INTRODUCTION
Lymphocytic colitis (LC), which was first described in 1989[1], belongs to a group of conditions known as microscopic colitides (MC). This disorder is characterised mainly by chronic or intermittent watery diarrhoea[2], which can be severe. Up to 25% of patients present with more than 10 bowel movements per day in addition to nocturnal diarrhoea[3]. Other symptoms such as cramping, abdominal pain and weight loss may occur[4]. The aetiology of LC is not yet clear, although several hypotheses have been suggested, such as association to various autoimmune conditions and drug induction[2]. LC has an incidence of 3.1 to 9.8 and prevalence of 14.2 per 100 000 people[5-12].
Sequential treatment for LC is recommended, in which a “therapeutic ladder” is followed. The suggested drugs in this ladder are: loperamide, bismuth salicylate, budesonide, cholestyramine 5-aminosalicylic acid preparations, prednisolone, azathioprine, 6-mercaptopurine methotrexate or octreotide[2].
In a previous study, a high density of colonic chromogranin A immunoreactive cells were reported in patients with LC[13]. Chromogranin A is a general marker for all endocrine cells[14-16], but it is not clear which types of colonic endocrine cells are responsible for the increase in the density of chromogranin A cells in patients with LC. Therefore, the current study was performed to identify the endocrine cell types involved.
MATERIALS AND METHODS
Patients and controls
Fifty-seven patients with a diagnosis of lymphocytic colitis during the period 2007-2010 were included in this study. The patients were diagnosed in all 3 hospitals of Helse-Fonna region in Western Norway, namely Stord, Haugesund and Odda. They were 41 females and 16 males, with an average age of 49 years (range 19-84 years). These patients did not show any clinical signs of other autoimmune disorders. They did not had coeliac disease tested either by serology or duodenal biopsies. Twenty-seven subjects that underwent colonoscopy with biopsies were used as controls. Twenty of these subjects underwent colonoscopy because of gastrointestinal bleeding, where the source of bleeding was identified as haemorrhoids (18), or angiodysplasia (2), and seven were examined because of health worries caused by a relative being diagnosed with colon carcinoma. They were 19 females and 8 males with an average age of 49 years (range 18-67 years). All these subjects had no other gastrointestinal complaints than those mentioned above.
The study was performed in accordance with the Declaration of Helsinki and was approved by the local Committee for Medical Research Ethics. All subjects gave oral and written consent.
Colonoscopy
Colonoscopies were performed for all patients and controls, and two biopsies were taken from the caecum, from the ascending colon and from the right half of the transverse colon. These biopsies were pooled together and were labelled as right colon. In addition, two biopsies were taken from the left half of the transverse colon, from the descending colon and from the sigmoid colon. These 6 biopsies were pooled together and labelled as left colon.
Histopathology and immunohistochemistry
Biopsies were fixed in 4% buffered paraformaldehyde overnight, embedded in paraffin and cut into 5 μm-thick sections. The sections were stained with haematoxylin-eosin and immunostained by the avidin-biotin-complex (ABC) method using the Vectastain ABC-kit (Vector laboratories) as described earlier in detail[17]. The primary antibodies used were: monoclonal anti-human leucocytes common antigen (Dako, CD 45, clone 2B11), monoclonal anti-human CD8 lymphocytes (Dako, CD 57, clone 2B01), monoclonal mouse anti-serotonin (Dako, code no. M869), polyclonal anti-porcine peptide YY (PYY) (Euro-diagnostica, code B52-1), polyclonal rabbit anti-synthetic human pancreatic polypeptide (PP) (Dako, code no. A619), polyclonal rabbit anti-synthetic human somatostatin, and polyclonal rabbit anti-porcine glucagon (Euro-diagnostica, code B31). The antibodies were used at dilutions of 1:100, 1:200, 1:1500, 1:1500, 1:1000, 1:1600 and 1:200, respectively. The anti-PYY cross reacts with PYY in all vertebrates including humans. It does not cross react with PP or neuropetide Y in immunohistochemical system. Anti-glucagon is directed to N-Terminus of glicentin (enteroglucagon) and does not cross react with glucagon, vasoactive intestinal polypeptide or gastric inhibitory polypeptide. The second layer biotinylated mouse anti-immunoglobulin G (IgG) and rabbit anti-IgG were obtained from Dako. Negative and positive controls were the same as those described previously[17].
Computerised image analysis
The number of immunoreactive cells and the area of the epithelial cells were measured using Olympus software: Cell ^D. When using ×40 objectives, the frame (field) on the monitor represents an area of 0.14 mm2 of the tissue. Measurements were performed in 10 randomly chosen fields for each individual and hormone. The X40 objective was used. The data from fields were tabulated, and the number of cells/mm2 of the epithelium was computed and statistically analysed. The immunostained sections of IBS patients and controls were coded and mixed, and measurements were made without the knowledge of sections identity.
Statistical analysis
The non-parametric Mann-Whitney test was performed. P < 0.05 was considered to be statistically significant.
RESULTS
Endoscopy, histopathology and immunohistochemistry
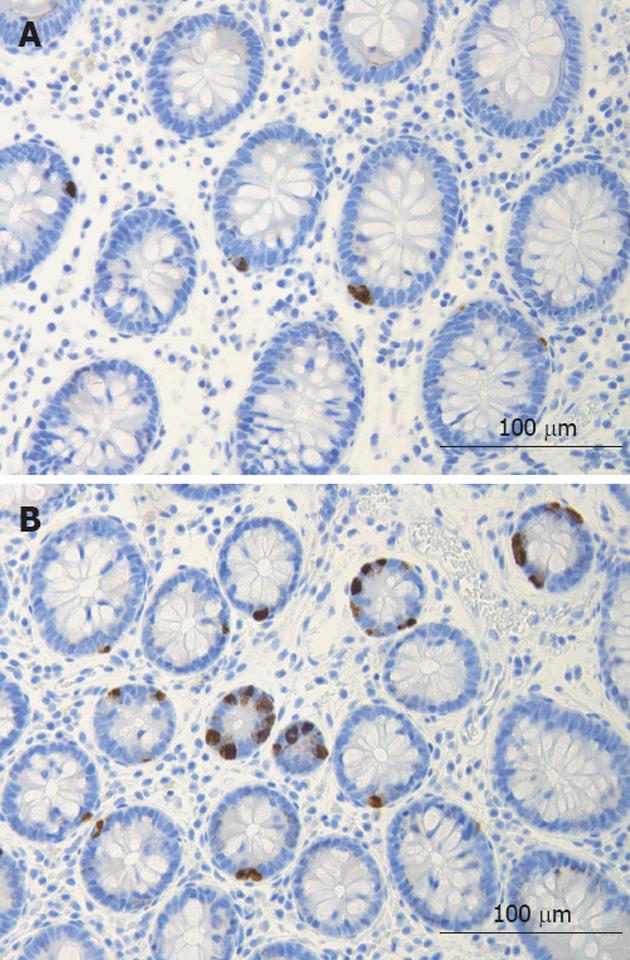
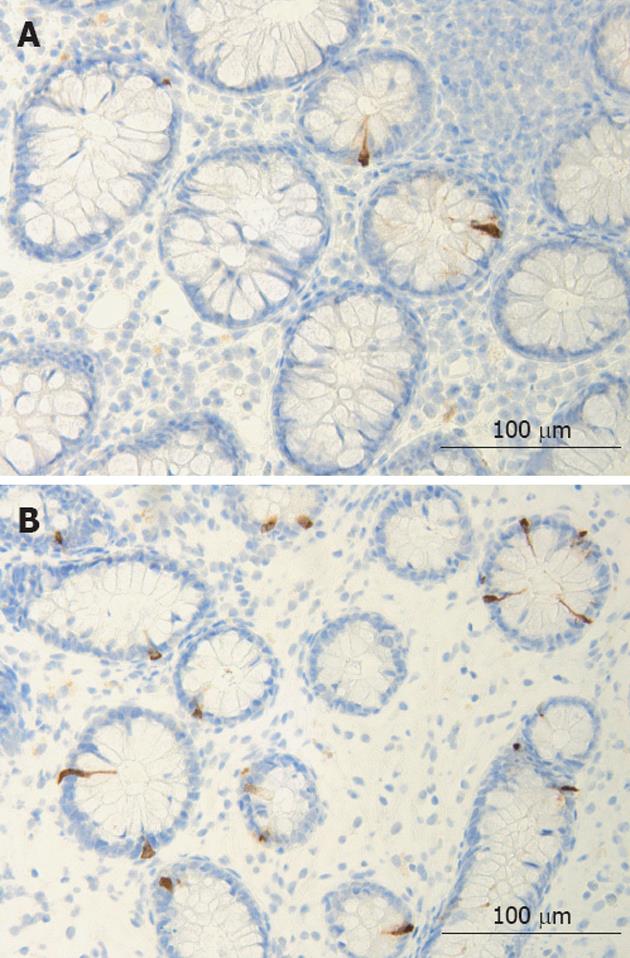
The colon of both the patient and the control subjects were macroscopically normal. Histopathological examination of colon biopsies from controls revealed normal histology. All patients fulfilled the diagnosis criteria required for of LC: an increase in intraepithelial lymphocytes (> 20 lymphocytes/100 epithelial cells) and surface epithelial damage with increased lamina propria plasma cells and absent or minimal crypt architectural distribution. In the colon of both patients and control subjects, serotonin-, PYY-, PP-, enteroglucagon- and somatostatin-immunoreactive cells were primarily located in the upper part of the crypts of Lieberkühn. These cells were basket- or flask-shaped (Figures 1 and 2).
Figure 1 Serotonin-immunoreactive cells in the colon of a control (A) and of a patient with lymphocytic colitis (B).
Figure 2 Colonic peptide YY -immunoreactive cells in a control (A) and in a patient with lymphocytic colitis (B).
Computerised image analysis
PP-, enteroglucagon- and somatostatin-immunoreactive cells were sparse in the biopsy material examined. This made it difficult to perform a reliable quantification of these cell types.
There was no statistically significant difference between the right and left colon in controls with regards to the densities of serotonin- and PYY-immunoreactive cells (P = 0.9 and 0.1, respectively).
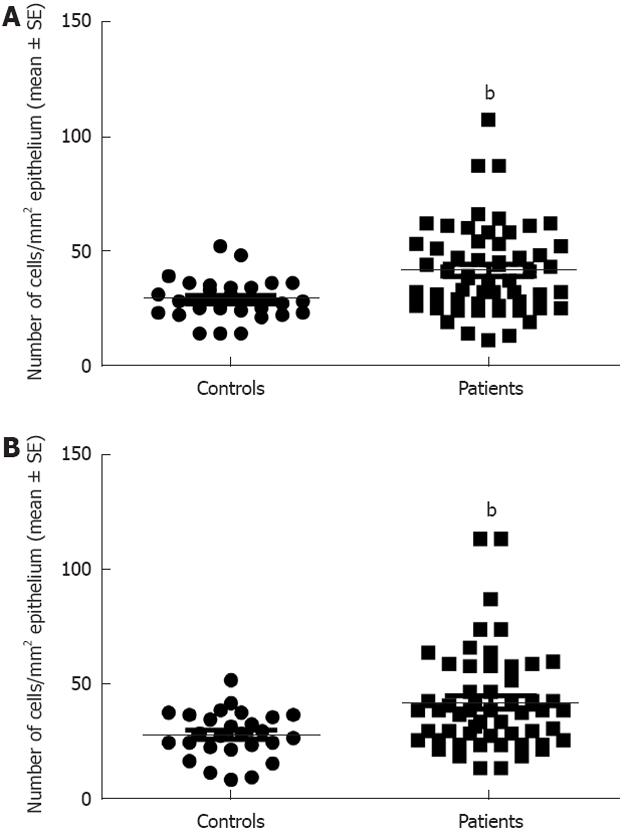
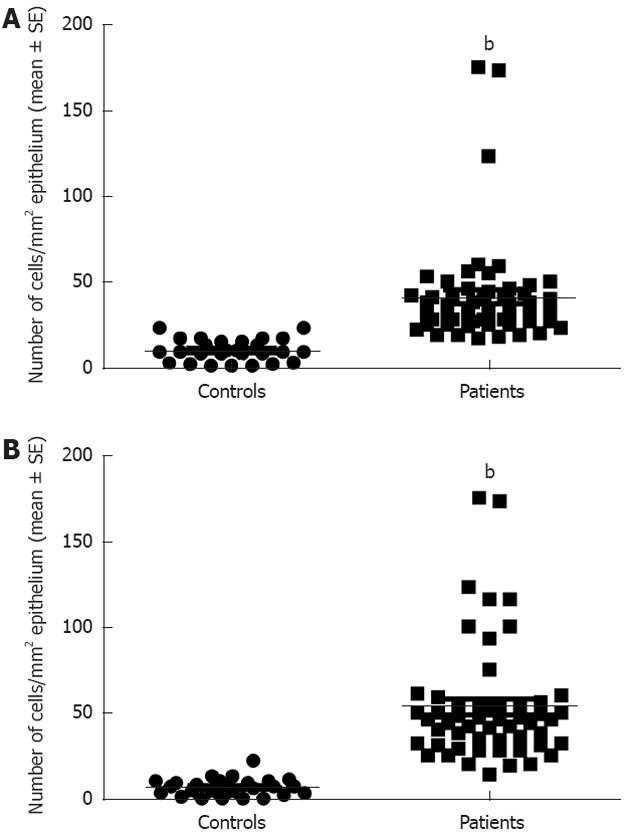
Serotonin cell density in the right colon in controls was 28.9 ± 1.8 (mean ± SE) and in LC patients 41.6 ± 2.6 (P = 0.008). In the left colon, the corresponding figures were 28.5 ± 1.9 and 42.4 ± 2.9, respectively (P = 0.009) (Figures 1 and 3). PYY cell density in the right colon of the controls was 10.1 ± 1 and of LC patients 41 ± 4 (P = 0.00006). In the left colon, PYY cell density in controls was 6.6 ± 1.2 and in LC patients 53.3 ± 4.6 (P = 0.00007) (Figures 2 and 4).
Figure 3 Serotonin cell density in the controls and patients with lymphocytic colitis in the right (A) and left colon (B).
bP < 0.01 vs controls.
Figure 4 Peptide YY cell density in controls and patients with lymphocytic colitis in the right (A) and left colon (B).
bP < 0.001 vs controls.
DISCUSSION
MC is a common cause of diarrhoea and 10% to 30% of older adults investigated for chronic diarrhoea have MC[18]. LC seems to be associated with several autoimmune diseases[19,20]. Furthermore, the prevalence of coeliac disease is high in patients with LC[21]. The information available on the gut endocrine cells in coeliac disease is restricted to the duodenum[22]. It is therefore difficult to compare the outcome of the present study with findings in coeliac disease.
The current study showed that serotonin and PYY cell densities were increased in both the right and left colon of patients with LC. Serotonin activates the submucosal sensory branch of the enteric nervous system, and controls gastrointestinal motility and chloride secretion via inter-neurons and motor neurons[23,24]. PYY stimulates the absorption of water and electrolytes and is a major regulator of the “ileal brake”[24]. There are several studies showing that inflammation and immune cells affect the neuroendocrine system of the gut[25]. Thus, serotonin secreted by enterochromaffin (EC) cells can be enhanced or attenuated by secretory products of immune cells, such as CD4+T[26]. Furthermore, serotonin modulates the immune response[26]. The EC are in contact with or very close to CD3+ and CD20+ lymphocytes, and several serotonergic receptors have been characterised in lymphocytes, monocytes, macrophages and dendritic cells[27].
One may speculate that the high density of serotonin cells in LC patients is caused by the interaction between the immune cells and serotonin cells in the epithelium and submucosa of LC patients. The increase in serotonin would cause accelerated colonic motility and visceral hypersensitivity. Accelerated colonic motility and visceral hypersensitivity can cause diarrhoea and abdominal pain, symptoms that occur in LC. It is probable that the increase in PYY cell density is secondary to the increase in serotonin cell density in order to compensate for accelerated motility by activating the ileal brake and by increasing the absorption of water. In support for this assumption are the findings that large intestinal serotonin and PYY cells as well plasma levels are affected in patients with ulcerative colitis and Crohn’s disease[28-32]. Similarly, these endocrine cells have been found to be affected in experimental animal model of colitis[33,34].
LC and post-infectious IBS (PI-IBS) show a striking similarity. They have the same clinical presentation and both can regress spontaneously[35,36]. Both LC and PI-IBS show intra-epithelial and submucosal infiltration of lymphocytes and mast cells, and exhibit a high density of colonic serotonin and PYY cells[36-41]. This raises the question as to whether LC and PI-IBS are actually the same disorder. If this is proven to be true, it would have an important clinical implication. Thus, PI-IBS can be treated by the same “therapeutic ladder” which is proven to be effective in LC.
COMMENTS
Background
Microscopic colitis is a chronic condition with watery diarrhoea as the cardinal symptom, but other symptoms such as cramping, abdominal pain and weight loss may occur Radiologic and endoscopic findings in these patients are normal. However, histopathological examinations of the colon reveal abnormal histology, which is of two distinctive types: lymphocytic colitis (LC) and collagenous colitis. LC exhibits an increased number of colonic intra-epithelial lymphocytes (> 20/100 epithelial cells), increased inflammatory cells within the lamina propria and preserved crypt architecture. LC and irritable bowel syndrome (IBS) have similar symptoms and both are without radiologic or endoscopic abnormalities. Thus, LC can be mistakenly diagnosed as IBS. In a study on the colonic chromogranin A cell density in IBS patients, nine patients out of 50 showed extremely high colonic chromogranin A cell density. This high density was in contrast to the low density of chromogranin cells in the rest of the IBS patients studied. Re-examination of these nine patients revealed that they suffered from LC. This unexpected finding was confirmed on a larger patient’s material. As chromogranin A is a common marker for endocrine cells, the present investigation was performed to identify the colonic endocrine cell-types that are affected.
Research frontiers
The current study is a further investigation of the unexpected observation that LC patients have high colonic chromogranin A cell density, which has been shown to be an excellent biomarker for the diagnosis of LC. This unexpected observation led to a novel approach toward LC, where the colonic hormones role in the symptom development in patients and their role in the pathogenesis of the disease come under focus. The current study showed that the endocrine cell-types that are affected in the colon of LC patients were peptide YY (PYY) and serotonin cells. This underlines the importance of theses two hormones in LC.
Innovations and breakthroughs
The present findings underline the importance of the interaction between the gut hormones and the local immune system in the gut (the endocrine/immune axis) and its role in the pathogenesis and symptom development in disease. Such interactions should be put under the spotlight in several gastrointestinal diseases, especially those with known inflammation such as inflammatory bowel disease and coeliac disease. The similarity in the endocrine changes between LC and post-infectious irritable bowel syndrome (PI-IBS) has drawn attention to other histopathological and clinical similarities such as: both LC and PI-IBS show intra-epithelial and submucosal infiltration of lymphocytes and mast cells; both have the same clinical presentation; and both can regress spontaneously. This lead to the notion that LC and PI-IBS may be the same disorder.
Applications
Understanding the interaction between gut hormones and the local immune system of the gut would result in better understanding of the pathogenesis of gut inflammatory diseases and possibly open a new avenue for treatment. If LC and PI-IBS are proven to be the same disorder, PI-IBS can be treated by the same “therapeutic ladder” that has been proven to be effective in LC. PI-IBS constitutes a large subset of IBS without any effective treatment.
Terminology
Chromogranin A: Chromogranin A is a 68-kDa protein comprising 439 amino-acid residues. Chromogranin is co-stored and co-released with monoamines and peptide hormones of the adrenal medulla, pituitary gland, parathyroid, thyroid C-cells, pancreatic islets, endocrine cells of the gastrointestinal tract and sympathetic nerves; PYY: PYY is localised in endocrine cells in the ileum and large intestine; Serotonin: Serotonin is a monoamine that is localised in the enterochromaffin cells throughout the entire gastrointestinal tract. It also occurs also in the enteric nervous system and acts as a hormone and a neurotransmitter.
Peer review
This is an excellent paper which shows new light on lymphocytic colitis. The study was performed to identify the endocrine cell types in the colonic epithelium of LC by immunostaining using representative 5 antibodies against serotonin, PYY, pancreatic polypeptide, somatostatin, and glucagon. The results were that serotonin and PYY cell densities were increased in both the right and left colon of patients with LC, when compared with controls. They concluded that the high density of serotonin cells in LC patients were caused by the interaction between immune cells and serotonin cells, which occurs in the epithelium and submucosa of LC patients, and that the increase in PYY density is secondary to the increase in serotonin cell density.
Peer reviewers: William Dickey, Altnagelvin Hospital, Londonderry, BT47 6SB Northern Ireland, United Kingdom; Mitsunori Yamakawa, Professor, Department of Pathological Diagnostics, Yamagata University, Faculty of Medicine, 2-2-2 Iida-Nishi, Yamagata 990-9585, Japan; Giuseppe Chiarioni, Dr, Gastroenterological Rehabilitation Division of the University of Verona, Valeggio sul Mincio Hospital, Azienda Ospedale di Valeggio s/M, 37067 Valeggio s/M, Italy
S- Editor Lv S L- Editor A E- Editor Li JY