Published online Nov 28, 2012. doi: 10.3748/wjg.v18.i44.6357
Revised: November 6, 2012
Accepted: November 14, 2012
Published online: November 28, 2012
Processing time: 133 Days and 23.5 Hours
Mucosal adaptation is an essential process in gut homeostasis. The intestinal mucosa adapts to a range of pathological conditions including starvation, short-gut syndrome, obesity, and bariatric surgery. Broadly, these adaptive functions can be grouped into proliferation and differentiation. These are influenced by diverse interactions with hormonal, immune, dietary, nervous, and mechanical stimuli. It seems likely that clinical outcomes can be improved by manipulating the physiology of adaptation. This review will summarize current understanding of the basic science surrounding adaptation, delineate the wide range of potential targets for therapeutic intervention, and discuss how these might be incorporated into an overall treatment plan. Deeper insight into the physiologic basis of adaptation will identify further targets for intervention to improve clinical outcomes.
- Citation: Shaw D, Gohil K, Basson MD. Intestinal mucosal atrophy and adaptation. World J Gastroenterol 2012; 18(44): 6357-6375
- URL: https://www.wjgnet.com/1007-9327/full/v18/i44/6357.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i44.6357
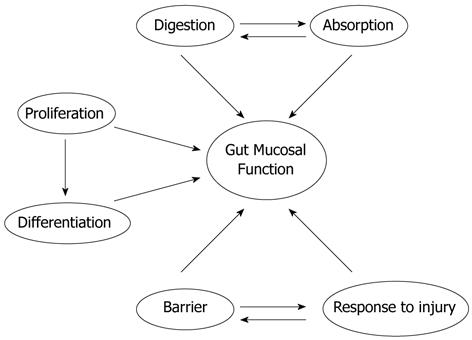
The small intestinal mucosa is adaptable but essential for survival. It has diverse biological roles including nutrient absorption, barrier function, injury response, and immunologic reservoir (Figure 1). Congenital or acquired diseases or medical and surgical interventions can alter intestinal mucosal mass and/or function with profound consequences to which the gut must adapt. The clinical challenges to gut adaptation include massive bowel resection, intestinal atresia, fasting, prolonged ileus, bariatric surgery, and total parenteral nutrition (TPN). The biology that regulates such adaptation represents a critical new frontier for gastroenterology and gastrointestinal (GI) surgery if outcomes are to be improved. This review discusses intestinal mucosal atrophy, hypertrophy, and barrier function, and how they may be influenced. We will begin this review by considering what is known about intestinal mucosal development, as this offers useful parallels to the intestinal mucosal response to pathology. Next, we will explore the biologic phenomena of mucosal atrophy and hypertrophy in more depth. Third, we will discuss the clinical syndrome of intestinal failure in more depth, and consider how our understanding of the biology of intestinal adaptation may guide therapeutic interventions. Finally we will discuss new frontiers in mucosal adaptation, focusing particularly on intersections with GI surgery, including both pro-absorptive procedures like intestinal lengthening procedures and anti-absorptive procedures like bariatric surgery, and on some lessons that may be learned in the future from recent scientific advances. Many potential approaches are available for facilitating absorption and mucosal homeostasis, but their optimal application may require a better understanding of the biology that regulates these processes.
Enterocytes are columnar cells with microvilli at their apices that form the intestinal brush border. The microvilli are covered by a glycocalyx coat that acts as a physical barrier and contains brush border enzymes. Enterocytes are joined by tight junctions to form a relatively impermeable membrane[1,2].
The small intestinal mucosa is folded to increase its surface area[1,2]. Submucosal folding forms plicae circularis that each include many crypt-villus units. Villi are mucosal surface modifications, finger-like extensions of lining epithelium formed by projections of lamina propria covered with epithelium. Each villus extends into lamina propria as an intestinal gland or crypt of Lieberkuhn[1,2]. Crypts have stem cells, paneth cells and enteroendocrine cells. Stem cells proliferate at the crypt base.
Mucosal growth and development are regulated by hormonal, nervous, immune, dietary and mechanical signals[3]. The small bowel mucosa ultimately develops in stereotypic crypt-villus units containing absorptive, secretory, progenitor and stem cells[4]. Intestinal stem cells maintained throughout life in the crypts give rise to progenitor cells that undergo a few cell divisions as they move out of the crypts toward the villi before final differentiation[5,6]. Small bowel ontogeny proceeds in three successive phases: morphogenesis and proliferation, cell differentiation, and functional maturation[7].
The field is beginning to identify molecular mechanisms that influence intestinal development[8]. For instance, homeobox (hox) genes are early regulators of proximal to distal organ-specific patterning[9]. Mucosal remodeling and villus formation precedes in a cranial-caudal direction[10]. A primitive endodermal gut tube surrounded by mesenchyme forms early in gestation[3]. Later, the endoderm transitions to stratified epithelium which ultimately matures to columnar epithelium starting at the apices of the developing villi. The intervillus epithelium differentiates last, and mitotic activity is restricted to intervillus regions and developing crypts by 16 wk[8,11]. This correlates with Wnt/β-catenin pathway activity that appears necessary for stem cell maintenance in fetuses and adults[12]. Fibroblast growth factor receptor (FGFR)-3 signaling regulates crypt epithelial stem cell expansion and crypt morphogenesis viaβ-catenin/Tcf-4 pathways[6].
Intestinal villus formation begins at embryonic week 8 in humans[8]. Influenced by Hedgehog and platelet-derived growth factor (PDGF) signals, mesenchymal cells condense under the epithelium and then grow toward the central lumen to form characteristic fingerlike inward projections-the villi[4,8,13,14]. With increasing age, villus epithelial turn-over, crypt depth, and villus height each increase[8,15].
Proliferation occurs in the crypts but differentiation occurs as cells migrate up the villus. Thus, differentiated cells populate the villi[6]. Each villus contains epithelium from the adjacent crypts[16]. Crypt formation occurs by differential growth of mesenchyme and the crypt-villus junction moves upwards towards lumen[17]. Crypt-base columnar cells are multipotent cells that differentiate into absorptive enterocytes and secretory mucous secreting goblet cells, entero-endocrine cells and paneth cells[18]. Notch pathways decide differentiation into absorptive vs secretory cells[19-21]. Crypt stem cells become monoclonal during development[22]. Critical functional differentiation into distinct apical, lateral and basal cells occurs. Apical cells express digestive enzymes and transporters, while lateral cells chiefly express transporters, and basal cells carry receptors for interaction with basement membrane[8]. The brush border complex formed by villin and myosin I appears at the apical surface of mature enterocytes[23,24].
Intestinal growth and differentiation are regulated by an intrinsic program; extrinsic mediators play secondary roles[25,26]. N-myc is an important regulator of proliferation[27]. Growth factors like epidermal growth factor (EGF), transforming growth factor (TGF)-α, and TGF-β, insulin-like growth factor (IGF)-2, hepatocyte growth factor (HGF), glucagon like peptide (GLP)-2 and their receptors have been detected in fetal human intestine and likely influence intestinal development[28]. Luminal and circulating factors are not necessary for fetal intestinal differentiation but may affect growth and maturation[26,29-31]. Three known triggers are weaning, thyroxine and glucocorticoids[3]. Regarding intrinsic regulation, it is thought that endoderm has an intrinsic program of regionalization of small intestine and it recruits other cells to complete the formation[32,33]. Hepatic nuclear factor (HNF)-3β, Cdx-1, Cdx-2, GATA transcription factor family and transcription factor CCAAT/enhancer-binding protein α are suggested to have important roles in intestinal development[8]. Specific regulatory regions of the fatty acid-binding protein (FABP) genes are responsible for appropriate temporal, crypt/villus and proximal/distal expression of genes[34]. Reciprocal permissive and instructive epithelial-mesenchymal interactions and signals direct organ-specific differentiation[35]. Endoderm can recruit mesenchymal elements[33]. Sonic hedgehog, bmp, Fkh6, H1x homeobox gene, tyrosine kinase receptors and fibroblast growth factors mediate intestinal growth by epithelial-mesenchymal interaction and direct regional patterning of the gut[35-40]. The extracellular matrix influences epithelial differentiation by receptor-mediated signaling[41] and by acting as reservoir for growth factors[42]. The matrix also drives the enterocyte response to growth factors and physical forces[43,44]. Matrix has a permissive effect on epithelial cells and is required for maximal differentiation[45].
Brush border membrane enzymes and brush border transport proteins represent the most important functional differentiation of small intestine. These enzymes provide digestive and absorptive functionality for carbohydrates, proteins, fats, minerals and vitamins. Brush border enzymes appear by week eight. Different disaccharidases, alkaline phosphatases, peptidases, and enterokinases mature at different rates in development[46-48]. Lactase-phlorizin hydrolase (LPH) cleaves lactose into glucose and galactose. Studies of LPH development suggest a proximal to distal gradient in functional maturation of the intestinal epithelium[46]. Although the human fetus expresses some enzymes and peptidases at levels similar to adults, many of these enzymes have different forms in the fetus[49-52]. Various transporters for sugar and amino acids appear during gestation in parallel with crypt and villus development[53,54]. Fetal intestine also starts developing the capacity to secrete lipoprotein fractions, chylomicrons, very-low-density lipoproteins and high-density lipoproteins[55,56]. Absorptive function is partially detectable at 26 wk[57].
The intestinal barrier exhibits immune and non-immune protective mechanisms. Although various mucosal defense systems appear early in gestation, immune function remains immature at birth[58-64]. Tight junctions between enterocytes and goblet cell mucins form a physical barrier by 12 wk[65].
In summary, the basal differentiation program in encoded in fetal endoderm and mediates spatial and cranio-caudal differentiation of intestinal epithelium via a complex intercellular communication network. Epithelial-mesenchymal interaction yields a specialized regional environment that influences gene expression and regulates the growth and differentiation of the intestinal mucosa into crypt-villus units.
Mucosal atrophy is characterized by diminished intestinal function as well as morphological changes including decreased villous height, crypt depth, surface area, and epithelial cell numbers[66]. Atrophy is most common in the absence of enteral nutrition, and is a known long-term consequence of starvation, an effect likely reduced with age[67]. Animal studies suggest incremental relief of atrophy with progressively greater intake, and that morphologic atrophy is most evident at the villous tip[68].
Atrophy occurs even if adequate parenteral nutrition is provided. Animal studies first demonstrated the physiology of atrophy during TPN[69], with atrophy of the proximal small intestinal mucosa with decreased intestinal weight and nitrogen content[69]. Absence of luminal contents due to either starvation or TPN similarly causes mucosal hypoplasia in rodents, mediated at least in part by an altered tumor necrosis factor (TNF)-α/EGF signaling pathway[70]. Additionally, rats receiving TPN have fewer Peyer’s patches and less total T cells than rats fed enterally, demonstrating an attenuating effect on the gut-associated lymphoid tissue[71]. Animal studies also demonstrate that TPN evokes enterocyte apoptosis via intraepithelial lymphocyte derived interferon-gamma, resulting in a loss of the overall barrier function[72]. Barrier function is further altered by TPN stimulation of ion secretion, an effect upon intestinal permeability further altered by interferon-gamma[72]. The effect of TPN on immunologic function (including that in the gut) may have profound clinical consequences. For instance, infectious complications were doubled in pancreaticoduodenectomy patients receiving TPN rather than jejunal feeding[73].
That the effects of fasting and TPN on the gut mucosa are not just from the TPN has been shown in animals. For instance, when a segment of rat jejunum is defunctionalized by a blind end Roux-en-y anastomosis and the rat is allowed to eat freely, the defunctionalized mucosa undergoes morphologic and biochemical atrophy, while the remainder of the gut mucosa remains intact[74]. This suggests that direct interaction with luminal chyme is required to sustain the mucosa. Indeed, the effects of the absence of enteral feeding may reflect not only the loss of luminal nutrients themselves, but also aberrations in the physical forces to which the mucosa is subjected during gut interactions with luminal chyme, either directly by peristaltic compression against the non-compressible liquid contents of the bowel or indirectly by villus motility[75-77]. It has long been known that luminal contents and distension influence postprandial intestinal motor activity[78] that leads to deformation of the bowel mucosa. In addition, villus motility is markedly stimulated by luminal amino acids and fatty acids (but not glucose)[79].
At the cellular level, atrophic loss of mucosal mass may reflect both decreased proliferation and increased apoptosis. EGF family cytokines are potent mitogens, but there are others, including GLP-2, while TNF-α and others mediate apoptosis. We found mostly decreased proliferation in defunctionalized rat intestine, perhaps because the incidence of apoptosis is too low to be readily measurable[74], except in chemotherapy-induced mucosal injury[80]. Fasting leads to jejunal mucosal atrophy with enhanced apoptosis in a mechanism related to increased nitric oxide[81]. Feng et al[70] recently explored the interaction between growth factor-stimulated proliferation and cytokine-driven apoptosis in a murine TPN model. BCl-2 expression acts on mitochondria to prevent cytochrome C release, and caspase 3 directed cell death. Bax in contrast, acts on mitochondria to cause caspase 3 release, leading to programmed cell death. The ratio between these determines overall cell survival[82,83]. Enterocytic differentiation is also impaired in mucosal atrophy, with decreased expression of brush border enzymes and other differentiation markers[74,84,85], but the mechanism by which this occurs is much less well understood. While translational science strives to find better mitogens to promote enterocytic proliferation, how to promote enterocytic differentiation in patients or animals with mucosal atrophy may represent an important question for basic science in the future.
Mucosal adaptation in many ways opposes atrophy, although that there may be subtle but important differences in the stimuli that prevent atrophy and maintain normal mucosal mass and those that induce adaptation. Teleologically, adaptation may be the attempt of the intestine to compensate for intestinal inadequacy. It has been best described after massive small bowel resection[86,87]. However, exogenously induced adaptation may reverse chemotherapeutically induced atrophy. For example, profound intestinal injury by methotrexate may be mitigated by supplementation with L-arginine or n-3 fatty acids[88,89].
Acute absence of nutrition alone cannot trigger full adaptation or fasting would cause intestinal hypertrophy rather than atrophy. The stimuli contributing to adaptation are diverse. Many cytokines facilitate adaptation, including PDGF-α, HGF, and interleukin (IL)-11[86,90,91]. As detailed later, hormones such as IGF-1 and growth hormone (GH) appear to exert strong adaptive influences[92,93]. Within enterocytes, intestinal resection invokes novel signals such as proline-rich protein 2 during wound healing[94], glutathione reductase during intestinal apoptosis[95], and basic Kruppel-like factor to activate the IGF-1 promoter[96].
The anatomic location of the bowel mucosa has an important relationship with adaptive biology. The small and large intestinal mucosa demonstrate many differences in histology, cell phenotype, and transport proteins that reflect their differences in normal function. In addition, the small and large intestinal mucosa respond differently to stimuli of malignant transformation. For example, the APC (Min) mouse is a dominant mutation that leads to multiple intestinal neoplasia[97]. Crypt cells express a balance of proliferation and differentiation, a process with aberrant regulation in these mutants. In mouse models with this mutation, small bowel neoplasms are much more common than colonic neoplasms, in contrast to the human condition in which colonic neoplasms are more common[98]. The reason for this regional difference is as yet unknown but further investigation may offer important clues into differentiated intestinal epithelial biology.
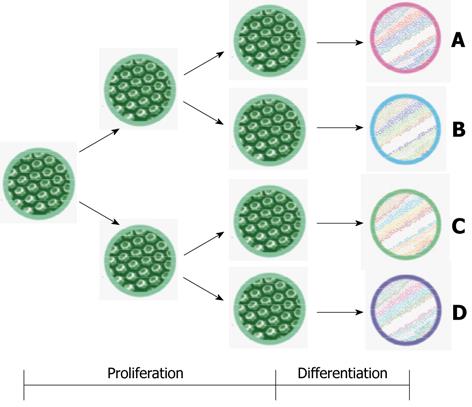
Finally, the role of nutrition in adaptation is not yet fully explained. Glutamine has been most studied[99] (Table 1). There may also be differences between the signals that stimulate the increase in mucosal mass and the signals that augment mucosal functionality during adaptation. Adaptation includes proliferation, augmentation of function and changes in intestinal epithelial phenotype (Figure 2).
| Cytokines | Intracellular transducers of physical force effects | Nutrients | |||
| Stimuli | Ref. | Stimuli | Ref. | Stimuli | Ref. |
| PDGF-α | Sukhotnik et al[86] | FAK-Tyr 925 | Chaturvedi et al[225] | L-arginine | Koppelmann et al[88] |
| HGF | Katz et al | Integrin-linked Kinase | Yuan et al[243] | Glutamine | Lardy et al[252] |
| Transforming GF-β | Sukhotnik et al[151] | RhoA | Chaturvedi et al[244] | Ornithine | Lardy et al[252] |
| IGF-1 | Lund et al[92] | ROCK | Chaturvedi et al[244] | Butyrate | Bartholome et al[161] |
| VEGF | Parvadia et al | mDial | Chaturvedi et al[244] | Short-chain fructooligosaccharide | Barnes et al[163] |
| EGF | Warner et al[90] | - | - | - | - |
| GLP-2 | Bortvedt et al[93] | - | - | - | - |
Proliferation is one mechanism of adaptation. Proliferation increases villus height, crypt depth, surface area, and intestinal wet weight[100]. The length of intestine resected correlates with the subsequent change in villus height in humans[101]. The site of resection also influences adaptation[102], with a notable increase in jejunal hyperplasia, and to some degree ileal hypertrophy. Animal studies also suggest increased intestinal stem cells after resection; these may contribute to increased crypt formation[103]. Growth factors and nutritional supplementation stimulate intestinal epithelial proliferation and turnover.
The intestine also adapts from a functional standpoint. Proliferation increases overall function just by creating more cells that can contribute to amino acid, glucose, and electrolyte uptake. Increased absorption via the H+/peptide co-transporter 1 after intestinal resection occurs because of hyperplasia and not upregulation of transporters[104]. Improved glucose uptake after massive small bowel resection is similarly driven by cellular proliferation rather than massive transporter upregulation[105]. In addition, we can see increase in Na+/glucose transporter (Sglt1), Na+/H+ exchangers (NHE2/3), and some brush border membrane enzymes[106].
Although most work has focused on enterocytes, adaptation also increases non-enterocytic mucosal epithelial cells. Goblet and paneth cells exhibit an early and sustained increase after bowel resection; these secretory cells may contribute to juxtacrine signaling that further stimulates intestinal adaptation[107]. Finally, the role of angiogenesis has been relatively understudied in mucosal adaptation. Bowel resection in rats induces angiogenesis within the adapting intestinal villi[108]. This may facilitate absorption, protect mucosal integrity and barrier function, and increase nutrients and oxygen delivery to the more rapidly proliferating mucosa.
To maintain and control epithelial cell homeostasis, proliferation and differentiation are transcriptionally regulated in a sequential and spatially defined manner[109]. The signals that control intestinal development also influence intestinal homeostasis. These include the canonical Wnt/β-catenin pathway[110], Notch[111], Hedgehog[112], the TGF-β family including bone morphometric proteins (BMP)[113], PI-3K[114] and Forkhead Box (FOX) and homeobox (HOX) genes[115]. These pathways use various transcription factors including HNF1α/β, HNF4α, GATA factors, ETS, and Cdx1/2, alone or in combination[116-120]. For instance, the combination of HNF1α, GATA4-6 and Cdx2 regulates sucrase isomaltase transcription[116] but in differentiated mouse epithelium; HNF4α regulates expression of genes upregulated during differentiation such as alkaline phosphatase[121]. GATA transcription factors are required for crypt cell proliferation and absorptive enterocyte gene expression[119]. HNF3β is expressed in small intestine and has critical role in foregut and midgut formation[122-124]. Finally, Cdx2, which is restricted to adult small intestine and colon[125], is necessary for maintenance of intestinal identity and differentiation of the small intestine epithelium (Table 2)[126].
| Cellular factors |
| Wnt/β-catenin |
| Notch |
| Hedgehog |
| PI3K |
| HNF1 α/β |
| GATA |
| ETS |
| Cdx2 |
| FGF4 |
| NEUROG3 |
| Schlafen-3 |
| Math1 |
A recent landmark study demonstrated the guidance of human pluripotent stem cells into intestinal tissue[127]. This study demonstrated that the activity of Wnt3a and FGF4 was adequate for hindgut patterning, specification and morphogenesis, with NEUROG3 transcription factor required for enteroendocrine cell development in vitro. Similarly, embryonic stem cells have been committed to intestine lineage in medium treated with Wnt3A, in a process that interestingly enough simulated the genes associated with distal gut-associated mesoderm (Foxf2, hlx, Hoxd8)[128]. This process was successful in that it allowed engraftment of these cells into murine colonic mucosa.
Intestinal failure occurs when the absorptive surface area falls below a critical level, either because of loss of bowel length or mucosal atrophy with severely flattened epithelium. Intestinal failure presents with diarrhea, dehydration, malabsorption, progressive malnutrition, and electrolyte disturbance[129].
TPN administration in starving animals or humans does not abrogate the atrophy observed in starvation alone. In addition, TPN may itself impair mucosal barrier function[130] beyond the effects of starvation on the epithelium. In adult trauma patients, this loss of barrier function may significantly increase sepsis[131]. Trauma patients receiving enteral nutrition have fewer pneumonias, intra-abdominal abscesses, and line sepsis and less infections overall compared to patients on TPN[131]. TPN-associated loss of epithelial barrier function may be related to altered mucosal lymphoid populations with increased interferon gamma and interleukin-10 expression, as well as loss of tight junctions and adherens junction proteins[72]. Prolonged starvation, as in chronic TPN, pancreatitis, or other medical conditions, can lead to intestinal failure via mucosal atrophy. Indeed, poor enteral intake can cause pancreatitis and intestinal mucosal atrophy[132] which in turn increases enterocyte apoptosis and alters glutamine and arginine transport[133,134]. Atrophy in turn creates a propensity for bacterial translocation and sepsis[135].
Massive small bowel resection can severely test the capacity of the remaining small bowel mucosa to adapt, resulting in short bowel syndrome, a devastating nutritional problem. The most common causes of short bowel syndrome in children include necrotizing enterocolitis, intestinal atresia, and midgut volvulus[136,137]. In adults, common causes include inflammatory bowel disease, mesenteric ischemia, small bowel obstruction, and radiation enteritis. The loss of mucosal area associated with short bowel syndrome causes substantial malabsorption, with attendant diarrhea, abdominal pain, and weight loss, electrolyte imbalance, and chronic malnutrition. Mucosal adaptation in such patients is a slow and gradual process that may require up to 1-2 years to reach maximum. The simplest and earliest phases of adaptation involve enterocytic proliferation and villous hyperplasia, which may manifest as a reduction in diarrhea and attendant fluid and electrolyte loss. Nutritional adaptation that addresses nutrient absorption and digestion sufficiently to permit weaning from TPN is slower and requires greater complexity.
Current medical management of intestinal failure: Intestinal failure is initially managed similarly whether due to atrophy or short gut. TPN supplies nutritional requirements while ways to transition to enteral feeding are sought. This is usually successful in mucosal atrophy, although it may be prolonged and difficult in some patients. Such transitions are less frequently successful in the short gut patient. The primary predictor of survival in adults with short gut is small bowel length. Eighty-three percent of adults with less than 50 cm of intestine require lifelong TPN; Twenty-five percent will die within 5 years[138]. In pediatric short gut patients, cholestasis and age-adjusted small bowel length less than 10% of expected length predict mortality; small bowel length and an intact ileocecal valve predict successful weaning from TPN[139]. The ileocecal valve slows transit through the small bowel, facilitating absorption and digestion. In addition, the colon may both absorb water and salvage energy in such patients, perhaps mitigating the need for parenteral nutrition if the small bowel is marginally adequate[140].
Whether as a bridge to enteral nutrition or as permanent maintenance, TPN is lifesaving in patients who cannot be nourished enterally. However, TPN has significant complications. TPN itself results in mucosal atrophy, impaired mucosal immunity with a proclivity towards intestinal infections, and dysfunction of the gut-associated lymphoid tissue[141]. It is unclear to what extent these phenomena reflect the lack of enteral feeding and to what extent they are consequences of the infusion of large quantities of hyperosmotic or hyperlipidemic nutrients into the circulation. TPN is also associated with chronic systemic problems including mechanical complications related to the catheter, recurrent infections, liver failure, and death. Randomized, controlled trials have demonstrated the benefits of enteral feeding over parenteral feeding for diverse conditions[142] with reductions in infection, intraabdominal abscess, anastomotic leak, hospital stay, and all other complications. Many enteral nutrients are essential for intestinal adaptation in both adult and pediatric populations[143,144]. In both acutely ill hospitalized patients and chronic short gut patients at home, some enteral nutrition is therefore desirable even if parenteral supplementation is required. Such enteral intake both maintains the mucosal barrier and supports the patient psychologically. The central theme of modern management is to provide the gut with at least some nutrients and consequent hormonal stimuli even if parenteral supplementation is required.
“Intestinal rehabilitation” for short bowel syndrome uses chronic home TPN as a bridge to maintain patients while seeking to adapt them to eventual enteral nutrition. Intestinal rehabilitation is a multidisciplinary approach aimed at achieving enteral autonomy, and keeping patients alive while still requiring TPN. Teams of GI and transplant surgeons, gastroenterologists, dieticians, pharmacists, nurses, and social workers collaborate to offer improved nutritional care and dietary manipulation, facilitated discussion about needs for surgical interventions, and formal monitoring and manipulation of essential medications including mucosal mitogens. This approach may improve survival compared to historical controls, although this could also reflect improved treatments over time[145].
In rats with short bowel syndrome, early enteral feeding affects not only cellular proliferation, but also overall gut weight and length[146]. Even marginal nutrition at the apical or luminal surfaces may improve human intestinal epithelial cell growth, motility, and absorption capacity[147]. Overall, enteral feeding induces significant intestinal adaptation. Further interest lies in trying to modify the nature of the diet.
Dietary lipids encourage intestinal adaptation through several mechanisms. At the simplest level, early feeding of a fatty diet increases lipid absorption in the remnant intestine[148]. Specific nutrients may be important. For instance, arachidonic acid stimulates intestinal adaptation more than linoleic acid[149]. Rats on high-fat diet demonstrate increased fat absorptive capacity compared to rats eating standard chow[150]. However there is some controversy about the role of enteral fatty acids. Other evidence does not demonstrate improved adaptation with enteral omega-3 fatty acids, but only with parenteral supplementation among rats[151]. Dietary fish oil appears to increase fat absorption without a concurrent increase in bile acid synthesis in rats following ileocecal resection[152].
Dietary fat intake might also modify gene expression and transport by altering the transcription and activation of signal proteins related to protein synthesis of nutrient transporters, including activation of peroxisome proliferator-activated receptors, HNF-4, and nuclear factor κ-B[153]. Dietary fat may activate intracellular signals to alter mRNA expression.
Diet also affects gut membrane permeability. Membrane fluidity is altered dramatically by the intrinsic fatty acid saturation and also by cholesterol and ganglioside/glycosphingolipid content, and can inhibit degradation of gut occluding tight junctions in rats[154]. Specialized parts of the membrane such as lipid rafts and caveolae affect signaling and protein intake in a manner altered by fatty acid intake[155].
Intestinal lipid transfer is relatively quickly influenced by diet. Rats fed a high-fat diet for only seven days undergo intestinal adaptation, reflected in dramatic increases in the expression of sterol regulatory element-binding protein (SREBP)-1c. The activation of SREBP-1 increases its synthesis and translocation to the nucleus in intestinal cells, altering lipid metabolism[156]. Further work is needed to identify the signals that influence short term and long term adaptation. This may have even morphological implications. Palmitic acid feeding increases rat bowel and mucosal weight after massive small bowel resection after only 14 d[157].
Interestingly, the colon also contributes to intestinal adaptation in malabsorption. In carbohydrate salvage, short-chain fatty acids (SCFA) produced by fermentation by anaerobic colonic bacteria are absorbed by the colonic mucosa, resulting in net energy absorption. These SCFA consist primarily of acetate, propionate, and butyrate. Luminal butyrate is the primary energy source of the colonocyte, and SCFA are trophic for the colonic mucosa. Adding SCFA to TPN prevents small bowel mucosal atrophy in fasting animals[158,159]. Adding butyrate to TPN also improves lymphocyte numbers, small intestinal IgA levels, and small intestinal surface area[159]. This demonstrates that intravenous nutrition can interact with luminal enterocytes, facilitating their function and altering their structure to promote digestion. Indeed, parenteral butyrate alone increases plasma GLP-2 and directly promotes GLUT2 activity[160,161]. Parenteral butyrate facilitates small bowel adaptation in piglets after massive resection, improving small intestinal morphology and reducing apoptosis[161]. Butyrate also must act independently of GLUT2 since it promotes enterocytic differentiation in isolated cells in culture[162]. Prebiotic supplementation with short-chain fructooligosaccharides may replace butyrate and also promote jejunal adaptation[163].
Traditionally attention has been placed on optimizing carbohydrate/fat/protein ratios to maximize nutrient delivery in short gut syndrome. However, enteral nutrients also influence intestinal adaptation. For example, dietary carbohydrate induces adaptation for monosaccharide absorption by increasing the quantity of carbohydrate transporters[164]. Dietary fiber may also be helpful in modulating nutrient uptake. In TPN-nourished rats with 85% small bowel resection, supplementation with dietary fiber along with GH synergistically enhanced intestinal adaptation[165].
Increased enteral protein content leads to adaptive amino acid uptake in the small bowel[166]. Glutamine is a conditionally essential amino acid is also the enterocyte’s primary energy source[141]. Providing parenterally fed rats with glutamine reduces mucosal atrophy[167], but this effect is less robust in enterally fed animals[165]. Glutamine supplementation also enhances mucosal immunity in rats with gut-derived sepsis[168]. However, animal results have been mixed[169]. Some studies showed glutamine to be effective only when combined with GH as discussed below[165]. One small uncontrolled study did report that glutamine promoted weaning from TPN, with increased growth and improved nutritional factors[170]. Human studies for the most part have not demonstrated much efficacy[171,172]. A recent prospective, randomized human study suggests that human GH may aid adaptation with or without glutamine, but only the patients who received GH along with glutamine maintained the reduction in parenteral nutrition at 3 mo[173]. This points to the need for multimodality therapy, and suggests caution with regard to glutamine supplementation alone.
Adaptation may be facilitated by retinoic acid. Retinoic acid administered intravenously has significant trophic effects in rats undergoing small bowel resection, apparently by inhibiting apoptosis and stimulating crypt cell proliferation[174]. Retinoic acid may act via changes in extracellular matrix[175], by acting on hedgehog signaling, by increasing Reg1 and Pap1 activity, and by acting on retinoid and peroxisome proliferators-activated receptor pathways. Convincing human data are lacking.
Polyamines can be either synthesized from ornithine or ingested. Diet supplementation with ornithine α-ketoglutarate increases intestinal adaptation after intestinal resection[176,177]. In one recent study, piglets with 80% small bowel resection were randomized to either parenteral nutrition alone or parenteral nutrition plus enteral feedings beginning on postoperative day 3[146]. The piglets with additional enteral feedings exhibited greater weight per length of intestine, as well as increased cellular proliferation index and ornithine decarboxylase activity. Response to enteral plus parenteral feedings was greater than the group with sham operation as well. In summary, with only a few days of enteral feeding piglets could undergo exceptional adaptation to extensive surgical resection as marked by polyamine synthesis and crypt cell proliferation. However, it remains unclear to what extent the polyamine synthesis was the critical mechanism for the trophic effects of enteral feedings in this study, and, as for retinoic acid, data suggesting that polyamine supplementation alone will be effective in humans are lacking at this time.
Enteral antibiotics are certainly effective in a very select group of short bowel patients in whom small bowel bacterial overgrowth potentiates malabsorption[178,179]. The inflammatory response to small bowel resection may also be a potential target for intervention. In massively bowel-resected rats with bowel segment reversal, oral antibiotics were associated with increased IGF-1and blunted increases in white blood cell count, IL-6, and serum nitric oxide. This demonstrated that antibiotics may attenuate the inflammatory response[180]. However, more clinical outcomes associated intervention with this have yet to be assessed. This represents an important frontier for future work, but should not justify indiscriminate antibiotic use in patients without demonstrable bacterial overgrowth.
Promoting enterocyte proliferation is an attractive strategy to treat short gut. Many agents have been promising in vitro and in animals. We will review several below. As of this writing, only GH and Teduglutide (outside the United States) are in clinical use. It remains unclear how substantial their effects are. In addition, the long term risks of treating the gut mucosa with mitogens over decades are unknown.
GH is an anabolic protein that initiates mitosis. It is released by the anterior pituitary and may act through IGF-1[181]. GH is perhaps the best studied short bowel mitogen. Experimental studies suggest that GH might have several beneficial effects on adaptation[182], including increases in mucosal hyperplasia and absorptive capacity[165,183], bowel growth, villus height, and crypt depth[184,185], and even increased length within the remaining intestine after extensive small bowel resection[92]. In humans, GH alone, or combined with high carbohydrate diets and glutamine supplementation, may increase nutrient absorption[186]. In children dependent on TPN for more than 50% of their nutritional needs, 12 wk of GH decreased TPN requirements. However, only two children (25%) were definitively weaned from TPN[187]. This suggests the need for multimodal interventions to achieve clinically meaningful endpoints. Combining GH with dietary modification and glutamine supplementation may permit weaning from TPN in some patients[183], and another prospective, double-blind randomized placebo-controlled trial demonstrated that a reduction in TPN use can persist for three months if GH is combined with glutamine[173]. Four randomized, double-blind, placebo-controlled studies have asked whether GH supplementation increases body weight in this setting[172,188-190]. These have yielded mixed results, although a recent Cochrane review found that glutamine overall increases weight, lean body mass, energy absorption, and nitrogen absorption[191]. Reported side effects[172] include myalgia, gynecomastia, insomnia, joint pain, and hyperglycemia. As of this writing, GH is the only FDA-approved agent to treat short bowel syndrome in the United States, but it is certainly not a panacea. To the extent to which GH is effective, it is most likely to benefit patients with 70-100 cm of small bowel remaining and without an intact colon. Side effects are significant.
Glucagon-like peptide-2: Glucagon-like peptide-1 physiologically is a humoral mediator of intestinal adaptation, normally secreted in response to enteral stimulus, especially by foods containing carbohydrates, fatty acids, and fibers[192,193]. It is a 33 amino acid peptide derived from proteolytic cleavage and modification of proglucagon in the pancreatic α-cells and intestinal L-cells[194].
GLP-2 production is most robust in the distal small bowel and large intestine[195]. Effects of GLP-2 are specific to different regions of the bowel and appear to stimulate morphologic adaptation with increase in microvillus height and overall surface area. This was demonstrated in an animal model with 80% small bowel resection, using animals given TPN with or without GLP-2. After only one week intestines were examined for morphology, crypt cell proliferation, apoptosis, SGLT-1 expression and GLUT-5 transport proteins. In addition to the expected finding of morphologic adaptation, GLP-2 increased the jejunal crypt apoptotic index without increasing transport protein expression[196].
Teduglutide (ALX-0600), a dipeptidyl peptidase IV (dpp-IV) resistant GLP-2analog, has been reported to promote intestinal growth in short bowel patients, increasing small intestinal villus height, crypt depth, and mitotic index and improving absorption over three weeks[197]. 11 short bowel syndrome patients with Crohn’s disease taking Teguglutide over 2 years demonstrated excellent compliance (93%), safety, and improved quality of life. The major reported side effects appear to be abdominal pain and obstructive symptoms, but it is difficult to determine the extent to which these side effects should be ascribed to Teduglutide or to the patients’ underlying Crohn’s disease. Whether other short gut patients will report less of these symptoms awaits study[198]. Teduglutide may also aid weaning from TPN. In a randomized placebo-controlled trial, low-dose Teduglutide promoted weaning from TPN, although puzzlingly high-dose Teduglutide did not have this effect[199] although secondary endpoints of villus height and body mass were increased by high-dose Teduglutide as well. This puzzling result was attributed to possible baseline differences between groups, although alternative explanations include difference in oral intake and side effects. In addition, a suprapharmacologic effect may limit efficacy. Teduglutide is in clinical use in some countries already, and will likely achieve broader distribution in the near future. Further studies with regard to the ideal dose, time course, and potential for synergy with other interventions would improve our understanding of how Teduglutide should be used (Table 3).
| Dose mg/kg per d | Side effects | Structure | Approval | |
| Growth hormone | 0.1 | Fluid retention, joint pain, hyperglycemia | 191-amino acid protein | FDA |
| EGF | NA1 | NA | 53-amino acid peptide | Not available commercially |
| GLP-2 | 0.1 | Abdominal pain/obstructive symptoms | dpp-IV resistant 33-amino acid peptide | Phase III clinical trials available in Europe |
IGF: IGF may also enhance enterocyte proliferation after small bowel resection is[200,201]. IGF-1 is produced primarily in the liver but it is also synthesized to a lesser extent within the intestine by subepithelial myofibroblasts[202]. IGF-1 may upregulate digestive enzymes including sucrase, maltase, and leucine aminopeptidases after small bowel resection in animals[203]. In addition, targeted overexpression of IGF-1 in transgenic mice leads to increased small bowel weight, length, and crypt cell proliferation[204]. In short bowel syndrome rats on parenteral nutrition, IGF-1 treatment induced jejunal hyperplasia[205]. In small bowel syndrome rats, IGF-1 increased jejunal mucosal mass by 20% and DNA content by 33%, reflecting increased enterocyte hyperplasia[206].
EGF: EGF is a 53-amino acid peptide in saliva and pancreaticobiliary secretions. EGF stimulates crypt cell proliferation and suppresses apoptosis[207]. EGF administration at the time of small bowel resection may facilitate intestinal adaptation, ameliorating weight loss and apoptosis[208,209]. EGF functions intraluminally as small bowel resection in animals increases salivary EGF without increasing plasma EGF, and either removal of salivary glands[209,210] or selective oral inhibition of the EGF receptor[106] attenuates adaptation after small bowel resection. The EGF receptor is regulated at the level of ligand expression during intestinal epithelial differentiation[211].
Leptin has been studied most regarding appetite and the obesity physiology. It is also a potential target to manipulate adaptation. Parenteral leptin may stimulate structural adaptation in short bowel rats by increasing cell proliferation and decreasing apoptosis. Leptin also increases GLUT-5 levels[212,213].
Bombesin is also being explored as a therapeutic target of interest. In rats, subcutaneous exposure to bombesin for 2 wk after massive small bowel resection enhanced enterocyte turnover with increased ileal transmural and mucosal weight, DNA and protein, villus height, crypt depth, and proliferation index. These rats also demonstrated increased ileal Bax and Bcl-2 and decreased apoptosis[214].
Ghrelin is secreted in the stomach and other tissues and influences food intake and nutrition. Plasma ghrelin is decreased in short bowel syndrome[215]. These changes are unrelated to hyperphagia. It is not yet known whether this decreased ghrelin is only reactive or has an adaptive function[216].
Glucocorticoids: The stress response may play a critical role in intestinal adaptation. In rats undergoing either 80% small bowel resection or sham operation, dexamethasone infusion reduced weight, DNA content, and mucosal protein content regardless of surgical status. IGF-1 was markedly decreased in the steroid-treated rats, demonstrating a potentially deleterious effect on adaptation[217]. In contrast, glucocorticoids may impact uptake of sugars by modulating uptake receptors with variable effects among the glucocorticoids[218]. How to modulate these effects without adversely affecting other physiologic parameters remains unknown.
Integrated multimodality treatment may prove the best strategy. For instance, just as GH may be more effective when combined with glutamine. GH and EGF in combination synergistically increased microvillus height and enhanced nutrient transport in a rabbit short bowel model[219].
Surgery is more acutely risky than medical intervention but also offers hope to patients who otherwise would be condemned to permanent TPN. Potential interventions include intestinal lengthening procedures such as the Bianchi procedure or the serial transverse enteroplasty (STEP) procedure and small bowel transplantation[141,220]. Intestinal lengthening procedures are more conservative.
The Bianchi procedure involves splitting a dilated bowel segment longitudinally and reanastomosing it. This could potentially double intestinal length. One institution recently reported a 40% TPN wean rate with Bianchi alone[221]. There is significant potential, however, for bowel loss in the event of technical misadventure. The STEP is an alternative that involves plicating the small bowel with staple lines alternating on the mesenteric and antimesenteric edges. One series reported a 60% TPN wean rate among adult and pediatric patients with short bowel syndrome using the STEP procedure[222]. On the horizon is the concept of using slow chronic intestinal distraction to stimulate intestinal mucosal and muscular proliferation and thus lengthen the small bowel slowly over time. This has been successfully performed in animal models with additional benefits including increased mucosal weight, and potentially improved function as given by increased disaccharidase activity[223]. This makes conceptual sense since pressure[224] and deformation[225] stimulate intestinal epithelial proliferation.
Small intestinal transplantation is more aggressive and risky but offers even more potential for weaning from TPN. The indications for small-bowel transplantation according to the American Society of Transplantation include the high risk of death related to the underlying disease as well as intestinal failure with increased morbidity or poor acceptance of parenteral nutrition. The United States Center for Medicare and Medicaid lists home TPN complications including impending liver failure, central venous access thrombosis of 2 or more central veins, recurrent line sepsis, and repeated episodes of dehydration[226]. We highlight the critical nature of these options to demonstrate the need for less morbid options. Risks specific to small intestine transplant include graft thrombosis, ischemia, infection related to immunosupression, and graft rejection. Outcomes appear to be improving with time. Graft and patient survival in carefully selected patients have recently been reported as high as 75% and 80%, respectively[226,227]. Although such surgical interventions are yielding increasingly impressive results, improved medical and nutritional therapy might obviate the need for such risky procedures.
Although we generally think about the intersection between mucosal function and surgery with regard to procedures to increase mucosal mass and digestive function, the biology of intestinal adaptation may also be relevant to morbid obesity surgery. Morbid obesity is a rising epidemic with profound cardiovascular, endocrine, and pulmonary systemic consequences[228]. Such patients typically do not respond well to conventional instructions to eat less and exercise more. The induction of artificial malabsorption using steatorrheic agents can cause mild weight loss but is associated with poor compliance[229]. Bariatric surgery has evolved as the most effective treatment for morbid obesity. Although many procedures have been described to induce weight loss, they can generally be categorized as to whether they have restrictive and/or malabsorptive components. Procedures including malabsorptive components, in which some of the small bowel is bypassed, generally achieve superior weight loss to purely restrictive procedures because malabsorption procedures create a functional short gut syndrome.
Such bariatric procedures generate initial weight loss, but many patients gain back weight later. Some failures are attributed to behavioral changes as patients learn to “outeat” the surgical procedure. However, it seems likely that postoperative intestinal adaptation to the functional short gut also ameliorates weight loss by increasing the absorptive capacity of the intestine that remains in continuity.
Intestinal adaptation does occur after malabsorptive obesity surgery. Humans undergoing classical jejunoileal bypass develop increased small bowel villus height and improved nutritional intake without increases in individual cell height or width, identifying epithelial hyperplasia as part of the adaptive mechanism[230]. However, the unbypassed functional segment of small intestine demonstrates not only an adapted morphologic appearance with increased villus height but also increased activity of brush border enzymes[231]. This suggests that individual intestinal epithelial cells, while not larger, are likely to be more functional, better able to absorb and digest nutrients, consistent with a bimodal model of adaptation in which the adapted intestine has not only more enterocytes but better enterocytes that more fully express characteristics needed for their function. It remains unclear whether similar changes occur in response to decreased nutrient consumption in the bowel of patients who undergo purely restrictive procedures such as laparoscopic gastric banding and gastric sleeve procedures. Human biopsies 11-22 mo after jejuno-ileal bypass reveal marked mucosal villus hypertrophy in the continuous segment of bowel, and atrophy within the bypassed segment[232]. Obesity surgery also alters gut hormone levels. For instance, 6 mo after Roux-en-Y gastric bypass, fasting leptin and insulin decrease while peptide YY, enteroglucagon, and GLP-1 increase[233] This coincides with sustained postprandial satiety that may also be related to intestinal adaptation. These effects may persist for years after surgery[234].
Mucosal atrophy and adaptation can also be reproduced in rat models for research. Adaptation in the remaining intestinal segment is well described in rats after massive small bowel resection. Conversely, we recently described a novel defunctionalizing Roux-en-Y anastomosis rat model in which the defunctionalized segment (not actually anastomosed proximally but just ligated at its proximal end) displays morphological and biochemical evidence of mucosal atrophy reminiscent of that seen in TPN-nourished animals despite enteral nutrition passing through the remaining gut[74]. Decreased mucosal mitogenic extracellular signal-regulated kinase (ERK) signaling correlates with decreased proliferation in this bypassed segment. This confirms that mucosal atrophy in the setting of TPN reflects loss of enteral nutrients in direct contact with the gut mucosa rather than loss of indirect neurohumoral effects associated with enteric food consumption.
Interestingly, more classical Roux-en-Y bypass rat models, in which the bypass limb receives continuous biliopancreatic secretions, result in increased villus height and crypt depth in the common limb as compared to the biliopancreatic limb, but decreased glucose transport overall, suggesting the importance of both overall mucosal mass and anatomic rearrangement in determining intestinal function[235]. Importantly, the biliary limb of this anastomosis exhibits partial adaptation with increased width and increased crypt cell proliferation without further mucosal adaptation. The alimentary and common channel exhibits full adaptation in bowel width, villus height, crypt depth and proliferation. This demonstrates the importance of direct contact and local factors required for full adaptation[236].
Some endocrine changes after obesity surgery probably contribute to the success of these procedures, beyond their anatomic restrictive and malabsorptive effects. However, some of the neurohumoral consequences of obesity surgery may act synergistically with the decrease in delivery of nutrients to the intestinal mucosa to promote intestinal adaptation which in turn undesirably enhances nutrient absorption and contributes to delayed weight gain. Altering this natural adaptation after obesity surgery could sustain weight loss with less frequent failure. If sufficiently severe mucosal atrophy could be pharmacologically induced, one might even create sufficient malabsorption to obviate the need for any surgical procedure.
The gut mucosa may not only be influenced by chemical interactions with growth factors, cytokines, and nutrients but also by exposure to physical forces such as repetitive deformation and pressure. These forces can originate from peristaltic contractions, villous motility, and physical interactions between the intestinal villi and the relatively non-compressible luminal chyme. In vitro, the proliferation of human intestinal epithelial cells is stimulated by rhythmic deformation, and this mitogenic effect is synergistic with the mitogenic effect of L-glutamine supplementation[237]. The mitogenic effects of strain are amplitude-dependent[75] as well as frequency-dependent[237], and occur not only in established cell lines but also in primary intestinal epithelial cells derived from human surgical specimens[44].
The pathway governing this mitogenic effect is complex. In vitro work suggests it includes a complex web of kinases[238] (while in vivo such signals are activated by repetitive deformation of the intestine in anesthetized animals[76]). Extracellular pressure may also be mitogenic for intestinal epithelial cells[224]. Pressure stimulates colon cancer cell proliferation via protein kinase C and tyrosine kinase signals. Supraphysiologic extracellular pressure inhibits intestinal epithelial wound healing independently of luminal nutrient flow[239]. Enterocytic differentiation is influenced by some of these same stimuli[240,241].
Interestingly, the intestinal epithelial response to repetitive deformation seems regulated by a matrix-dependent switch. Under basal circumstances, repetitive deformation induces proliferation and differentiation consistent with the ideal enterocytic phenotype. However, when fibronectin is added to the matrix substrate or medium in vitro[44] or deposited into the extracellular matrix in vivo during inflammation or injury[239], then deformation promotes a shift to a migratory phenotype and more rapid cell motility to close the resultant mucosal defect and maintain the mucosal barrier. The signal pathways that regulate this motogenic effect are similar to those by which deformation is mitogenic in the absence of fibronectin, but exhibit subtle but important differences that may permit selective targeting of each effect[242]. For instance, repetitive deformation promotes epithelial motility across fibronectin via a FAK-Tyr 925-phosphorylation that occurs independently of Src, while the FAK-Tyr 925-phosphorylation that occurs in response to strain in the absence of fibronectin requires Src[225]. We recently demonstrated that integrin-linked kinase, in association with focal adhesion kinase and Src, modifies the downstream response to strain, perhaps implicating ILK as a useful molecular target for intervention[243].
Animal studies are beginning to validate these in vitro observations. The defunctionalized gut, deprived of chemical and physical interactions with luminal nutrients, displays mucosal atrophy throughout its length[74] and slower mucosal wound healing in wounded areas into which fibronectin has been deposited[239]. Targeting otherwise deformation-activated signals such as ILK[243] or small GTP-binding proteins like RhoA, rho-associated kinases and the formin homology protein mDia[244] may someday maintain the gut mucosa despite fasting or ileus.
While most therapeutic efforts have emphasized modulating intestinal epithelial proliferation, it may also be useful and important to modulate intestinal epithelial differentiation to achieve a fully and optimally functional intestinal mucosa. Schlafen-3 and Math-1 are potentially interesting targets.
Schlafen-3 is a member of the Schlafen superfamily, a poorly understood heterogenous group of proteins first described in 1998 as a family of growth regulatory genes that modulate thymocyte development[245]. Schlafen-3 is not expressed in humans, but could have a functional human ortholog. We recently demonstrated that the Schlafens could play an important role in modulating intestinal adaptation. Schlafen-3 levels increase in parallel with expression of differentiation markers like dipeptidyl dipeptidase activity and villin expression when non-transformed rat intestinal epithelial IEC-6 cells are induced to differentiate in response to repetitive deformation, TGF-beta, or sodium butyrate. More importantly, reducing Schlafen-3 by specific siRNA prevents the differentiating effect of each of these stimuli[240]. This suggests that Schlafen-3 may represent an important common or convergent node in the differentiating signal pathways invoked by these three very different stimuli. Schlafen-3 is also downregulated in the intestinal mucosa of aging rats[246], but conversely substantially increases in expression between the fetal state over the first few days after birth when the rat intestine is maturing[247].
Although Patel et al[246] reported that Schlafen-3 slows proliferation, we observed no effect on basal or EGF-stimulated proliferation when modulating Schlafen-3[240]. This discrepancy awaits exploration in vivo. Tracing and triggering the Schlafen-3-dependent pathway may offer a way to selectively promote intestinal epithelial differentiation, either without affecting proliferation or perhaps even synergistically with agents such as GH or Teduglutide to promote both proliferation and differentiation.
Targeting differentiation is clinically important because altering the function of naive cells may promote intestinal function. A recent piglet study replicated multiple previous findings of increased total villus cell numbers over 6 wk of adaptation to massive small bowel resection. However, this study demonstrated a disconnect between early proliferation and the absence of increased early weight gain. This suggests that the early proliferation of immature enterocytes alone may not suffice for nutrition, and highlights the need to encourage earlier differentiation to optimize clinical gains[248].
Although the Schlafen superfamily may promote differentiation toward an enterocytic phenotype, intestinal stem cells also differentiate into goblet cells, enteroendocrine cells, and paneth cells in addition to enterocytes. Although less abundant in the mucosa, these intestinal epithelial cells are also important for optimal mucosal function. Murine knockout studies have identified Math1 as a transcription factor that influences the differentiation of these secretory cell types[249]. In fact, deletion of Math1 does not disturb the capacity for self-renewal in intestinal epithelium at the crypt base[250].
Further terminal differentiation is still under intensive investigation. A series of downstream targets influence differentiation toward the endocrine lineage, including NGN3, BETA2, Pax4, and Pax6[251]. Manipulating these targets may also be important in the future to promote a fully functional mucosa.
Intestinal adaptation is an extraordinary phenomenon, induced by diverse pathological and surgical conditions, but not always successful in recreating adequate mucosal function. The long term consequences of such deficits in intestinal function highlight the need for more effective therapy for short gut syndrome directed by investigation into the physiological basis of adaptation. Despite recent progress, current targets are limited. We propose not only continued efforts to stimulate small bowel mucosal proliferation, but also increased investigation into the role of differentiation in adaptation (Table 4). Addressing both proliferation and differentiation multimodally could greatly improve patient outcomes.
| What is the ideal timing for influence of proliferative and differentiation phases in small bowel adaptation with targeted therapy? |
| What are useful combination regimens of multimodality therapy? |
| What dietary supplementation is essential for optimization of adaptation? |
| What is an appropriate algorithm for advanced medical compared to surgical treatment of small bowel syndrome ? |
| What is the precise role for glutamine supplementation in facilitating adaptation? |
| What is the ideal dose of Teduglutide? |
| Can other agents such as polyamines or retinoic acid be useful? |
| How can surgical procedures best be combined with multimodal therapy? |
| How can the role of physical force in adaptation be replaced pharmacologically? |
| Why is neoplasm more common in the large intestine than in the small intestine? |
| Why do APC (Min) mice have more common neoplasms in the small bowel? |
Peer reviewers: Haruhiko Sugimura, MD, PhD, Professor, Department of Pathology, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan; Satoshi Osawa, MD, PhD, Assistant Professor, First Department of Medicine, Hamamatsu University School of Medicine, 1-20-1 Handayama, Higashi-ku, Hamamatsu 431-3192, Japan
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Eroschenko VP, Fiore MSHd. Di Fiore's atlas of histology with functional correlations. 8th ed. Media: Lippincott Williams & Wilkins 1996; . |
| 2. | Gartner LP, Hiatt JL. Color textbook of histology. 2nd ed. Philadelphia: W.B. Saunders 2001; . |
| 3. | Pácha J. Development of intestinal transport function in mammals. Physiol Rev. 2000;80:1633-1667. [PubMed] |
| 4. | Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 170] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Noah TK, Donahue B, Shroyer NF. Intestinal development and differentiation. Exp Cell Res. 2011;317:2702-2710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 258] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 6. | Holnthoner W, Pillinger M, Groger M, Wolff K, Ashton AW, Albanese C, Neumeister P, Pestell RG, Petzelbauer P. Fibroblast growth factor-2 induces Lef/Tcf-dependent transcription in human endothelial cells. J Biol Chem. 2002;277:45847-45853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Colony PC. Successive phases of human fetal intestinal development. New York: Raven 1983; 3-28. |
| 8. | Montgomery RK, Mulberg AE, Grand RJ. Development of the human gastrointestinal tract: twenty years of progress. Gastroenterology. 1999;116:702-731. [PubMed] |
| 9. | James R, Kazenwadel J. Homeobox gene expression in the intestinal epithelium of adult mice. J Biol Chem. 1991;266:3246-3251. [PubMed] |
| 10. | Trier JS, Moxey PC. Morphogenesis of the small intestine during fetal development. Ciba Found Symp. 1979;3-29. [PubMed] |
| 11. | Browning TH, Trier JS. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969;48:1423-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Kim BM, Mao J, Taketo MM, Shivdasani RA. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | van den Brink GR. Hedgehog signaling in development and homeostasis of the gastrointestinal tract. Physiol Rev. 2007;87:1343-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 14. | Moon HW. Epithelial cell migration in the alimentary mucosa of the suckling pig. Proc Soc Exp Biol Med. 1971;137:151-154. [PubMed] |
| 15. | Schmidt GH, Wilkinson MM, Ponder BA. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985;40:425-429. [PubMed] |
| 16. | Wilson TJ, Ponder BA, Wright NA. Use of a mouse chimaeric model to study cell migration patterns in the small intestinal epithelium. Cell Tissue Kinet. 1985;18:333-344. [PubMed] |
| 17. | Calvert R, Pothier P. Migration of fetal intestinal intervillous cells in neonatal mice. Anat Rec. 1990;227:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1039] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Zecchini V, Domaschenz R, Winton D, Jones P. Notch signaling regulates the differentiation of post-mitotic intestinal epithelial cells. Genes Dev. 2005;19:1686-1691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci USA. 2005;102:12443-12448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 696] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 22. | Ponder BA, Schmidt GH, Wilkinson MM, Wood MJ, Monk M, Reid A. Derivation of mouse intestinal crypts from single progenitor cells. Nature. 1985;313:689-691. [PubMed] |
| 23. | Costa de Beauregard MA, Pringault E, Robine S, Louvard D. Suppression of villin expression by antisense RNA impairs brush border assembly in polarized epithelial intestinal cells. EMBO J. 1995;14:409-421. [PubMed] |
| 24. | Moxey PC, Trier JS. Development of villus absorptive cells in the human fetal small intestine: a morphological and morphometric study. Anat Rec. 1979;195:463-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Winter HS, Hendren RB, Fox CH, Russell GJ, Perez-Atayde A, Bhan AK, Folkman J. Human intestine matures as nude mouse xenograft. Gastroenterology. 1991;100:89-98. [PubMed] |
| 26. | Savidge TC, Morey AL, Ferguson DJ, Fleming KA, Shmakov AN, Phillips AD. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Meichle A, Philipp A, Eilers M. The functions of Myc proteins. Biochim Biophys Acta. 1992;1114:129-146. [PubMed] |
| 28. | Podolsky DK. Regulation of intestinal epithelial proliferation: a few answers, many questions. Am J Physiol. 1993;264:G179-G186. [PubMed] |
| 29. | Jolma VM, Kendall K, Koldovský O. Differences in the development of jejunum and ileum as observed in fetal rat isografts. Possible implications related to the villus size gradient. Am J Anat. 1980;158:211-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Montgomery RK, Sybicki MA, Grand RJ. Autonomous biochemical and morphological differentiation in fetal rat intestine transplanted at 17 and 20 days of gestation. Dev Biol. 1981;87:76-84. [PubMed] |
| 31. | Rubin DC, Roth KA, Birkenmeier EH, Gordon JI. Epithelial cell differentiation in normal and transgenic mouse intestinal isografts. J Cell Biol. 1991;113:1183-1192. [PubMed] |
| 32. | Duluc I, Freund JN, Leberquier C, Kedinger M. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211-221. [PubMed] |
| 33. | Del Buono R, Fleming KA, Morey AL, Hall PA, Wright NA. A nude mouse xenograft model of fetal intestine development and differentiation. Development. 1992;114:67-73. [PubMed] |
| 34. | Cohn SM, Simon TC, Roth KA, Birkenmeier EH, Gordon JI. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol. 1992;119:27-44. [PubMed] |
| 35. | Kaestner KH, Silberg DG, Traber PG, Schütz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583-1595. [PubMed] |
| 36. | Hentsch B, Lyons I, Li R, Hartley L, Lints TJ, Adams JM, Harvey RP. Hlx homeo box gene is essential for an inductive tissue interaction that drives expansion of embryonic liver and gut. Genes Dev. 1996;10:70-79. [PubMed] |
| 37. | Sonnenberg E, Gödecke A, Walter B, Bladt F, Birchmeier C. Transient and locally restricted expression of the ros1 protooncogene during mouse development. EMBO J. 1991;10:3693-3702. [PubMed] |
| 38. | Partanen J, Mäkelä TP, Eerola E, Korhonen J, Hirvonen H, Claesson-Welsh L, Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991;10:1347-1354. [PubMed] |
| 39. | Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Dev Biol. 1995;172:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1101] [Cited by in RCA: 1045] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 40. | Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163-3174. [PubMed] |
| 41. | Basson MD, Turowski G, Emenaker NJ. Regulation of human (Caco-2) intestinal epithelial cell differentiation by extracellular matrix proteins. Exp Cell Res. 1996;225:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res. 1992;202:1-8. [PubMed] |
| 43. | Basson MD, Modlin IM, Madri JA. Human enterocyte (Caco-2) migration is modulated in vitro by extracellular matrix composition and epidermal growth factor. J Clin Invest. 1992;90:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Zhang J, Li W, Sanders MA, Sumpio BE, Panja A, Basson MD. Regulation of the intestinal epithelial response to cyclic strain by extracellular matrix proteins. FASEB J. 2003;17:926-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Sanderson IR, Ezzell RM, Kedinger M, Erlanger M, Xu ZX, Pringault E, Leon-Robine S, Louvard D, Walker WA. Human fetal enterocytes in vitro: modulation of the phenotype by extracellular matrix. Proc Natl Acad Sci USA. 1996;93:7717-7722. [PubMed] |
| 46. | Lacroix B, Kedinger M, Simon-Assmann P, Haffen K. Early organogenesis of human small intestine: scanning electron microscopy and brush border enzymology. Gut. 1984;25:925-930. [PubMed] |
| 47. | Antonowicz I, Lebenthal E. Developmental pattern of small intestinal enterokinase and disaccharidase activities in the human fetus. Gastroenterology. 1977;72:1299-1303. [PubMed] |
| 48. | Auricchio S, Stellato A, De Vizia B. Development of brush border peptidases in human and rat small intestine during fetal and neonatal life. Pediatr Res. 1981;15:991-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Wang Y, Harvey C, Rousset M, Swallow DM. Expression of human intestinal mRNA transcripts during development: analysis by a semiquantitative RNA polymerase chain reaction method. Pediatr Res. 1994;36:514-521. [PubMed] |
| 50. | Triadou N, Zweibaum A. Maturation of sucrase-isomaltase complex in human fetal small and large intestine during gestation. Pediatr Res. 1985;19:136-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Auricchio S. "Fetal" forms of brush border enzymes in the intestine and meconium. J Pediatr Gastroenterol Nutr. 1983;2 Suppl 1:S164-S171. [PubMed] |
| 52. | Auricchio S, Caporale C, Santamaria F, Skovbjerg H. Fetal forms of oligoaminopeptidase, dipeptidylaminopeptidase IV, and sucrase in human intestine and meconium. J Pediatr Gastroenterol Nutr. 1984;3:28-36. [PubMed] |
| 53. | Malo C, Berteloot A. Proximo-distal gradient of Na+-dependent D-glucose transport activity in the brush border membrane vesicles from the human fetal small intestine. FEBS Lett. 1987;220:201-205. [PubMed] |
| 54. | Mahraoui L, Rousset M, Dussaulx E, Darmoul D, Zweibaum A, Brot-Laroche E. Expression and localization of GLUT-5 in Caco-2 cells, human small intestine, and colon. Am J Physiol. 1992;263:G312-G318. [PubMed] |
| 55. | Levy E, Thibault L, Ménard D. Intestinal lipids and lipoproteins in the human fetus: modulation by epidermal growth factor. J Lipid Res. 1992;33:1607-1617. [PubMed] |
| 56. | Thibault L, Ménard D, Loirdighi N, Levy E. Ontogeny of intestinal lipid and lipoprotein synthesis. Biol Neonate. 1992;62:100-107. [PubMed] |
| 57. | Lebenthal A, Lebenthal E. The ontogeny of the small intestinal epithelium. JPEN J Parenter Enteral Nutr. 1999;23:S3-S6. [PubMed] |
| 58. | Brandtzaeg P, Nilssen DE, Rognum TO, Thrane PS. Ontogeny of the mucosal immune system and IgA deficiency. Gastroenterol Clin North Am. 1991;20:397-439. [PubMed] |
| 59. | Orlic D, Lev R. An electron microscopic study of intraepithelial lymphocytes in human fetal small intestine. Lab Invest. 1977;37:554-561. [PubMed] |
| 60. | Spencer J, Dillon SB, Isaacson PG, MacDonald TT. T cell subclasses in fetal human ileum. Clin Exp Immunol. 1986;65:553-558. [PubMed] |
| 61. | Husband AJ, Gleeson M. Developmental aspects of gut associated immunity: a comparative review. Boca Raton: CRC 1990; 83-116. |
| 62. | Perkkiö M, Savilahti E. Time of appearance of immunoglobulin-containing cells in the mucosa of the neonatal intestine. Pediatr Res. 1980;14:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 84] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Klockars M, Reitamo S, Adinolfi M. Ontogeny of human lysozyme. Distribution in fetal tissues. Biol Neonate. 1977;32:243-249. [PubMed] |
| 64. | Mallow EB, Harris A, Salzman N, Russell JP, DeBerardinis RJ, Ruchelli E, Bevins CL. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996;271:4038-4045. [PubMed] |
| 65. | Chambers JA, Hollingsworth MA, Trezise AE, Harris A. Developmental expression of mucin genes MUC1 and MUC2. J Cell Sci. 1994;107:413-424. [PubMed] |
| 66. | Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134:1467-1474. [PubMed] |
| 67. | Song J, Wolf SE, Wu XW, Finnerty CC, Gauglitz GG, Herndon DN, Jeschke MG. Starvation-induced proximal gut mucosal atrophy diminished with aging. JPEN J Parenter Enteral Nutr. 2009;33:411-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 68. | Chappell VL, Thompson MD, Jeschke MG, Chung DH, Thompson JC, Wolf SE. Effects of incremental starvation on gut mucosa. Dig Dis Sci. 2003;48:765-769. [PubMed] |
| 69. | Roos KA, Meurling S, Sandberg G. Growth and nitrogen utilization in rats on continuous and intermittent parenteral nutrition with and without fat (Intralipid 20%). Acta Chir Scand. 1981;147:459-464. [PubMed] |
| 70. | Feng Y, Teitelbaum DH. Epidermal growth factor/TNF-α transactivation modulates epithelial cell proliferation and apoptosis in a mouse model of parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2012;302:G236-G249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 71. | Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995;39:44-51; discussion 51-2. [PubMed] |
| 72. | Yang H, Fan Y, Teitelbaum DH. Intraepithelial lymphocyte-derived interferon-gamma evokes enterocyte apoptosis with parenteral nutrition in mice. Am J Physiol Gastrointest Liver Physiol. 2003;284:G629-G637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 73. | Gerritsen A, Besselink MG, Cieslak KP, Vriens MR, Steenhagen E, van Hillegersberg R, Borel Rinkes IH, Molenaar IQ. Efficacy and complications of nasojejunal, jejunostomy and parenteral feeding after pancreaticoduodenectomy. J Gastrointest Surg. 2012;16:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 74. | Kovalenko PL, Basson MD. Changes in morphology and function in small intestinal mucosa after Roux-en-Y surgery in a rat model. J Surg Res. 2012;177:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Basson MD, Li GD, Hong F, Han O, Sumpio BE. Amplitude-dependent modulation of brush border enzymes and proliferation by cyclic strain in human intestinal Caco-2 monolayers. J Cell Physiol. 1996;168:476-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 76. | Basson MD, Coppola CP. Repetitive deformation and pressure activate small bowel and colonic mucosal tyrosine kinase activity in vivo. Metabolism. 2002;51:1525-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Basson MD. Effects of repetitive deformation on intestinal epithelial cells. Inflammopharmacology. 2007;15:109-114. [PubMed] |
| 78. | Johnson CP, Sarna SK, Baytiyeh R, Zhu YR, Cowles VE, Telford GL, Roza AM, Adams MB. Postprandial motor activity and its relationship to transit in the canine ileum. Surgery. 1997;121:182-189. [PubMed] |
| 79. | Womack WA, Barrowman JA, Graham WH, Benoit JN, Kvietys PR, Granger DN. Quantitative assessment of villous motility. Am J Physiol. 1987;252:G250-G256. [PubMed] |
| 80. | Owari M, Wasa M, Oue T, Nose S, Fukuzawa M. Glutamine prevents intestinal mucosal injury induced by cyclophosphamide in rats. Pediatr Surg Int. 2012;28:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 81. | Ito J, Uchida H, Yokote T, Ohtake K, Kobayashi J. Fasting-induced intestinal apoptosis is mediated by inducible nitric oxide synthase and interferon-{gamma} in rat. Am J Physiol Gastrointest Liver Physiol. 2010;298:G916-G926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 668] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 83. | Merritt AJ, Potten CS, Watson AJ, Loh DY, Nakayama K, Nakayama K, Hickman JA. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995;108:2261-2271. [PubMed] |
| 84. | Bernal NP, Stehr W, Coyle R, Erwin CR, Warner BW. Epidermal growth factor receptor signaling regulates Bax and Bcl-w expression and apoptotic responses during intestinal adaptation in mice. Gastroenterology. 2006;130:412-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 85. | Guedon C, Schmitz J, Lerebours E, Metayer J, Audran E, Hemet J, Colin R. Decreased brush border hydrolase activities without gross morphologic changes in human intestinal mucosa after prolonged total parenteral nutrition of adults. Gastroenterology. 1986;90:373-378. [PubMed] |
| 86. | Sukhotnik I, Mogilner JG, Pollak Y, Blumenfeld S, Bejar J, Coran AG. PDGF-α stimulates intestinal epithelial cell turnover after massive small bowel resection in a rat. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1274-G1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 87. | Wang W, Xiao W, Sun L, Zhang C, Chen G, Yang H. Inhibition of ACE activity contributes to the intestinal structural compensation in a massive intestinal resection rat model. Pediatr Surg Int. 2012;28:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I. Dietary L-arginine supplementation reduces Methotrexate-induced intestinal mucosal injury in rat. BMC Gastroenterol. 2012;12:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, Sukhotnik I. Reversal of severe methotrexate-induced intestinal damage using enteral n-3 fatty acids. Br J Nutr. 2012;1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 90. | Warner BW, Erwin CR. Critical roles for EGF receptor signaling during resection-induced intestinal adaptation. J Pediatr Gastroenterol Nutr. 2006;43 Suppl 1:S68-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Fiore NF, Ledniczky G, Liu Q, Orazi A, Du X, Williams DA, Grosfeld JL. Comparison of interleukin-11 and epidermal growth factor on residual small intestine after massive small bowel resection. J Pediatr Surg. 1998;33:24-29. [PubMed] |
| 92. | Lund PK. Molecular basis of intestinal adaptation: the role of the insulin-like growth factor system. Ann N Y Acad Sci. 1998;859:18-36. [PubMed] |
| 93. | Bortvedt SF, Lund PK. Insulin-like growth factor 1: common mediator of multiple enterotrophic hormones and growth factors. Curr Opin Gastroenterol. 2012;28:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 94. | Stern LE, Erwin CR, Falcone RA, Huang FS, Kemp CJ, Williams JL, Warner BW. cDNA microarray analysis of adapting bowel after intestinal resection. J Pediatr Surg. 2001;36:190-195. [PubMed] |
| 95. | Madesh M, Benard O, Balasubramanian KA. Apoptotic process in the monkey small intestinal epithelium: 2. Possible role of oxidative stress. Free Radic Biol Med. 1999;26:431-438. [PubMed] |
| 96. | Baksheev L, Fuller PJ. Gene expression in the adapting small bowel after massive small bowel resection. J Gastroenterol. 2006;41:1041-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 97. | Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391-400. [PubMed] |
| 98. | Moser AR, Dove WF, Roth KA, Gordon JI. The Min (multiple intestinal neoplasia) mutation: its effect on gut epithelial cell differentiation and interaction with a modifier system. J Cell Biol. 1992;116:1517-1526. [PubMed] |
| 99. | Matarese LE, Seidner DL, Steiger E. Growth hormone, glutamine, and modified diet for intestinal adaptation. J Am Diet Assoc. 2004;104:1265-1272. [PubMed] |
| 100. | Drozdowski LA, Clandinin MT, Thomson AB. Morphological, kinetic, membrane biochemical and genetic aspects of intestinal enteroplasticity. World J Gastroenterol. 2009;15:774-787. [PubMed] |
| 101. | McDuffie LA, Bucher BT, Erwin CR, Wakeman D, White FV, Warner BW. Intestinal adaptation after small bowel resection in human infants. J Pediatr Surg. 2011;46:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 102. | Haxhija EQ, Yang H, Spencer AU, Sun X, Teitelbaum DH. Intestinal epithelial cell proliferation is dependent on the site of massive small bowel resection. Pediatr Surg Int. 2007;23:379-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 103. | Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1013-G1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 104. | Madhavan S, Scow JS, Chaudhry RM, Nagao M, Zheng Y, Duenes JA, Sarr MG. Intestinal adaptation for oligopeptide absorption via PepT1 after massive (70%) mid-small bowel resection. J Gastrointest Surg. 2011;15:240-247; discussion 240-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 105. | Iqbal CW, Qandeel HG, Zheng Y, Duenes JA, Sarr MG. Mechanisms of ileal adaptation for glucose absorption after proximal-based small bowel resection. J Gastrointest Surg. 2008;12:1854-164; discussion 1854-164;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | O'Brien DP, Nelson LA, Williams JL, Kemp CJ, Erwin CR, Warner BW. Selective inhibition of the epidermal growth factor receptor impairs intestinal adaptation after small bowel resection. J Surg Res. 2002;105:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 107. | Helmrath MA, Fong JJ, Dekaney CM, Henning SJ. Rapid expansion of intestinal secretory lineages following a massive small bowel resection in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G215-G222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Martin CA, Perrone EE, Longshore SW, Toste P, Bitter K, Nair R, Guo J, Erwin CR, Warner BW. Intestinal resection induces angiogenesis within adapting intestinal villi. J Pediatr Surg. 2009;44:1077-182; discussion 1083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | VanDussen KL, Carulli AJ, Keeley TM, Patel SR, Puthoff BJ, Magness ST, Tran IT, Maillard I, Siebel C, Kolterud Å. Notch signaling modulates proliferation and differentiation of intestinal crypt base columnar stem cells. Development. 2012;139:488-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 110. | Pinto D, Clevers H. Wnt control of stem cells and differentiation in the intestinal epithelium. Exp Cell Res. 2005;306:357-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 199] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 111. | Okamoto R, Tsuchiya K, Nemoto Y, Akiyama J, Nakamura T, Kanai T, Watanabe M. Requirement of Notch activation during regeneration of the intestinal epithelia. Am J Physiol Gastrointest Liver Physiol. 2009;296:G23-G35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 112. | Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 969] [Cited by in RCA: 967] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 113. | Hardwick JC, Van Den Brink GR, Bleuming SA, Ballester I, Van Den Brande JM, Keller JJ, Offerhaus GJ, Van Deventer SJ, Peppelenbosch MP. Bone morphogenetic protein 2 is expressed by, and acts upon, mature epithelial cells in the colon. Gastroenterology. 2004;126:111-121. [PubMed] |
| 114. | Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem. 2002;277:8226-8234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 131] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 115. | Richmond CA, Breault DT. Regulation of gene expression in the intestinal epithelium. Prog Mol Biol Transl Sci. 2010;96:207-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Boudreau F, Rings EH, van Wering HM, Kim RK, Swain GP, Krasinski SD, Moffett J, Grand RJ, Suh ER, Traber PG. Hepatocyte nuclear factor-1 alpha, GATA-4, and caudal related homeodomain protein Cdx2 interact functionally to modulate intestinal gene transcription. Implication for the developmental regulation of the sucrase-isomaltase gene. J Biol Chem. 2002;277:31909-31917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 189] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 117. | Gautier-Stein A, Domon-Dell C, Calon A, Bady I, Freund JN, Mithieux G, Rajas F. Differential regulation of the glucose-6-phosphatase TATA box by intestine-specific homeodomain proteins CDX1 and CDX2. Nucleic Acids Res. 2003;31:5238-5246. [PubMed] |
| 118. | Jedlicka P, Sui X, Sussel L, Gutierrez-Hartmann A. Ets transcription factors control epithelial maturation and transit and crypt-villus morphogenesis in the mammalian intestine. Am J Pathol. 2009;174:1280-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 119. | Beuling E, Bosse T, aan de Kerk DJ, Piaseckyj CM, Fujiwara Y, Katz SG, Orkin SH, Grand RJ, Krasinski SD. GATA4 mediates gene repression in the mature mouse small intestine through interactions with friend of GATA (FOG) cofactors. Dev Biol. 2008;322:179-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 120. | Boyd M, Hansen M, Jensen TG, Perearnau A, Olsen AK, Bram LL, Bak M, Tommerup N, Olsen J, Troelsen JT. Genome-wide analysis of CDX2 binding in intestinal epithelial cells (Caco-2). J Biol Chem. 2010;285:25115-25125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Babeu JP, Darsigny M, Lussier CR, Boudreau F. Hepatocyte nuclear factor 4alpha contributes to an intestinal epithelial phenotype in vitro and plays a partial role in mouse intestinal epithelium differentiation. Am J Physiol Gastrointest Liver Physiol. 2009;297:G124-G134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 122. | Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561-574. [PubMed] |
| 123. | Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575-588. [PubMed] |
| 124. | Dufort D, Schwartz L, Harpal K, Rossant J. The transcription factor HNF3beta is required in visceral endoderm for normal primitive streak morphogenesis. Development. 1998;125:3015-3025. [PubMed] |
| 125. | Mutoh H, Satoh K, Kita H, Sakamoto H, Hayakawa H, Yamamoto H, Isoda N, Tamada K, Ido K, Sugano K. Cdx2 specifies the differentiation of morphological as well as functional absorptive enterocytes of the small intestine. Int J Dev Biol. 2005;49:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Hryniuk A, Grainger S, Savory JG, Lohnes D. Cdx function is required for maintenance of intestinal identity in the adult. Dev Biol. 2012;363:426-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 127. | Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1668] [Cited by in RCA: 1436] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 128. | Cao L, Gibson JD, Miyamoto S, Sail V, Verma R, Rosenberg DW, Nelson CE, Giardina C. Intestinal lineage commitment of embryonic stem cells. Differentiation. 2011;81:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 129. | Tappenden KA. Emerging therapies for intestinal failure. Arch Surg. 2010;145:528-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 130. | Peterson CA, Carey HV, Hinton PL, Lo HC, Ney DM. GH elevates serum IGF-I levels but does not alter mucosal atrophy in parenterally fed rats. Am J Physiol. 1997;272:G1100-G1108. [PubMed] |
| 131. | Kudsk KA, Croce MA, Fabian TC, Minard G, Tolley EA, Poret HA, Kuhl MR, Brown RO. Enteral versus parenteral feeding. Effects on septic morbidity after blunt and penetrating abdominal trauma. Ann Surg. 1992;215:503-11; discussion 511-3. [PubMed] |
| 132. | Hernandez G, Velasco N, Wainstein C, Castillo L, Bugedo G, Maiz A, Lopez F, Guzman S, Vargas C. Gut mucosal atrophy after a short enteral fasting period in critically ill patients. J Crit Care. 1999;14:73-77. [PubMed] |
| 133. | Fukuyama K, Iwakiri R, Noda T, Kojima M, Utsumi H, Tsunada S, Sakata H, Ootani A, Fujimoto K. Apoptosis induced by ischemia-reperfusion and fasting in gastric mucosa compared to small intestinal mucosa in rats. Dig Dis Sci. 2001;46:545-549. [PubMed] |
| 134. | Sarac TP, Souba WW, Miller JH, Ryan CK, Koch M, Bessey PQ, Sax HC. Starvation induces differential small bowel luminal amino acid transport. Surgery. 1994;116:679-85; discussion 685-6. [PubMed] |
| 135. | Deitch EA, Winterton J, Li M, Berg R. The gut as a portal of entry for bacteremia. Role of protein malnutrition. Ann Surg. 1987;205:681-692. [PubMed] |
| 136. | Warner BW, Ziegler MM. Management of the short bowel syndrome in the pediatric population. Pediatr Clin North Am. 1993;40:1335-1350. [PubMed] |
| 137. | Collins JB, Georgeson KE, Vicente Y, Kelly DR, Figueroa R. Short bowel syndrome. Semin Pediatr Surg. 1995;4:60-72; discussion 72-3. [PubMed] |
| 138. | Messing B, Crenn P, Beau P, Boutron-Ruault MC, Rambaud JC, Matuchansky C. Long-term survival and parenteral nutrition dependence in adult patients with the short bowel syndrome. Gastroenterology. 1999;117:1043-1050. [PubMed] |
| 139. | Spencer AU, Neaga A, West B, Safran J, Brown P, Btaiche I, Kuzma-O'Reilly B, Teitelbaum DH. Pediatric short bowel syndrome: redefining predictors of success. Ann Surg. 2005;242:403-49; discussion 403-49;. [PubMed] |
| 140. | Jeppesen PB, Mortensen PB. The influence of a preserved colon on the absorption of medium chain fat in patients with small bowel resection. Gut. 1998;43:478-483. [PubMed] |
| 141. | Sax HC. Management of Short Bowel Syndrome. Current Surgical Therapy. 10th ed. Philadelphia: Elsevier 2010; . |
| 142. | Mazaki T, Ebisawa K. Enteral versus parenteral nutrition after gastrointestinal surgery: a systematic review and meta-analysis of randomized controlled trials in the English literature. J Gastrointest Surg. 2008;12:739-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 143. | Jeejeebhoy KN. Management of short bowel syndrome: avoidance of total parenteral nutrition. Gastroenterology. 2006;130:S60-S66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Modi BP, Langer M, Ching YA, Valim C, Waterford SD, Iglesias J, Duro D, Lo C, Jaksic T, Duggan C. Improved survival in a multidisciplinary short bowel syndrome program. J Pediatr Surg. 2008;43:20-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 146. | Dodge ME, Bertolo RF, Brunton JA. Enteral feeding induces early intestinal adaptation in a parenterally fed neonatal piglet model of short bowel syndrome. JPEN J Parenter Enteral Nutr. 2012;36:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 147. | Perdikis DA, Basson MD. Basal nutrition promotes human intestinal epithelial (Caco-2) proliferation, brush border enzyme activity, and motility. Crit Care Med. 1997;25:159-165. [PubMed] |
| 148. | Poirier H, Niot I, Degrace P, Monnot MC, Bernard A, Besnard P. Fatty acid regulation of fatty acid-binding protein expression in the small intestine. Am J Physiol. 1997;273:G289-G295. [PubMed] |
| 149. | Kollman-Bauerly KA, Thomas DL, Adrian TE, Lien EL, Vanderhoof JA. The role of eicosanoids in the process of adaptation following massive bowel resection in the rat. JPEN J Parenter Enteral Nutr. 2001;25:275-281. [PubMed] |
| 150. | Sukhotnik I, Mor-Vaknin N, Drongowski RA, Miselevich I, Coran AG, Harmon CM. Effect of dietary fat on early morphological intestinal adaptation in a rat with short bowel syndrome. Pediatr Surg Int. 2004;20:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 151. | Sukhotnik I, Shany A, Bashenko Y, Hayari L, Chemodanov E, Mogilner J, Coran AG, Shaoul R. Parenteral but not enteral omega-3 fatty acids (Omegaven) modulate intestinal regrowth after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2010;34:503-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 152. | Yang Q, Lan T, Chen Y, Dawson PA. Dietary fish oil increases fat absorption and fecal bile acid content without altering bile acid synthesis in 20-d-old weanling rats following massive ileocecal resection. Pediatr Res. 2012;72:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Jump DB, Clarke SD. Regulation of gene expression by dietary fat. Annu Rev Nutr. 1999;19:63-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 434] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 154. | Park EJ, Suh M, Ramanujam K, Steiner K, Begg D, Clandinin MT. Diet-induced changes in membrane gangliosides in rat intestinal mucosa, plasma and brain. J Pediatr Gastroenterol Nutr. 2005;40:487-495. [PubMed] |
| 155. | Yaqoob P, Shaikh SR. The nutritional and clinical significance of lipid rafts. Curr Opin Clin Nutr Metab Care. 2010;13:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 156. | Hernández Vallejo SJ, Alqub M, Luquet S, Cruciani-Guglielmacci C, Delerive P, Lobaccaro JM, Kalopissis AD, Chambaz J, Rousset M, Lacorte JM. Short-term adaptation of postprandial lipoprotein secretion and intestinal gene expression to a high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2009;296:G782-G792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 157. | Kessel A, Toubi E, Pavlotzky E, Mogilner J, Coran AG, Lurie M, Karry R, Sukhotnik I. Treatment with glutamine is associated with down-regulation of Toll-like receptor-4 and myeloid differentiation factor 88 expression and decrease in intestinal mucosal injury caused by lipopolysaccharide endotoxaemia in a rat. Clin Exp Immunol. 2008;151:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 158. | Koruda MJ, Rolandelli RH, Settle RG, Zimmaro DM, Rombeau JL. Effect of parenteral nutrition supplemented with short-chain fatty acids on adaptation to massive small bowel resection. Gastroenterology. 1988;95:715-720. [PubMed] |
| 159. | Murakoshi S, Fukatsu K, Omata J, Moriya T, Noguchi M, Saitoh D, Koyama I. Effects of adding butyric acid to PN on gut-associated lymphoid tissue and mucosal immunoglobulin A levels. JPEN J Parenter Enteral Nutr. 2011;35:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 160. | Hartmann B, Thulesen J, Hare KJ, Kissow H, Orskov C, Poulsen SS, Holst JJ. Immunoneutralization of endogenous glucagon-like peptide-2 reduces adaptive intestinal growth in diabetic rats. Regul Pept. 2002;105:173-179. [PubMed] |
| 161. | Bartholome AL, Albin DM, Baker DH, Holst JJ, Tappenden KA. Supplementation of total parenteral nutrition with butyrate acutely increases structural aspects of intestinal adaptation after an 80% jejunoileal resection in neonatal piglets. JPEN J Parenter Enteral Nutr. 2004;28:210-222; discussion 222-223. [PubMed] |
| 162. | Basson MD, Turowski GA, Rashid Z, Hong F, Madri JA. Regulation of human colonic cell line proliferation and phenotype by sodium butyrate. Dig Dis Sci. 1996;41:1989-1993. [PubMed] |
| 163. | Barnes JL, Hartmann B, Holst JJ, Tappenden KA. Intestinal adaptation is stimulated by partial enteral nutrition supplemented with the prebiotic short-chain fructooligosaccharide in a neonatal intestinal failure piglet model. JPEN J Parenter Enteral Nutr. 2012;36:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 164. | Diamond JM, Karasov WH, Cary C, Enders D, Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984;349:419-440. [PubMed] |
| 165. | Zhou X, Li YX, Li N, Li JS. Effect of bowel rehabilitative therapy on structural adaptation of remnant small intestine: animal experiment. World J Gastroenterol. 2001;7:66-73. [PubMed] |
| 166. | Scharrer E. Adaptation of intestinal amino acid transport. Experientia. 1972;28:267. [PubMed] |
| 167. | Ziegler TR, Mantell MP, Chow JC, Rombeau JL, Smith RJ. Gut adaptation and the insulin-like growth factor system: regulation by glutamine and IGF-I administration. Am J Physiol. 1996;271:G866-G875. [PubMed] |
| 168. | Lai YN, Yeh SL, Lin MT, Shang HF, Yeh CL, Chen WJ. Glutamine supplementation enhances mucosal immunity in rats with Gut-Derived sepsis. Nutrition. 2004;20:286-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 169. | Alavi K, Kato Y, Yu D, Schwartz MZ. Enteral glutamine does not enhance the effects of hepatocyte growth factor in short bowel syndrome. J Pediatr Surg. 1998;33:1666-1669. [PubMed] |
| 170. | Guo M, Li Y, Li J. Role of growth hormone, glutamine and enteral nutrition in pediatric short bowel syndrome: a pilot follow-up study. Eur J Pediatr Surg. 2012;22:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 171. | van den Berg A, van Elburg RM, Teerlink T, Lafeber HN, Twisk JW, Fetter WP. A randomized controlled trial of enteral glutamine supplementation in very low birth weight infants: plasma amino acid concentrations. J Pediatr Gastroenterol Nutr. 2005;41:66-71. [PubMed] |
| 172. | Szkudlarek J, Jeppesen PB, Mortensen PB. Effect of high dose growth hormone with glutamine and no change in diet on intestinal absorption in short bowel patients: a randomised, double blind, crossover, placebo controlled study. Gut. 2000;47:199-205. [PubMed] |
| 173. | Byrne TA, Wilmore DW, Iyer K, Dibaise J, Clancy K, Robinson MK, Chang P, Gertner JM, Lautz D. Growth hormone, glutamine, and an optimal diet reduces parenteral nutrition in patients with short bowel syndrome: a prospective, randomized, placebo-controlled, double-blind clinical trial. Ann Surg. 2005;242:655-661. [PubMed] |
| 174. | Wang L, Tang Y, Rubin DC, Levin MS. Chronically administered retinoic acid has trophic effects in the rat small intestine and promotes adaptation in a resection model of short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1559-G1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 175. | Swartz-Basile DA, Wang L, Tang Y, Pitt HA, Rubin DC, Levin MS. Vitamin A deficiency inhibits intestinal adaptation by modulating apoptosis, proliferation, and enterocyte migration. Am J Physiol Gastrointest Liver Physiol. 2003;285:G424-G432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 176. | Dumas F, De Bandt JP, Colomb V, Le Boucher J, Coudray-Lucas C, Lavie S, Brousse N, Ricour C, Cynober L, Goulet O. Enteral ornithine alpha-ketoglutarate enhances intestinal adaptation to massive resection in rats. Metabolism. 1998;47:1366-1371. [PubMed] |
| 177. | Tappenden KA, Thomson AB, Wild GE, McBurney MI. Short-chain fatty acids increase proglucagon and ornithine decarboxylase messenger RNAs after intestinal resection in rats. JPEN J Parenter Enteral Nutr. 1996;20:357-362. [PubMed] |
| 178. | Castiglione F, Rispo A, Di Girolamo E, Cozzolino A, Manguso F, Grassia R, Mazzacca G. Antibiotic treatment of small bowel bacterial overgrowth in patients with Crohn's disease. Aliment Pharmacol Ther. 2003;18:1107-1112. [PubMed] |
| 179. | Cole CR, Ziegler TR. Small bowel bacterial overgrowth: a negative factor in gut adaptation in pediatric SBS. Curr Gastroenterol Rep. 2007;9:456-462. [PubMed] |
| 180. | Lee CH, Lo HC, Chou MC, Tsai HR. Oral antibiotics attenuate bowel segment reversal-induced systemic inflammatory response and body weight loss in massively bowel-resected rats. JPEN J Parenter Enteral Nutr. 2007;31:397-405. [PubMed] |
| 181. | Laron Z. Growth hormone therapy: emerging dilemmas. Pediatr Endocrinol Rev. 2011;8:364-373. [PubMed] |
| 182. | Nucci AM, Finegold DN, Yaworski JA, Kowalski L, Barksdale EM. Results of growth trophic therapy in children with short bowel syndrome. J Pediatr Surg. 2004;39:335-39; discussion 335-39;. [PubMed] |
| 183. | Gu Y, Wu ZH, Xie JX, Jin DY, Zhuo HC. Effects of growth hormone (rhGH) and glutamine supplemented parenteral nutrition on intestinal adaptation in short bowel rats. Clin Nutr. 2001;20:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 184. | Cukier C, Waitzberg DL, Borges VC, Silva Mde L, Gama-Rodrigues J, Pinotti HW. Clinical use of growth hormone and glutamine in short bowel syndrome. Rev Hosp Clin Fac Med Sao Paulo. 1999;54:29-34. [PubMed] |
| 185. | Fadrique B, Lopez JM, Bermudez R, Gomez de Segura IA, Vazquez I, De Miguel E. Growth hormone plus high protein diet promotes adaptation after massive bowel resection in aged rats. Exp Gerontol. 2001;36:1727-1737. [PubMed] |
| 186. | Byrne TA, Persinger RL, Young LS, Ziegler TR, Wilmore DW. A new treatment for patients with short-bowel syndrome. Growth hormone, glutamine, and a modified diet. Ann Surg. 1995;222:243-54; discussion 254-5. [PubMed] |
| 187. | Goulet O, Dabbas-Tyan M, Talbotec C, Kapel N, Rosilio M, Souberbielle JC, Corriol O, Ricour C, Colomb V. Effect of recombinant human growth hormone on intestinal absorption and body composition in children with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2010;34:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 188. | Scolapio JS. Effect of growth hormone, glutamine, and diet on body composition in short bowel syndrome: a randomized, controlled study. JPEN J Parenter Enteral Nutr. 1999;23:309-312; discussion 312-313. [PubMed] |
| 189. | Ellegård L, Bosaeus I, Nordgren S, Bengtsson BA. Low-dose recombinant human growth hormone increases body weight and lean body mass in patients with short bowel syndrome. Ann Surg. 1997;225:88-96. [PubMed] |
| 190. | Seguy D, Vahedi K, Kapel N, Souberbielle JC, Messing B. Low-dose growth hormone in adult home parenteral nutrition-dependent short bowel syndrome patients: a positive study. Gastroenterology. 2003;124:293-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 191. | Wales PW, Nasr A, de Silva N, Yamada J. Human growth hormone and glutamine for patients with short bowel syndrome. Cochrane Database Syst Rev. 2010;CD006321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 192. | van Goudoever JB, Stoll B, Hartmann B, Holst JJ, Reeds PJ, Burrin DG. Secretion of trophic gut peptides is not different in bolus- and continuously fed piglets. J Nutr. 2001;131:729-732. [PubMed] |
| 193. | Martin GR, Wallace LE, Sigalet DL. Glucagon-like peptide-2 induces intestinal adaptation in parenterally fed rats with short bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2004;286:G964-G972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 194. | Orskov C, Holst JJ, Knuhtsen S, Baldissera FG, Poulsen SS, Nielsen OV. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986;119:1467-1475. [PubMed] |
| 195. | Thulesen J, Hartmann B, Orskov C, Jeppesen PB, Holst JJ, Poulsen SS. Potential targets for glucagon-like peptide 2 (GLP-2) in the rat: distribution and binding of i.v. injected (125)I-GLP-2. Peptides. 2000;21:1511-1517. [PubMed] |
| 196. | Sigalet DL, Bawazir O, Martin GR, Wallace LE, Zaharko G, Miller A, Zubaidi A. Glucagon-like peptide-2 induces a specific pattern of adaptation in remnant jejunum. Dig Dis Sci. 2006;51:1557-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 197. | Jeppesen PB, Sanguinetti EL, Buchman A, Howard L, Scolapio JS, Ziegler TR, Gregory J, Tappenden KA, Holst J, Mortensen PB. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 343] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 198. | Jeppesen PB, Lund P, Gottschalck IB, Nielsen HB, Holst JJ, Mortensen J, Poulsen SS, Quistorff B, Mortensen PB. Short bowel patients treated for two years with glucagon-like peptide 2 (GLP-2): compliance, safety, and effects on quality of life. Gastroenterol Res Pract. 2009;2009:425759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 199. | Jeppesen PB, Gilroy R, Pertkiewicz M, Allard JP, Messing B, O'Keefe SJ. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902-914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 304] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 200. | Dahly EM, Guo Z, Ney DM. IGF-I augments resection-induced mucosal hyperplasia by altering enterocyte kinetics. Am J Physiol Regul Integr Comp Physiol. 2003;285:R800-R808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 201. | McMellen ME, Wakeman D, Longshore SW, McDuffie LA, Warner BW. Growth factors: possible roles for clinical management of the short bowel syndrome. Semin Pediatr Surg. 2010;19:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 202. | Ørskov C, Hartmann B, Poulsen SS, Thulesen J, Hare KJ, Holst JJ. GLP-2 stimulates colonic growth via KGF, released by subepithelial myofibroblasts with GLP-2 receptors. Regul Pept. 2005;124:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 166] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 203. | Vanderhoof JA, McCusker RH, Clark R, Mohammadpour H, Blackwood DJ, Harty RF, Park JH. Truncated and native insulinlike growth factor I enhance mucosal adaptation after jejunoileal resection. Gastroenterology. 1992;102:1949-1956. [PubMed] |
| 204. | Wang J, Niu W, Nikiforov Y, Naito S, Chernausek S, Witte D, LeRoith D, Strauch A, Fagin JA. Targeted overexpression of IGF-I evokes distinct patterns of organ remodeling in smooth muscle cell tissue beds of transgenic mice. J Clin Invest. 1997;100:1425-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 155] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 205. | Ney DM. Effects of insulin-like growth factor-I and growth hormone in models of parenteral nutrition. JPEN J Parenter Enteral Nutr. 1999;23:S184-S189. [PubMed] |
| 206. | Knott AW, Juno RJ, Jarboe MD, Profitt SA, Erwin CR, Smith EP, Fagin JA, Warner BW. Smooth muscle overexpression of IGF-I induces a novel adaptive response to small bowel resection. Am J Physiol Gastrointest Liver Physiol. 2004;287:G562-G570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 207. | Barnard JA, Beauchamp RD, Russell WE, Dubois RN, Coffey RJ. Epidermal growth factor-related peptides and their relevance to gastrointestinal pathophysiology. Gastroenterology. 1995;108:564-580. [PubMed] |
| 208. | Thompson JS. Epidermal growth factor and the short bowel syndrome. JPEN J Parenter Enteral Nutr. 1999;23:S113-S116. [PubMed] |
| 209. | Helmrath MA, Shin CE, Fox JW, Erwin CR, Warner BW. Adaptation after small bowel resection is attenuated by sialoadenectomy: the role for endogenous epidermal growth factor. Surgery. 1998;124:848-854. [PubMed] |
| 210. | Shin CE, Helmrath MA, Falcone RA, Fox JW, Duane KR, Erwin CR, Warner BW. Epidermal growth factor augments adaptation following small bowel resection: optimal dosage, route, and timing of administration. J Surg Res. 1998;77:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 211. | Kuwada SK, Li XF, Damstrup L, Dempsey PJ, Coffey RJ, Wiley HS. The dynamic expression of the epidermal growth factor receptor and epidermal growth factor ligand family in a differentiating intestinal epithelial cell line. Growth Factors. 1999;17:139-153. [PubMed] |
| 212. | Pearson PY, O'Connor DM, Schwartz MZ. Novel effect of leptin on small intestine adaptation. J Surg Res. 2001;97:192-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 213. | Sukhotnik I, Vadasz Z, Coran AG, Lurie M, Shiloni E, Hatoum OA, Mogilner JG. Effect of leptin on intestinal re-growth following massive small bowel resection in rat. Pediatr Surg Int. 2006;22:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 214. | Sukhotnik I, Slijper N, Karry R, Shaoul R, Coran AG, Lurie M, Shiloni E, Mogilner JG. Bombesin stimulates enterocyte turnover following massive small bowel resection in a rat. Pediatr Surg Int. 2007;23:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 215. | Krsek M, Rosická M, Haluzík M, Svobodová J, Kotrlíková E, Justová V, Lacinová Z, Jarkovská Z. Plasma ghrelin levels in patients with short bowel syndrome. Endocr Res. 2002;28:27-33. [PubMed] |
| 216. | Compher CW, Kinosian BP, Metz DC. Ghrelin does not predict adaptive hyperphagia in patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2009;33:428-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 217. | Park JH, McCusker RH, Mohammadpour H, Blackwood DJ, Hrbek M, Vanderhoof JA. Dexamethasone inhibits mucosal adaptation after small bowel resection. Am J Physiol. 1994;266:G497-G503. [PubMed] |
| 218. | Thiesen A, Wild GE, Keelan M, Clandinin MT, Thomson AB. Locally and systemically active glucocorticosteroids modify intestinal absorption of sugars in rats. J Appl Physiol. 2003;94:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 219. | Iannoli P, Miller JH, Ryan CK, Gu LH, Ziegler TR, Sax HC. Epidermal growth factor and human growth hormone accelerate adaptation after massive enterectomy in an additive, nutrient-dependent, and site-specific fashion. Surgery. 1997;122:721-78; discussion 721-78;. [PubMed] |
| 220. | Bianchi A. Intestinal lengthening: an experimental and clinical review. J R Soc Med. 1984;77 Suppl 3:35-41. [PubMed] |
| 221. | Walker SR, Nucci A, Yaworski JA, Barksdale EM. The Bianchi procedure: a 20-year single institution experience. J Pediatr Surg. 2006;41:113-19; discussion 113-19;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 222. | Sudan D, Thompson J, Botha J, Grant W, Antonson D, Raynor S, Langnas A. Comparison of intestinal lengthening procedures for patients with short bowel syndrome. Ann Surg. 2007;246:593-601; discussion 601-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 223. | Safford SD, Freemerman AJ, Safford KM, Bentley R, Skinner MA. Longitudinal mechanical tension induces growth in the small bowel of juvenile rats. Gut. 2005;54:1085-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 224. | Walsh MF, Woo RK, Gomez R, Basson MD. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif. 2004;37:427-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 225. | Chaturvedi LS, Gayer CP, Marsh HM, Basson MD. Repetitive deformation activates Src-independent FAK-dependent ERK motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2008;294:C1350-C1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 226. | Fishbein TM. Intestinal transplantation. N Engl J Med. 2009;361:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 227. | Hanto DW, Fishbein TM, Pinson CW, Olthoff KM, Shiffman ML, Punch JD, Goodrich NP. Liver and intestine transplantation: summary analysis, 1994-2003. Am J Transplant. 2005;5:916-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 228. | Lew EA, Garfinkel L. Variations in mortality by weight among 750,000 men and women. J Chronic Dis. 1979;32:563-576. [PubMed] |
| 229. | Padwal R, Li SK, Lau DC. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst Rev. 2004;CD004094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 102] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 230. | Iversen BM, Schjonsby H, Skagen DW, Solhaug JH. Intestinal adaptation after jejuno-ileal bypass operation for massive obesity. Eur J Clin Invest. 1976;6:355-360. [PubMed] |
| 231. | Asp NG, Gudmand-Höyer E, Andersen B, Berg NO, Dahlqvist A. Distribution of disaccharidases, alkaline phosphatase, and some intracellular enzymes along the human small intestine. Scand J Gastroenterol. 1975;10:647-651. [PubMed] |
| 232. | Dudrick SJ, Daly JM, Castro G, Akhtar M. Gastrointestinal adaptation following small bowel bypass for obesity. Ann Surg. 1977;185:642-648. [PubMed] |
| 233. | Beckman LM, Beckman TR, Sibley SD, Thomas W, Ikramuddin S, Kellogg TA, Ghatei MA, Bloom SR, le Roux CW, Earthman CP. Changes in gastrointestinal hormones and leptin after Roux-en-Y gastric bypass surgery. JPEN J Parenter Enteral Nutr. 2011;35:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 234. | Pournaras DJ, Osborne A, Hawkins SC, Mahon D, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. The gut hormone response following Roux-en-Y gastric bypass: cross-sectional and prospective study. Obes Surg. 2010;20:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 235. | Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297:G950-G957. [PubMed] |
| 236. | Taqi E, Wallace LE, de Heuvel E, Chelikani PK, Zheng H, Berthoud HR, Holst JJ, Sigalet DL. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 237. | Murnin M, Kumar A, Li GD, Brown M, Sumpio BE, Basson MD. Effects of glutamine isomers on human (Caco-2) intestinal epithelial proliferation, strain-responsiveness, and differentiation. J Gastrointest Surg. 2000;4:435-442. [PubMed] |
| 238. | Chaturvedi LS, Marsh HM, Shang X, Zheng Y, Basson MD. Repetitive deformation activates focal adhesion kinase and ERK mitogenic signals in human Caco-2 intestinal epithelial cells through Src and Rac1. J Biol Chem. 2007;282:14-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 239. | Flanigan TL, Craig DH, Gayer CP, Basson MD. The effects of increased extracellular deformation, pressure, and integrin phosphorylation on fibroblast migration. J Surg Res. 2009;156:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 240. | Yuan L, Yu Y, Sanders MA, Majumdar AP, Basson MD. Schlafen 3 induction by cyclic strain regulates intestinal epithelial differentiation. Am J Physiol Gastrointest Liver Physiol. 2010;298:G994-G1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 241. | Han O, Li GD, Sumpio BE, Basson MD. Strain induces Caco-2 intestinal epithelial proliferation and differentiation via PKC and tyrosine kinase signals. Am J Physiol. 1998;275:G534-G541. [PubMed] |
| 242. | Gayer CP, Craig DH, Flanigan TL, Reed TD, Cress DE, Basson MD. ERK regulates strain-induced migration and proliferation from different subcellular locations. J Cell Biochem. 2010;109:711-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 243. | Yuan L, Sanders MA, Basson MD. ILK mediates the effects of strain on intestinal epithelial wound closure. Am J Physiol Cell Physiol. 2011;300:C356-C367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 244. | Chaturvedi LS, Marsh HM, Basson MD. Role of RhoA and its effectors ROCK and mDia1 in the modulation of deformation-induced FAK, ERK, p38, and MLC motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C1224-C1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 245. | Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a new family of growth regulatory genes that affect thymocyte development. Immunity. 1998;9:657-668. [PubMed] |
| 246. | Patel VB, Yu Y, Das JK, Patel BB, Majumdar AP. Schlafen-3: a novel regulator of intestinal differentiation. Biochem Biophys Res Commun. 2009;388:752-756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 247. | Walsh MF, Hermann R, Sun K, Basson MD. Schlafen 3 changes during rat intestinal maturation. Am J Surg. 2012;204:598-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 248. | Pereira-Fantini PM, Thomas SL, Wilson G, Taylor RG, Sourial M, Bines JE. Short- and long-term effects of small bowel resection: a unique histological study in a piglet model of short bowel syndrome. Histochem Cell Biol. 2011;135:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 249. | Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155-2158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 718] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 250. | Durand A, Donahue B, Peignon G, Letourneur F, Cagnard N, Slomianny C, Perret C, Shroyer NF, Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1). Proc Natl Acad Sci USA. 2012;109:8965-8970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 251. | Schonhoff SE, Giel-Moloney M, Leiter AB. Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev Biol. 2004;270:443-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 252. | Lardy H, Mouillé B, Thomas M, Darcy-Vrillon B, Vaugelade P, Blachier F, Bernard F, Cherbuy C, Robert V, Corriol O. Enterocyte metabolism during early adaptation after extensive intestinal resection in a rat model. Surgery. 2004;135:649-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |