INTRODUCTION
Peliosis hepatis (PH) is a rare benign condition characterized by the presence of multiple, randomly distributed, blood filled cystic areas of variable size within the liver parenchyma[1]. PH has been associated with malignancies, immunosuppression, infections and medications. PH is difficult to recognize, and the diagnosis is often missed or delayed because its imaging findings are often non-specific and the condition may be mistaken for neoplasm, metastases or multiple abscesses. Here, we present a case of focal PH in a colon cancer patient mistaken for liver metastases in the initial diagnosis. A review of the literature on PH in relation to etiology and imaging diagnosis was performed, and the presentation and management of this rare condition are discussed.
CASE REPORT
A 75-year-old female with a previous history of colon cancer treated with radical resection, was admitted to Shanghai East Hospital when a liver mass in the right liver lobe was found 11 mo after surgery during the follow-up period. The pathological diagnosis of colon cancer was moderately differentiated adenocarcinoma. After colon cancer surgery, the patient received a number of chemotherapeutic protocols including capecitabine (Xeloda) and oxaliplatin. She had a history of type 2 diabetes for over 20 years. She took gliclazide for the treatment of diabetes, and glucose was well-controlled. She had no history of viral hepatitis or alcohol abuse.
Laboratory evaluations revealed hemoglobin of 120 g/L, and a normal white cell and platelet count. Prothrombin time, electrolytes, BUN and creatinine were normal. Liver chemistry revealed aspartate aminotransferase 24 IU/L, alanine aminotransaminase 16 IU/L, total bilirubin (TB) 10.6 μmol/L, direct bilirubin (DB) 4.5 μmol/L, alkaline phosphatase 97 IU/L, lactate dehydrogenase 184 IU/L and albumin 44 g/L. Hepatitis B virus examination showed: hepatitis B surface antigen, hepatitis B e antigen, hepatitis B e antibody negative, hepatitis B surface antibody, hepatitis B core antibody positive and HBV DNA < 500 copies/mL. α-fetoprotein was 2.78 ng/mL, carcinoembryonic antigen (CEA) 3.32 ng/mL, CA125 24.50 U/mL, CA153 12.29 U/mL, CA199 52.49 U/mL, and CA724 1.24 U/mL. Thyroid function tests were normal. Human immunodeficiency virus was negative.
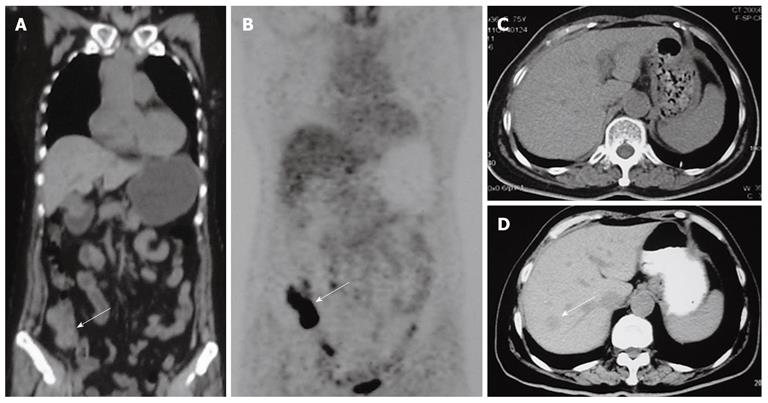
CT and magnetic resonance imaging (MRI) scan of the abdomen were performed. Plain CT scan at the level of the upper abdomen (Figure 1D) showed the presence of one hypodense lesion in the right liver lobe, which was not present on a previous positron emission tomography and computed tomography (PET/CT) or CT scan performed 11 mo previously (Figure 1A-C).
Figure 1 Positron emission tomography plus computed tomography and computed tomography scan findings in our patient.
A: Positron emission tomography plus computed tomography (PET/CT) scan showed thickening of the ileocecal region (white arrow), and no liver lesions; B: PET/CT scan showed increased metabolic areas in the ileocecal region (white arrow), no increased metabolic areas in the liver; C: Six months later a follow-up CT scan showed no liver lesions; D: Eleven months later a follow-up CT scan showed one low-density lesion in the right liver lobe (white arrow).
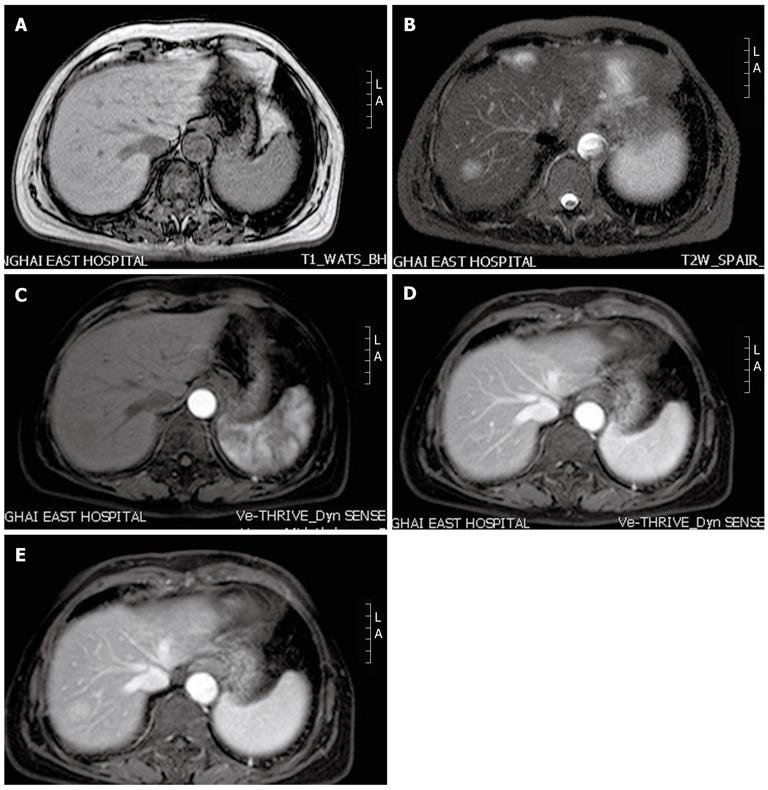
MRI T1-weighted images (Figure 2A) of the upper abdomen showed that the hepatic lesion demonstrated low signal intensity compared with adjacent liver tissue, while on T2-weighted images (Figure 2B) hyperintense signal intensity was observed. Following administration of gadolinium contrast medium (Figure 2C) the mass remained unenhanced. During the portal (Figure 2D), and delayed (Figure 2E) phases, the lesion displayed peripheral enhancement with a centripetal progression.
Figure 2 Magnetic resonance imaging scan findings in our patient.
A: On T1-weighted images, the hepatic lesion shows low signal intensity; B: On T2-weighted images, the lesion shows hyperintense signal intensity; C: In the arterial phase, the lesion remains unenhanced; D, E: During the portal and delayed phases, the lesion displays peripheral enhancement with a centripetal progression. L: Left; A: Ahead.
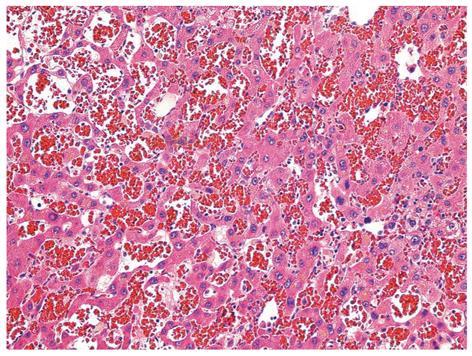
Because the patient had a history of colon cancer, the initial diagnosis was possible metastatic hepatocellular carcinoma. After informed consent was obtained, the patient underwent excision of hepatic segment VII, where the nodule was located. The pathological diagnosis of the surgical specimen was PH (Figure 3). The patient had an uneventful postoperative recovery without complications, and was discharged one week after surgery.
Figure 3 Photomicrograph of a liver section from our case showing variable-sized, blood-filled cystic spaces (hematoxylin and eosin, 200 ×).
DISCUSSION
PH was first reported in the German literature in 1861 by Wagner, and named by Schoenlank in 1916. The first description in the English literature was in 1950 by Zak[2]. Although the exact origins of this disorder are unknown, PH has been associated with the prolonged use of a number of drugs and infectious causes such as Bartonella henselae[3], tuberculosis, acquired immunodeficiency syndrome[4], gummatous syphilis[5] and several debilitating illnesses such as hematological malignancies[6]. In this case, PH was found during the follow-up period after capecitabine and oxaliplatin treatment which suggests that chemotherapy could be responsible for the development of PH. Drugs reported to be associated with PH include thiopurine[7,8], azathioprine[9], 6-thioguanine[10], tamoxifen[11], androgen[12], oral contraceptives, diethylstilbestrol and toxin (e.g., polyvinyl chloride, arsenic, thorium oxide) exposure[13]. To our knowledge, PH associated with capecitabine and oxaliplatin treatment has not been reported.
The clinical presentation and laboratory data in patients with PH are non-specific. Its clinical presentation is variable, ranging from asymptomatic cases discovered at autopsy to progressive cases with cholestasis, liver failure, portal hypertension or even spontaneous rupture[14]. Death may result from hepatic failure or intraperitoneal hemorrhage. PH regresses after drug withdrawal, cessation of steroid therapy, or resolution of an associated infectious disease. Its variable clinical presentation makes the correct diagnosis of PH important, because misdiagnosis could lead to inappropriate treatment in asymptomatic cases, and in advanced cases could lead to progression and a fatal outcome if appropriate treatment is not given. Cohen et al[15] reported a case of PH simulating a hepatic abscess both clinically and radiographically in a patient with sepsis. CT-guided drainage of the presumed liver abscess led to a fatal outcome.
The definitive diagnosis of PH is by histology. A percutaneous needle biopsy can also be used to confirm the diagnosis. However, even when ultrasound-guided, the procedure has a high risk of life-threatening hemorrhage[16]. The imaging appearance of PH is difficult to differentiate from multiple abscesses, adenoma, focal nodular hyperplasia, hemangiomatosis, and metastases. CT findings of PH have been reported to display early globular enhancement centrally with centrifugal progression and eventual homogenous enhancement on the delayed phase[17]. Wannesson et al[18] reported CT findings in a case of PH which displayed peripheral enhancement with a centripetal progression between the arterial and portal phases. Iannaccone et al[13] reported PH lesions with typical centrifugal progression of contrast enhancement, however, centripetal enhancement can also be observed. The enhancement pattern of PH varies depending on the freshness of the blood filling the peliotic cavities. Fresh circulating blood within the peliotic cavities is associated with marked enhancement, whereas retention of old blood is associated with mild or no enhancement[19]. Characteristic MRI findings of PH include T1 hypointense and T2 hyperintense lesions, which show early peripheral and late diffuse contrast enhancement on dynamic imaging. Additionally, several T1 and T2 hyperintense hemorrhagic lesions may be detected due to hemorrhage[20].
There is no specific treatment for PH. Treatment is primarily symptomatic and includes discontinuation of offending medications, partial hepatectomy or transarterial embolization[21] due to liver rupture causing intraabdominal bleeding, or occasionally liver transplantation[22] in patients with irreversible liver insufficiency.
In our case, points supporting metastatic liver cancer were: (1) a new liver lesion on follow-up; (2) a history of colon cancer; and (3) MRI imaging compatible with a metastasis. Points not supporting metastatic liver cancer were: (1) the presence of just one lesion; and (2) normal CEA level. An uncertain diagnosis in this situation led to antitumor therapies.
In conclusion, it is likely that PH is underdiagnosed in radiologic studies. PH should be considered in the differential diagnosis of new liver lesions in patients whose clinical settings do not clearly favor metastasization. Clinicians and radiologists must recognize these lesions to minimize the probability of misdiagnosis and inappropriate treatment.