifested a profound loss of organic anion transport (e.g., PAH) both ex vivo (in isolated renal slices) as well as in vivo (as indicated by loss of renal secretion). The loss of renal secretion in knockout animals resulted in impaired diuretic responsiveness to furosemide[63]. These results indicate an important role for the OAT1 transporter in the handling of organic anions by the classical pathway.
It has been reported that upregulation of OAT1 protein expression in rats at an early phase of acute obstructive cholestasis might explain the increased renal elimination of PAH and FS[57]. We have also reported an increase in the systemic clearance of PAH associated with an increase in the abundance of OAT1 in renal cortex homogenates in rats during the early phase of acute extrahepatic cholestasis[26,88].
FS is a loop diuretic secreted through the organic anion transport system. The capacity of the organic anion transport system to secrete a diuretic determines its intraluminal concentration, which is decisive of the diuretic activity. OAT1 and OAT3 are responsible for FS delivery to its site of action, since these proteins are involved in the renal tubular secretion of this diuretic[55,89]. The protein expression of OAT1 was significantly increased both in cortical homogenates and in basolateral membranes from kidneys after 21 h of BDL[26,57]. In fact, clearance and urinary excretion of PAH were both higher in BDL rats. Connected to this, PAH uptake rate[57] was increased in basolateral membrane vesicles from BDL rats. OAT1 upregulation was also associated with a concomitant increase of systemic and renal FS clearance.
Similar studies were performed by Brandoni et al[59] after 3 d of obstructive cholestasis (the peak of elevation of serum bile acids and bilirubin). After this time, BDL rats displayed a decrease in the renal elimination of PAH. OAT1 protein expression in kidney homogenates was not modified, but was decreased in basolateral membranes. This study demonstrated, once more, the key role of OAT1 expression in the impaired elimination of PAH after 3 d of BDL. So, the evolution time of obstructive cholestasis has an important impact in the regulation of OAT1.
It has been described that bile acids and high bilirubin levels can activate PKC[55,90]. We therefore postulate that the peak of elevation of bile acids and bilirubin (demonstrated after 3 d of BDL) can also trigger PKC activation. It has been described that PKC induces OAT1 downregulation through carrier retrieval from the cell membrane. This PKC activation may cause the phosphorylation of caveolin-2, which may induce the internalization of caveolae with OAT1 protein anchored with caveolin, as has been described by Kwak et al[91].
In summary, in this experimental model during an early phase of acute extrahepatic cholestasis where no evident renal cell injury exists yet, OAT1 upregulation associated with an increase in renal organic anion elimination was demonstrated. The protein expression of OAT1 was significantly increased both in cortical homogenates and in basolateral membranes from kidneys after 21 h of BDL, which might suggest an increase in the synthesis or a decrease in the degradation of this membrane transporter. In this experimental model, OAT1 upregulation may occur in order to enhance renal secretion of toxic compounds that are not eliminated by the liver in a pathological state. Connected to this, Tanaka et al[24] found that bilirubin ditaurate, sulfate conjugated bile acids, and some components of human bile upregulate the expression of MRP2 in human renal tubular cells. Moreover, we found an increased 3H-PAH uptake by S2 cells expressing OAT1 incubated with bilirubin ditaurate[92].
Contrary to what has been described in an early phase of acute extrahepatic cholestasis, OAT1 expression significantly decreased in the basolateral membranes from kidneys after 3 d of BDL[59]. It has been suggested that there is an increase in the internalization of membrane transporters or an inhibition in the recruitment of preformed transporters into the membranes. This study stressed, once more, the critical role of OAT1 renal expression in the excretion of organic anions, such as PAH, in a model of extrahepatic cholestasis in rats.
ASBT
ASBT is expressed in the ileum, in the cholangiocytes and in the kidneys[93-96]. Bile acids, after secretion with bile into the small intestine, are nearly completely reabsorbed in the terminal ileum. They return with the portal venous blood to the liver where they are taken up and re-secreted into the bile. About 10%-50% of the reabsorbed bile acids avoid hepatic uptake and enter in the peripheral circulation. Around 10%-30% of the bile acids, present in the blood plasma, are subject to glomerular filtration. Urinary excretion of bile acids is much smaller than the amount filtered due to the tubular reabsorption of the filtered bile acids[10,97]. Thus, the kidneys also take part in the recirculation process and aid conservation of bile acids. Tubular reabsorption of bile acids is accomplished by ASBT, which is expressed in the apical membrane of proximal tubule cells[95,96].
Lee et al[13] have described decreased taurocholate transport in brush border membrane vesicles derived from rat kidneys after 14 d of BDL. The reduced taurocholate uptake was associated with a reduction of renal ASBT protein expression.
After one day of obstructive cholestasis, Schlattjan et al[98] demonstrated reduced taurocholate transport in proximal tubular cells without changes in the amount of the transport protein in these cells. The diminished taurocholate transport in this early phase of cholestasis may be mediated by a change in the phosphorylation status and hence the activity of ASBT and/or by a redistribution of the transporter between the plasma membrane and intracellular compartments of the proximal tubular cells. These studies have demonstrated that there is a functional adaptive downregulation of renal ASBT leading to enhance renal clearance of bile acids during the early phase of obstructive cholestasis.
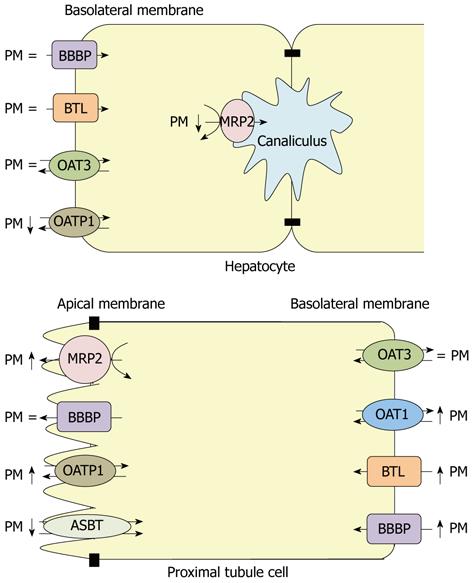
Figure 2 shows a summary of the protein expression of the above mentioned transporters in liver and kidney from rats with extrahepatic cholestasis of 21 h.
Figure 2 Protein expression of organic anion transporters in hepatocytes and proximal renal tubule cells from a rat with extrahepatic cholestasis of 21 h.
PM: Plasma membranes; MRP2: Multidrug resistance-associated protein 2; OATP1: Organic anion transporting polypeptide 1; OAT: Organic anion transporter; BTL: Bilitranslocase; BBBP: Bilirubin binding protein; ASBT: Sodium dependent bile salt transporter.
CONCLUSION
Altered expression of different transporter proteins of organic anions have been described in liver and kidney from rats with extrahepatic cholestasis. MRP2 and OATP1 abundances were decreased while no changes were detected for BBBP, BTL and OAT3 protein expressions in hepatocytes from BDL rats. On the other hand, in proximal tubule renal cells, increased protein expressions were observed for OAT1, BTL, BBBP; MRP2 and OATP1. In contrast, a diminished abundance of renal ASBT was found in this experimental model to prevent reabsorption of excess bile acids that were eliminated in the urine. This pattern in renal cells may be a compensatory mechanism to increase the elimination of compounds that could not be excreted by the liver in this pathological state.
ACKNOWLEDGMENTS
The authors thank Professor Endou H and Anzai N (Department of Pharmacology and Toxicology, Kyorin University School of Medicine, Tokyo, Japan) for kindly providing OAT1 and OAT3 specific antibodies, to Professor Passamonti S (Department of Life Science, Trieste University, Italy) for kindly providing BTL specific antibodies and to Professor Stremmel W (Medizinische Universitatsklinik, Heidelberg University, Germany) for kindly providing BBBP specific antibodies.