INTRODUCTION
A primary aortoenteric fistula (AEF) is a communication between the aorta and the intestines, and occurs in a setting without prior aortic surgery. It is a rare but potentially lethal condition with an incidence of 0.04% to 0.07%[1]. Primary fistulas, are in most cases (90%), the result of erosion of the bowel wall, caused by an abdominal aortic aneurysm (AAA)[2]. The majority occur between the aorta and the duodenum[3]. Primary aortoduodenal fistulas (ADF) have been reported in the presence of various conditions, including underlying atherosclerotic aortic aneurysm disease, gallstone erosion, foreign body ingestion, and invasive intra-abdominal malignancies[4]. However, it has been reported that an aortic aneurysm can be a part of a spectrum of immunoglobulin G4 (IgG4)-related sclerosing disease[5]. Most reported cases of IgG4-related inflammatory aortic aneurysm of abdominal aorta (IAAA) have no association with other IgG4-related sclerosing diseases. Without apparent pathological conditions in other organs, confirmation of the existence of IgG4-related IAAA requires histopathological evidence[6-10]. An ADF as a complication of IgG4-related disease has not yet been reported. Endovascular aortic repair is an effective alternative to standard open surgical management of primary aortoenteric fistulae, especially in situations where an open surgical procedure is either difficult or contraindicated[11]. In this article we present the urgent endovascular repair of an ADF in a patient with high serum IgG4 levels.
CASE REPORT
A 56-year-old Caucasian male was admitted to our institution due to severe anemia caused by gastrointestinal hemorrhage. He had chronic fatique and at least one episode of melena every day of one month duration. At admission he had anemia with a red blood cell count (RBC) of 2.8 million per μL, hematocrit (HCT) of 25% and blood pressure of 90/70 mmHg with a heart rate of 110 beats/min. No abdominal pulsating mass was found on physical examination. A digital rectal examination showed signs of melena.
The patient had undergone previous abdominal surgery in another medical institution (9 years before), due to acute generalized abdominal pain. He had no medical documentation, and we were unable to obtain more information about his previous condition.
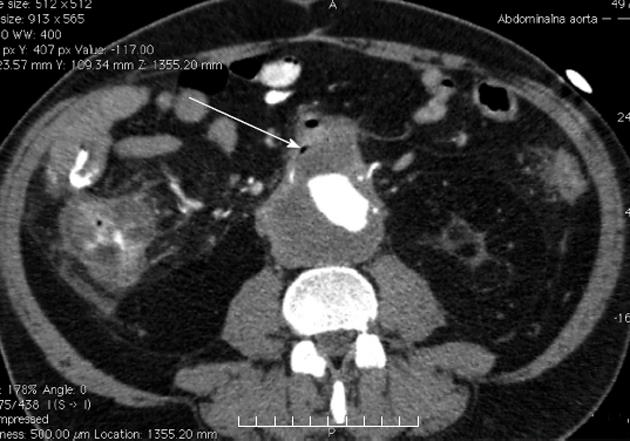
Urgent upper endoscopy (EGDS) did not show any signs of bleeding. Abdominal ultrasound showed an infrarenal AAA that was verified on computed tomography (CT) angiography (Figure 1). During the same CT evaluation a gas collection in a suspected thrombotic mass in an aneurysmatic sac was noted, suggesting an AEF. The CT findings excluded the presence of autoimmune pancreatitis.
Figure 1 An infrarenal aneurysm (arrow) of the abdominal aorta with gas collection in a suspected thrombotic mass in the aneurysmatic sac, suggesting an aortoenteric fistula.
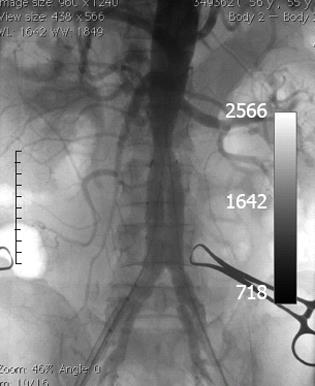
Because there was a suspicion of Meckel’s diverticulum, we performed abdominal scintigraphy, without any pathological findings. Colonoscopy findings were normal. In addition to administering blood and blood product substitution therapy, standard therapy was also administered, however, melena persisted. Control EGDS findings were normal. Due to worsening of the patient’s general condition [persistent melena followed by anemia; RBC 3.1 million per μL, HCT 27% and C-reactive protein (CRP) 82 mg/L], the case was presented at a meeting of gastroenterologists, vascular and abdominal surgeons, radiologists and anesthesiologists, and it was decided that operative treatment was necessary. We performed an emergency endovascular repair of the AAA using an Excluder Stent Graft (Excluder®, WL Gore and Associates, Flagstaff, Arizona, United States) (Figure 2).
Figure 2 Intraoperative computed tomography angiography after endovascular repair of abdominal aortic aneurysm with excluder stent graft.
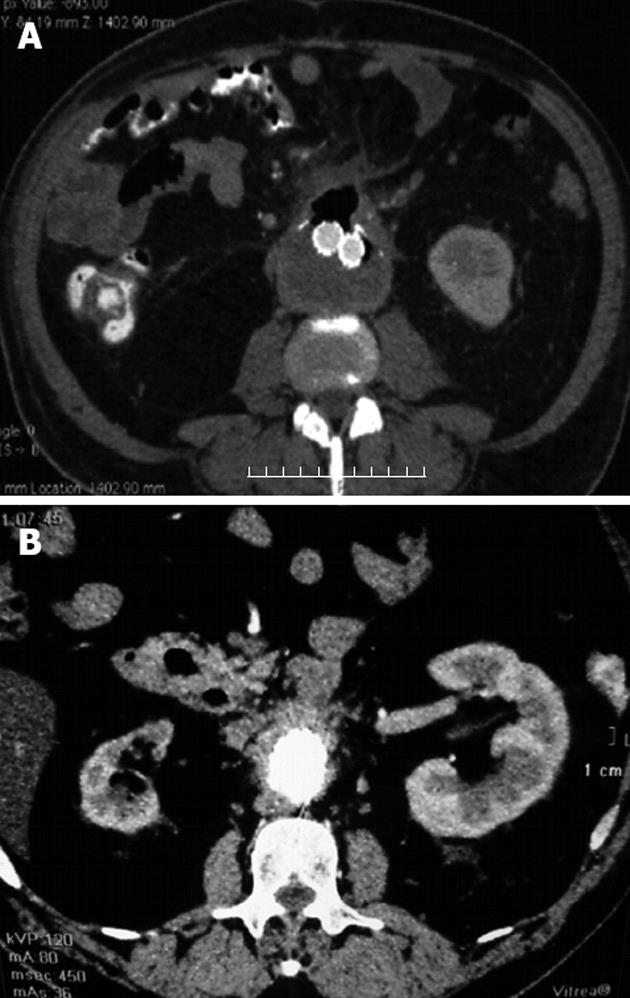
We initiated empiric broad spectrum intravenous antibiotics (imipenem in combination with vancomycin) preoperatively, and continued with the same medication for 4 wk after endovascular repair. The patient then continued taking an oral antibiotic for the next two months (ciprofloxacin 1000 mg per day for 4 wk then doxycycline 100 mg per day for another 4 wk). The postoperative course was without complications and without further blood loss. After the patient was introduced to per os food intake, control CT angiography showed no endoleaks and a reduction in thrombotic mass volume in the aneurysm with regression of the aneurysm diameter, however, the gas collection persisted (Figure 3A).
Figure 3 Control computed tomography angiography.
A: Control computed tomography (CT) angiography shows no endoleaks and reduction of thrombotic mass volume in the aneurysmatic sac with persistence of gas collection; B: Control CT angiography after 6 mo showed no endoleaks and near total reduction of thrombotic mass in the aneurysmatic sac, without gas collection.
On the tenth day after endovascular repair, RBC and HCT values were normal (RBC 4.2 million per μL and HCT 35%). The patient had improved, but the laboratory tests showed a CRP serum level of 56 mg/L and a high serum IgG level of 20.2 g/L and IgG4 of 4.24 g/L, respectively. Autoantibodies against lactoferrin and carbonic anhydrase II were negative, and autoantibodies including anti-nuclear antibodies, antineutrophil cytoplasmic antibodies and rheumatoid factor were not found. Markers for viral hepatitis Bs infection, hepatitis C virus, hepatitis A virus, human immunodeficiency viruses 1 and 2 were negative. Due to lack of histopathological findings, but elevated serum levels of IgG, it could not be determined with confidence that this was a systemic disease. It was decided that the patient should start steroid therapy with oral prednisolone at 40 mg per day for 4 wk, and then decrease the dosage by 5 mg every week. Four wk after initiation of steroid therapy, serum levels of IgG (16 g/L) and IgG4 (0.7 g/L) as well as CRP were normal. In the control examination conducted 6 mo after the patient’s discharge from hospital (almost 3 mo after the end of steroid therapy), the levels of serum immunoglobulins and CRP were in the normal ranges and control CT angiography showed no endoleaks, near total reduction of the thrombotic mass in the aneurysmatic sac, and no gas collection (Figure 3B).
DISCUSSION
Primary ADF represents a serious condition and is much less frequent than a secondary ADF which occurs as a result of previous aortic aneurysm grafting. The first described case of primary ADF was more than a century ago, and since then about 250 cases have been reported. In the absence of treatment, the mortality rate is almost 100%. With surgical intervention, survival ranges from 18%-93%, but 40% of operated cases develop complications with an overall postoperative mortality rate greater than 30%[12]. Diagnosis of primary ADF is also difficult. Only 33% to 50% of AEF are diagnosed preoperatively[3]. Gastrointestinal bleeding and abdominal pain have been described in all cases, but the classic trio of abdominal pain, palpable mass and gastrointestinal bleeding was found in only 6% to 27.8% of patients[2,13,14]. Our patient only had gastrointestinal bleeding presenting as melena. Diagnostic procedures include EGDS, CT scan, and arteriography. Endoscopy is considered the modality of choice for initial evaluation of upper gastrointestinal bleeding, but has the potential risk of inducing massive hemorrhage by dislodging fresh thrombus in the fistula[15,16]. In our case, EGDS findings were without signs of gastrointestinal hemorrhage. CT angiography was reliable in diagnosing AEF but may be somewhat challenging in unstable patients.
The surgical treatment of ADF consists of repairing the duodenal defect and performing a prosthetic repair of the aorta with graft. Where contamination is present, or in the case of mycotic aneurysm, an extraanatomical aortic graft is preferred and extensive debridement is required[17]. Although the endovascular approach is increasingly utilized in the repair of secondary AEF, only a few primary AEF treated using this method have been reported[4,11,12,18,19]. In the case of any applied vascular procedure, long-term broad spectrum antibiotic therapy is necessary[11,12]. We believe that patients with persistent anemia who undergo laparotomy without diagnosis is hazardous.
Primary ADF is the result of inflammatory destruction of an aortic aneurysm arising from an atherosclerotic AAA. This is an etiological factor in 73% of all primary ADFs, while 26% are caused by traumatic or mycotic aneurysms[2,17,20]. Rarer causes such as radiation, tumors, ulcers and ingestion of foreign bodies account of 1% or less[4,12]. A very small number of AEF occurin the absence of an aortic aneurysm. In comparison to atherosclerotic AAA, IAAA occurs in only 2% to 10% of all AAA, and has different clinical characteristics, such as younger age of patients, non-specific symptoms, and large aneurysm diameter. Recent reports showed a close relationship between IgG4 and sclerosing lesions of various organs [21]. IgG4-related sclerosing lesions, which are also called IgG4-related sclerosing disease, was first reported with regard to autoimmune pancreatitis[22].The clinical characteristics of IgG4-related sclerosing disease are a frequent occurence in adult male patients, and include elevation of serum IgG4 levels, and steroid sensitivity. In addition to affected organs in IgG4-related disease, elevated IgG4 levels have been described in atopic dermatitis, asthma, some parasitic diseases, pemphigus vulgaris, pemphigus foliaceus and pancreatic cancer[23]. Our patient had no clinical signs of these diseases. The correct diagnosis of IAAA as part of IgG4-related disease is based on histopathological findings[21]. According to these recommendations, the diagnosis of IAAA as part of IgG4-related disease in our patient couldnot be established due to lack of histopathological findings, as endovascular repair of AAA, was successful. If the AAA in our patient was an IAAA, this would be the first case with ADF in IgG4-related sclerosing disease. In addition, the patient had a positive response to steroid therapy. The question remains as to how it is possible to make a diagnosis of IgG4-related IAAA in such cases where urgent minor surgery is the treatment of choice. In our case, endovascular repair of ADF was successful, and this may be an alternative treatment, especially in patients with high operative risk. Broad spectrum antibiotic therapy is as important as the endovascular treatment in ADF.
In conclusion, primary ADF is a rare complication of aortic aneurysms and is a rare cause of gastrointestinal bleeding. It can be associated with IgG4-related sclerosing disease, whether it occurs as a complication of IAAA or not. CT angiography is a good diagnostic test for an ADF. This case demonstrates that endovascular grafting of primary ADF as an effective, minimally invasive alternative to standard surgical repair. Other supportive therapy such as antibiotics are very important in the treatment of this condition. High serum IgG4 levels and a positive response to steroid therapy suggest the existence of an ADF in IgG4-related IAAA, without histopathological examination.
Peer reviewers: Fausto Catena, MD, Department of General, Emergency and Transplant Surgery, St. Orsola-Malpighi University Hospital, Via Massarenti 9, 40139 Bologna, Italy; Dr. Beata Jabońska, Digestive Tract Surgery, Medical University of Silesia, Medyków 14 St., 40-752 Katowice, Poland
S- Editor Gou SX L- Editor Webster JR E- Editor Li JY