Published online Nov 21, 2012. doi: 10.3748/wjg.v18.i43.6183
Revised: September 25, 2012
Accepted: September 28, 2012
Published online: November 21, 2012
Processing time: 133 Days and 14.5 Hours
Hepatitis C virus (HCV) infects approximately 200 million people worldwide. Interferon-based therapies have dominated over the past two decades. However, the overall response rates remain suboptimal. Thanks to the tremendous effort from both academia and industry, two serine protease inhibitors telaprevir and boceprevir for treating chronic hepatitis C have finally reached the clinic. Although these compounds are only approved for combination use with interferon and ribavirin in genotype 1 HCV infected chronic patients, the management of HCV patients however is now evolving incredibly. Here, we overviewed a series of landmark studies, regarding the clinical development of telaprevir and boceprevir. We discussed the mechanism-of-action of telaprevir/boceprevir and their potential application in HCV-positive liver transplantation patients. We further emphasized some emerging concerns with perspective of further development in this field.
- Citation: Pan Q, Peppelenbosch MP, Janssen HL, Knegt RJ. Telaprevir/boceprevir era: From bench to bed and back. World J Gastroenterol 2012; 18(43): 6183-6188
- URL: https://www.wjgnet.com/1007-9327/full/v18/i43/6183.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i43.6183
Since the discovery of hepatitis C virus (HCV) in 1989[1], the lack of good model systems has hampered the research at the early stages. Even though still little is known about the virus, interferon was found to be effective for treating chronic HCV and the first interferon-α (IFN-α) is approved by the United States Food and Drug Administration (FDA) to treat HCV in 1991[2]. Interferons have a vital role for immune modulation of the host cells and involve the induction of a multitude of interferon-stimulated genes to establish an antiviral status[3]. Through a series of optimizations, the current pegylated interferon (peg-IFN) in combination with ribavirin has dramatically improved the outcome, but still only approximately 50% of the patients can develop a sustained virologic response (SVR)[4,5].
Thanks to the development of various cell culture models and the important evolvement of biotechnology[6], research in the HCV field has been flourishing both in academy and industry, over the past decade. This progress has enabled further improvement on the current interferon-based standard antiviral therapy and the development of novel antivirals with distinct mechanism of action. A range of directly acting antivirals agents (DAAs), including protease and polymerase inhibitors, have been in various stages of clinical development[7]. The serine protease inhibitors telaprevir and boceprevir are the most advanced in clinic. Both had been approved by FDA in May 2011 for treating chronic genotype 1 HCV infection and later were also approved by the European Medicines Agency[7].
The HCV genome composes of a single open reading frame, encoding a polyprotein precursor of approximately 3000 amino acids, flanked by 5’ and 3’ non-coding region (NCR). Translation of the viral polyprotein is mediated by an internal ribosome entry site located within the highly conserved 5’ NCR. The synthesized polyprotein is subsequently cleaved into four structural (core, E1, E2 and p7) and six non-structural (NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins[8]. Processing of structural proteins, including the p7/NS2 junction, is carried out by at least two host signal peptidases and non-structural proteins and is matured by two viral proteases, NS2 and NS3/4A[8,9].
The NS3/4A serine protease is a non-covalent, heterodimer complex formed by the catalytic subunit of the N-terminal serine protease domain of NS3 and the activation subunit of the NS4A cofactor. Unique to the NS3 protease is an extended polydentate substrate binding cleft, which ensures substrate specificity[10]. It is responsible for the proteolytic cleavage of NS3/NS4A self cleavage, NS4A/NS4B, NS4B/NS5A and NS5A/NS5B. The multi-functional property of NS3/4A protease has attracted for targeted drug development. Current protease inhibitors can be divided into covalent and non-covalent peptidomimetic inhibitors. Telaprevir and boceprevir are covalent linear inhibitors, discovered by using structure-based drug design approach[11,12]. Both act via formation of a reversible covalent interaction with serine-139. The assailment of electrophilic carbonyl of the ketoamide by the catalytic serine leads to tetrahedral formation which mimics the transition state of peptide bond cleavage. This is further stabilized by additional ionic interactions with the active site[11,12]. However, telaprevir and boceprevir were designed to target the protease of genotype 1 HCV and therefore their activity varies among genotypes. Experimental data has shown that both were active against genotypes 2, 5 and 6, but not 3[13]. Clinical evidence suggested that telaprevir was effective against genotype 2 but not genotype 3 and uncertain for genotype 4[14,15].
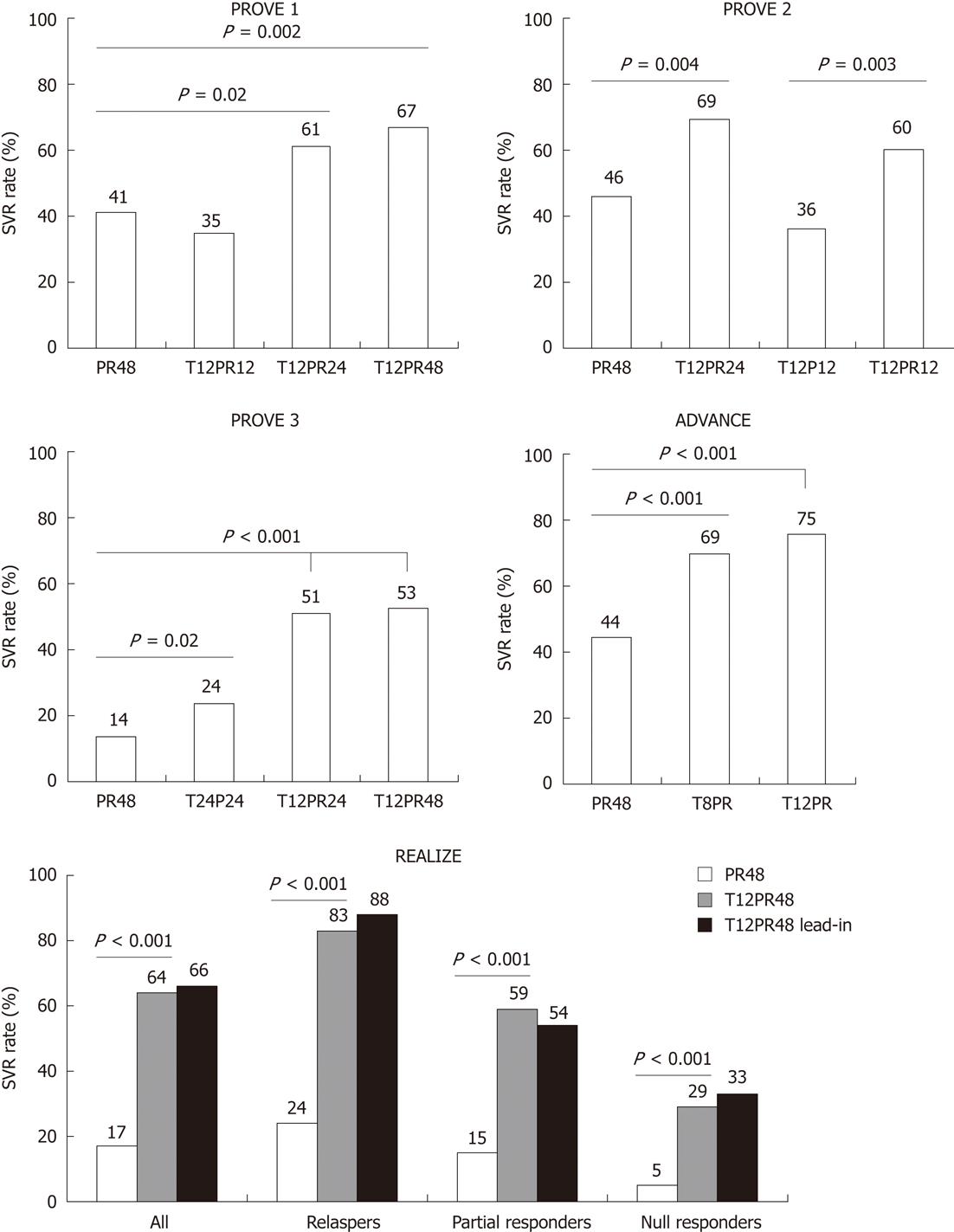
Two weeks monotherapy with telaprevir has demonstrated substantial antiviral activity in patients with genotype 1 HCV in a phase 1 trial (3 log drop in viral load in majority of patients). However, some patients showed resistance to telaprevir can be seen from viral breakthrough during dosing and were associated with selection of the variants[16]. Therefore, combination therapy with peg-IFN-α/ribavirin was required for the subsequent larger multicentre phase 2, PROVE 1[17], PROVE 2[18] and PROVE 3[19] trials. Overall, telaprevir increased 20%-30% SVR rates in both naïve and genotype 1 treated patients (Figure 1). Ribavirin was an essential component of this triple therapy (Figure 1).
Phase 3 trials in the treatment of naïve or experienced patients were carried out in the ADVANCE[20] and REALIZE[21] studies, respectively. Superior SVR rates with treatment of telaprevir-based regimens were confirmed in both naïve and experienced genotype 1 patients. The illuminate phase 3 study supported the concept of response-guided telaprevir combination treatment (RGT) in naïve patients. This study summarized that treatment of telaprevir in combination with peg-IFN/ribavirin in the first 12 and 24 wk was noninferior to the same regimen for 48 wk in patients with undetectable HCV RNA at weeks 4 and 12[22].
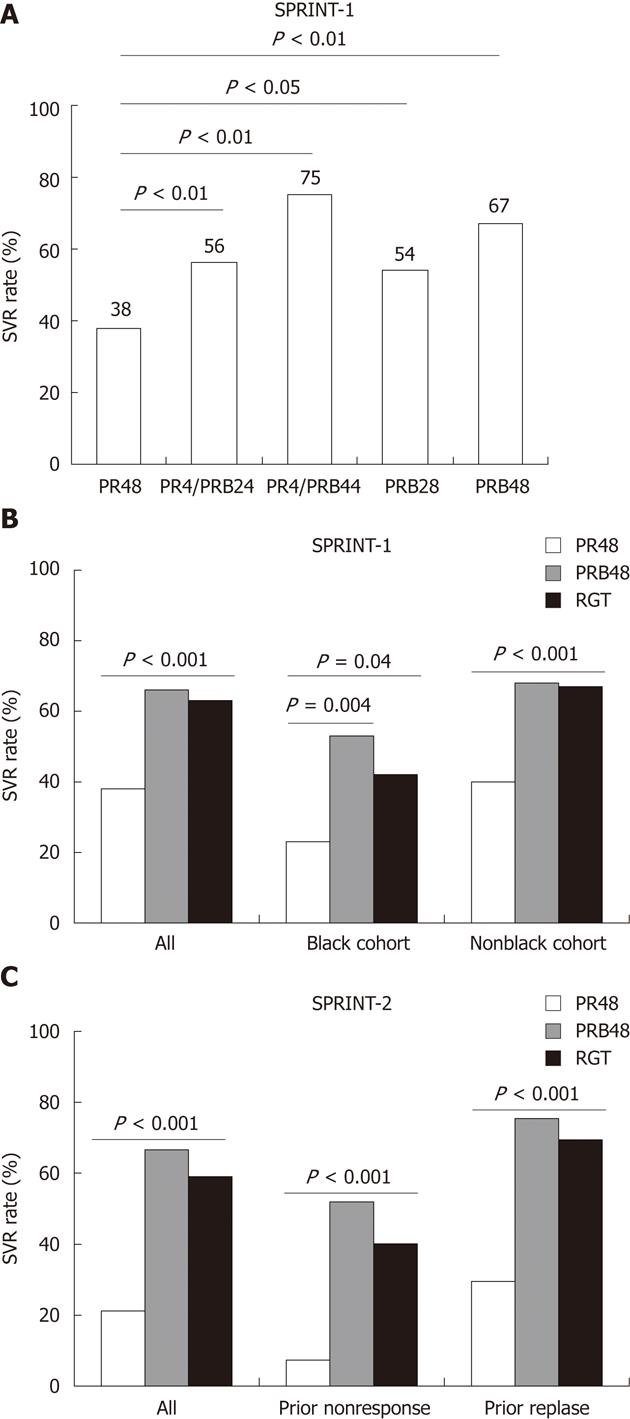
One week monotherapy with boceprevir resulted in an average HCV RNA load reduction of about 1.0 and 1.6 log10 IU/mL in nonresponders of peg-IFN/ribavirin at dose of 200 mg and 400 mg, 3 times a day, respectively[23]. However, during boceprevir monotherapy, resistance mutations at six positions within the NS3 protease were detected. All mutations were associated with reduced replicative fitness estimated by mathematical modeling with cross-resistance to telaprevir[24]. Similarly, the successful clinical development of boceprevir treatment was used in combination with peg-IFN/ribavirin both in naïve and experienced genotype 1 patients, as demonstrated in the phase 2b SPRINT-1 trial (Figure 2)[25], the phase 3 SPRINT-2 trial (Figure 2)[26] and the phase 3 RESPOND-2 trial (Figure 2)[27].
The most serious adverse effects of telaprevir were rash and anemia and of boceprevir were anemia, dysgusea, neutropenia and anal discomfort.
End-stage liver disease caused by chronic HCV infection is currently the leading indication for liver transplantation[28]. However, HCV re-infection of the graft occurs universally and often results in accelerated recurrence of liver fibrosis and early development of cirrhosis[29]. In general, peg-IFN/ribavirin is much less effective in transplant patients with approximate SVR rates of only 20%[30]. An aggravated course of infection and more resistance to antiviral therapy have been attributed to several host and viral factors, in particular the application of specific immunosuppressive medication[31].
The suppression of HCV viraemia in pre-transplant patients may attenuate the risk of viral recurrence. However, the tolerability and efficacy of peg-IFN/ribavirin antiviral therapy is compromised in decompensated cirrhosis patients[32]. For telaprevir or boceprevir, a few patients with advanced fibrosis or compensated cirrhosis have been included in these phase 3 trials[20-21,26-27]. However, the response rates in patients with advanced fibrosis were significantly lower than in patients with mild to moderate fibrosis but there is no experience in patients with decompensated cirrhosis so far.
Although prophylactic treatment, preemptive treatment or treatment of established HCV recurrence after transplantation with peg-IFN/ribavirin is common, its tolerability and efficacy remain unsatisfactory[33]. Therefore, there is much expectation of telaprevir and boceprevir for improving the outcome in liver transplant patients. In the 18th annual congress of the International Liver Transplantation Society this year in San Francisco, United States, the first series of clinical data of treating HCV recurrence using telaprevir or boceprevir in liver transplant patients were revealed, although these were rather small clinical studies. In a multicenter study of 7 patients, 2 patients had an undetectable viral load and 2 had viral load below 2 log at week 12 after treatment with boceprevir in combination with peg-IFN/ribavirin. However, all patients had severe anemia requiring erythropoietin and 4 patients required granulocyte colony stimulating factor. The dosage of peg-IFN and/or ribavirin was reduced in 4 patients and treatment was stopped prematurely in 3 patients[34]. In one of the telaprevir-based triple therapy studies, 2 out of 9 patients achieved rapid viral response, however hematological adverse events were common, including anemia and leucopenia[35]. In another telaprevir-based triple therapy study, 4 out of 5 patients were telaprevir tolerated but 1 patient stopped the treatment due to complications. Two out of these 4 patients obtained SVR after 5 wk of therapy. Another 2 patients achieved 2-log and 3-log viral reductions in the 1st week follow-up, respectively[36]. Triple combination therapy has shown substantial antiviral efficacy but larger properly designed trials are definitely required to further evaluate the use of telaprevir and boceprevir in this setting. However, these studies have consistently raised the concerns of poor tolerability of these compounds in transplant patients. Clinical studies of additional DAAs peri-transplant are in progress and should be monitored closely.
In addition to the adverse events, potential interactions between telaprevir/boceprevir with immunosuppressive drugs are other concerns. As reported in a phase 1 study, telaprevir interferes the metabolism of both cyclosporin A (CsA) and tacrolimus by inhibition of cytochrome P450 3A enzymatic activity[37]. Telaprevir caused a significant increase in blood concentrations of both immunosuppressants and could potentially lead to serious or even life-threatening adverse effects. McCashland et al[35] has specifically evaluated the drug level profile of CsA during telaprevir therapy in liver transplant patients, however no negative impact in achieving CsA target levels was observed. In fact, different immunosuppressive drugs can also have distinct effects on peg-IFN/ribavirin, such as CsA and MPA can promote the antiviral effects of interferon whereas tacrolimus does not[31,38,39]. Thus, more attention from both basic and clinical perspective is needed, regarding drug to drug interaction with telaprevir/boceprevir.
Monotherapy with telaprevir or boceprevir not only been associated with resistance but also the antiviral potency is not sufficient for complete eradication of the virus[16,24]. Therefore, combination treatment with peg-IFN/ribavirin has emerged as the ultimate solution to resolve these limitations. A specific issue which would be concerned is that whether the IL28B genetic variation as one of the important SVR prediction factors to peg-IFN/ribavirin, is still relevant to telaprevir/boceprevir-based triple combination therapy or not. In treatment of naïve patients, the IL28B rs12979860-CC genotype was a positive predictor to select appropriate candidates for RGT in the ADVANCE and SPRINT-2 trials[20,26]. In treatment of experienced patients, the CC genotype however is less frequent than in general population, IL28B therefore is expected to be less important for response prediction. However, data remain limited to address the exact prediction value of IL28B genotype in telaprevir/boceprevir-based regimens. Another clear evidence from the PROVE and SPRINT trials suggested that the treatment regimens with low-dose or without ribavirin were associated with lower SVR rates, probably due to viral breakthrough and viral relapse after therapy[18-19,25].
An emerging concept is the development of interferon-free regimens, which aims to limit the contraindications and adverse effects of peg-IFN. The combination of different reagents offers the potential for interferon-free therapy including the development of various DAAs such as protease, NS4B, NS5A, polymerase inhibitors and host-targeting agents including cyclophilin inhibitors and anti-miR-122 oligonucleotides[40]. In current clinical trials, different DAA combinations with or without ribavirin are the main scenario. It is not surprising that with or without interferon, a study of combining a non-nucleoside polymerase inhibitor with a protease inhibitor clearly showed the benefit of ribavirin[41]. In spite of the mechanism of how ribavirin can synergize interferon that has been extensively extrapolated[42-44], the mechanism of how ribavirin works with DAAs are poorly investigated. Notably, a recent study has shown that high SVR rate could be achieved with two DAAs (the NS5A inhibitor daclatasvir and the NS3 inhibitor asunaprevir) only, although higher SVR rates were achieved when combined with peg-IFN/ribavirin[45]. Therefore, it remains an open question regarding the future of ribavirin in interferon-free-based DAA combination therapy. Further basic and clinical research will conceivably help to figure out this issue.
The approval of telaprevir and boceprevir by FDA and the European Medicines Agency in last year (2011) has indeed changed the management of chronic HCV. From these series of clinical landmark studies, a 20%-30% increase of SVR rates were obtained with the additional telaprevir/boceprevir to the conventional standard peg-IFN/ribavirin therapy. Despite these exciting results, certainly there are several remaining concerns. An apparent limitation of telaprevir/boceprevir as well as most of the pipeline DDAs (such as protease inhibitors asunaprevir, BI 201335 and ABT-450) is genotype 1 specificity, whereas other HCV genotypes also prevail all over the world. Secondly, these compounds have very low genetic barrier to the development of resistant viral variants. Thirdly, although telaprevir/boceprevir are generally well tolerated, their adverse effects are still hampering the successful application in specific populations, particularly for liver transplant patients.
Perspectively, these issues could likely be minimized by further optimizing the clinical protocol. In addition, by the development of other compounds, more optimal combinations will be available. As being expected, the dream of achieving high SVR rates with all-oral interferon-free regimens will be no longer far from clinical reality.
Peer reviewers: Dr. Dahlene N Fusco, Gastrointestinal Unit, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA 02114, United States; Akihito Tsubota, MD, Department of Gastroenterology, Toranomon Hospital, 2-2-2 Toranomon, Minato-ku, Tokyo 105-8470, Japan
S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4996] [Cited by in RCA: 4657] [Article Influence: 129.4] [Reference Citation Analysis (0)] |
| 2. | Hess G. Treatment of chronic hepatitis C. J Hepatol. 1991;13 Suppl 1:S17-S20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Sun L, Liu S, Chen ZJ. SnapShot: pathways of antiviral innate immunity. Cell. 2010;140:436-436.e2. [PubMed] |
| 4. | Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 575] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 5. | Zeuzem S, Buti M, Ferenci P, Sperl J, Horsmans Y, Cianciara J, Ibranyi E, Weiland O, Noviello S, Brass C. Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia. J Hepatol. 2006;44:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 364] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 6. | Boonstra A, van der Laan LJ, Vanwolleghem T, Janssen HL. Experimental models for hepatitis C viral infection. Hepatology. 2009;50:1646-1655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 7. | Casey LC, Lee WM. Hepatitis C therapy update. Curr Opin Gastroenterol. 2012;28:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Penin F, Dubuisson J, Rey FA, Moradpour D, Pawlotsky JM. Structural biology of hepatitis C virus. Hepatology. 2004;39:5-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 458] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Bartenschlager R, Ahlborn-Laake L, Mous J, Jacobsen H. Kinetic and structural analyses of hepatitis C virus polyprotein processing. J Virol. 1994;68:5045-5055. [PubMed] |
| 10. | Berger A, Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970;257:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 296] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Perni RB, Almquist SJ, Byrn RA, Chandorkar G, Chaturvedi PR, Courtney LF, Decker CJ, Dinehart K, Gates CA, Harbeson SL. Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease. Antimicrob Agents Chemother. 2006;50:899-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 299] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Malcolm BA, Liu R, Lahser F, Agrawal S, Belanger B, Butkiewicz N, Chase R, Gheyas F, Hart A, Hesk D. SCH 503034, a mechanism-based inhibitor of hepatitis C virus NS3 protease, suppresses polyprotein maturation and enhances the antiviral activity of alpha interferon in replicon cells. Antimicrob Agents Chemother. 2006;50:1013-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 233] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Gottwein JM, Scheel TK, Jensen TB, Ghanem L, Bukh J. Differential efficacy of protease inhibitors against HCV genotypes 2a, 3a, 5a, and 6a NS3/4A protease recombinant viruses. Gastroenterology. 2011;141:1067-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Foster GR, Hézode C, Bronowicki JP, Carosi G, Weiland O, Verlinden L, van Heeswijk R, van Baelen B, Picchio G, Beumont M. Telaprevir alone or with peginterferon and ribavirin reduces HCV RNA in patients with chronic genotype 2 but not genotype 3 infections. Gastroenterology. 2011;141:881-889.e1. [PubMed] |
| 15. | Schaefer EA, Chung RT. Anti-hepatitis C virus drugs in development. Gastroenterology. 2012;142:1340-1350.e1. [PubMed] |
| 16. | Reesink HW, Zeuzem S, Weegink CJ, Forestier N, van Vliet A, van de Wetering de Rooij J, McNair L, Purdy S, Kauffman R, Alam J. Rapid decline of viral RNA in hepatitis C patients treated with VX-950: a phase Ib, placebo-controlled, randomized study. Gastroenterology. 2006;131:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 301] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 17. | McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, McNair L, Alam J, Muir AJ. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med. 2009;360:1827-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 809] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 18. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 794] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 19. | McHutchison JG, Manns MP, Muir AJ, Terrault NA, Jacobson IM, Afdhal NH, Heathcote EJ, Zeuzem S, Reesink HW, Garg J. Telaprevir for previously treated chronic HCV infection. N Engl J Med. 2010;362:1292-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 535] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 20. | Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, Marcellin P, Muir AJ, Ferenci P, Flisiak R. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364:2405-2416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1905] [Cited by in RCA: 1862] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 21. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1214] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 22. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 592] [Cited by in RCA: 602] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 23. | Sarrazin C, Rouzier R, Wagner F, Forestier N, Larrey D, Gupta SK, Hussain M, Shah A, Cutler D, Zhang J. SCH 503034, a novel hepatitis C virus protease inhibitor, plus pegylated interferon alpha-2b for genotype 1 nonresponders. Gastroenterology. 2007;132:1270-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 233] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 24. | Susser S, Welsch C, Wang Y, Zettler M, Domingues FS, Karey U, Hughes E, Ralston R, Tong X, Herrmann E. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology. 2009;50:1709-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 25. | Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 517] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 26. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1996] [Cited by in RCA: 1981] [Article Influence: 141.5] [Reference Citation Analysis (0)] |
| 27. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1306] [Cited by in RCA: 1308] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 28. | Brown RS. Hepatitis C and liver transplantation. Nature. 2005;436:973-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 257] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 29. | Yilmaz N, Shiffman ML, Stravitz RT, Sterling RK, Luketic VA, Sanyal AJ, Mills AS, Contos MJ, Coterell A, Maluf D. A prospective evaluation of fibrosis progression in patients with recurrent hepatitis C virus following liver transplantation. Liver Transpl. 2007;13:975-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Samuel D, Bizollon T, Feray C, Roche B, Ahmed SN, Lemonnier C, Cohard M, Reynes M, Chevallier M, Ducerf C. Interferon-alpha 2b plus ribavirin in patients with chronic hepatitis C after liver transplantation: a randomized study. Gastroenterology. 2003;124:642-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Pan Q, Tilanus HW, Metselaar HJ, Janssen HL, van der Laan LJ. Virus-drug interactions--molecular insight into immunosuppression and HCV. Nat Rev Gastroenterol Hepatol. 2012;9:355-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Bruno S, Shiffman ML, Roberts SK, Gane EJ, Messinger D, Hadziyannis SJ, Marcellin P. Efficacy and safety of peginterferon alfa-2a (40KD) plus ribavirin in hepatitis C patients with advanced fibrosis and cirrhosis. Hepatology. 2010;51:388-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 33. | Bzowej N, Nelson DR, Terrault NA, Everson GT, Teng LL, Prabhakar A, Charlton MR. PHOENIX: A randomized controlled trial of peginterferon alfa-2a plus ribavirin as a prophylactic treatment after liver transplantation for hepatitis C virus. Liver Transpl. 2011;17:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Coilly A, Roche B, Botta-Fridlund D, Leroy V, Pageaux PG, Si-Ahmed SN, Antonini TM, Samuel D, Duclos-Vallee JC. A First Multicentric Experience of Protease Inhibitors for Severe Hepatitis C Recurrence after Liver Transplantation. Liver Transpl. 2012;18:S84-S85. |
| 35. | McCashland TM, Olivera-Martinez MA, de Sicilia MGS, Mukherjee S, Rochling FA, Schafer DF, Sorrell MF. Early Experience with Triple Drug Therapy (Telaprevir, Pegylated Interferon alpha 2A and Ribavirin) in Patients on Cyclosporine A for Hepatitis C Recurrence after Liver Transplantation. Liver Transpl. 2012;18:S99. |
| 36. | Shin HJ, Pereira AD, Safdar A, Tobias H, Gelb B, Morgan G, Diflo T, Teperman L. Initial Experience of Telaprevir for Recurrent Hepatitis C in Post Liver Transplant Patients. Liver Transpl. 2012;18:S130. |
| 37. | Garg V, van Heeswijk R, Lee JE, Alves K, Nadkarni P, Luo X. Effect of telaprevir on the pharmacokinetics of cyclosporine and tacrolimus. Hepatology. 2011;54:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 38. | Pan Q, de Ruiter PE, Metselaar HJ, Kwekkeboom J, de Jonge J, Tilanus HW, Janssen HL, van der Laan LJ. Mycophenolic acid augments interferon-stimulated gene expression and inhibits hepatitis C Virus infection in vitro and in vivo. Hepatology. 2012;55:1673-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 39. | Pan Q, Metselaar HJ, de Ruiter P, Kwekkeboom J, Tilanus HW, Janssen HL, van der Laan LJ. Calcineurin inhibitor tacrolimus does not interfere with the suppression of hepatitis C virus infection by interferon-alpha. Liver Transpl. 2010;16:520-526. [PubMed] |
| 40. | Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012;61 Suppl 1:i36-i46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Zeuzem S, Buggisch P, Agarwal K, Marcellin P, Sereni D, Klinker H, Moreno C, Zarski JP, Horsmans Y, Mo H. The protease inhibitor, GS-9256, and non-nucleoside polymerase inhibitor tegobuvir alone, with ribavirin, or pegylated interferon plus ribavirin in hepatitis C. Hepatology. 2012;55:749-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augmenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011;53:32-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Pan Q, Tilanus HW, Janssen HL, van der Laan LJ. Ribavirin enhances interferon-stimulated gene transcription by activation of the interferon-stimulated response element. Hepatology. 2011;53:1400-1401; author reply 1402. [PubMed] |
| 44. | Thomas E, Ghany MG, Liang TJ. The application and mechanism of action of ribavirin in therapy of hepatitis C. Antivir Chem Chemother. 2012;23:1-12. [PubMed] |
| 45. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 476] [Article Influence: 36.6] [Reference Citation Analysis (0)] |