Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5990
Revised: September 3, 2012
Accepted: September 12, 2012
Published online: November 7, 2012
A 57-year-old man presented with a 2-wk history of painless jaundice and weight loss. He had a large ill-defined enhancing mass-like lesion in the uncinate process of the pancreas with stricture of the distal common bile duct. Aspiration cytology of the pancreatic mass demonstrated inflammatory cells without evidence of malignancy. Total serum immunoglobulin G level was slightly elevated, but IgG4 level was normal. After the 2-wk 40 mg prednisolone trial, the patient’s symptoms and bilirubin level improved significantly. A follow-up computed tomography (CT) scan showed a dramatic resolution of the pancreatic lesion. A low dose steroid was continued. After six months he self-discontinued prednisolone for 3 wk, and was presented with jaundice again. A CT scan showed newly developed intrahepatic biliary dilatation and marked concentric wall thickening of the common hepatic duct and the proximal common bile duct without pancreatic aggravation. The patient’s IgG4 level was elevated to 2.51 g/L. Prednisolone was started again, after which his serum bilirubin level became normal and the thickening of the bile duct was resolved. This case suggests that autoimmune pancreatitis can progress to other organs that are not involved at the initial diagnosis, even with sustained pancreatic remission.
- Citation: Kim JH, Chang JH, Nam SM, Lee MJ, Maeng IH, Park JY, Im YS, Kim TH, Kim CW, Han SW. Newly developed autoimmune cholangitis without relapse of autoimmune pancreatitis after discontinuing prednisolone. World J Gastroenterol 2012; 18(41): 5990-5993
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5990.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5990
Autoimmune pancreatitis (AIP) is a type of chronic pa-ncreatitis characterized by an autoimmune inflammatory process in which prominent lymphocyte infiltration with associated fibrosis of the pancreas leads to organ dysfunction. Steroids are the first choice of therapy in patients with AIP and the response to steroid therapy is usually dramatic[1]. However, 20% to 60% of patients with AIP are found to relapse after the initial course of corticosteroid therapy[2]. The relapse can occur with or without the involvement of other organs such as the bile duct, retroperitoneum, kidneys, salivary or lacrimal gland[3]. However, cholangitis in the proximal extrahepatic bile duct without pancreatic relapse has rarely been reported. Herein we report a case of newly developed autoimmune cholangitis in a patient with AIP with sustained pancreatic remission.
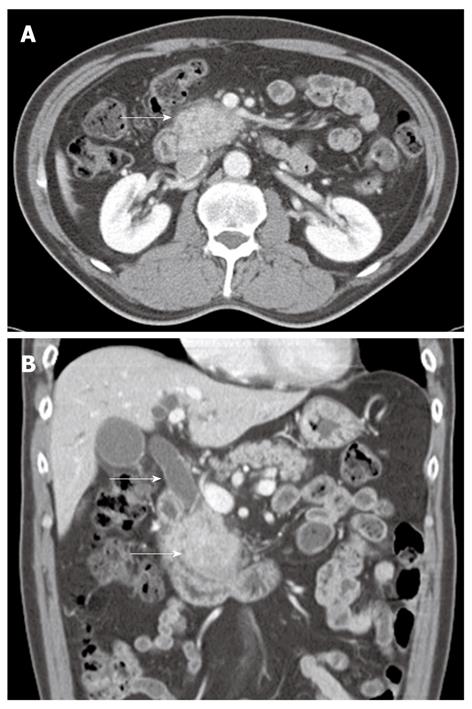
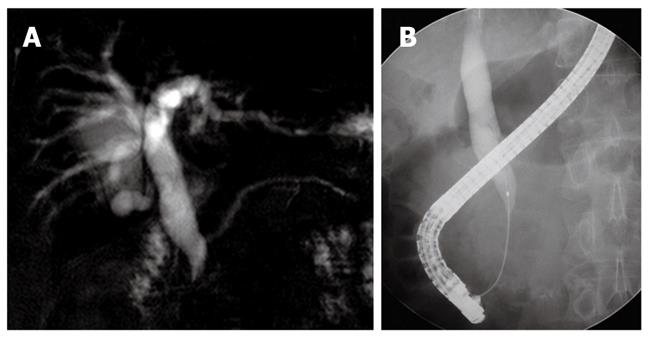
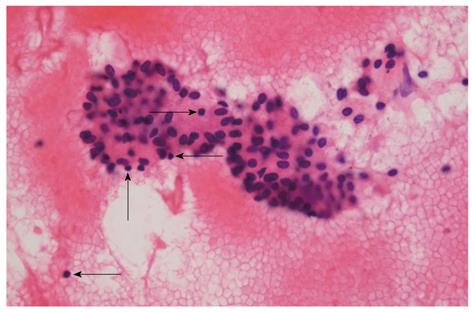
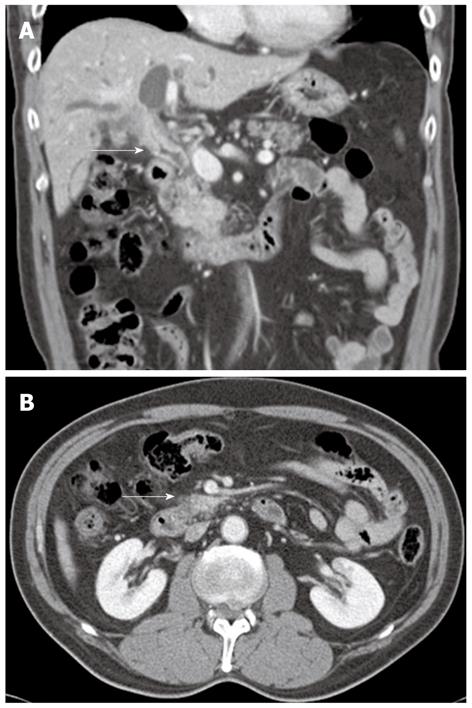
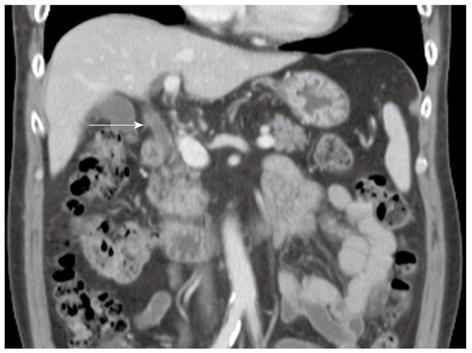
A 57-year-old man presenting with painless jaundice and 2 kg weight loss in 2 wk, was admitted to our hospital. He was an inactive carrier of hepatitis B and his liver function test was within the normal limits at a regular follow up 6 mo ago. He drank socially and had a 30-pack-year history of smoking. On physical examination, he was deeply jaundiced, but other clinical examinations were unremarkable. Laboratory tests revealed white blood cell count of 5850/mm3, aspartate aminotransferase 112 U/L, alanine aminotransferase 230 U/L, alkaline phosphatase (ALP) 326 IU/L, gamma-glutamyl transpeptidase (GGT) 584 U/L, total bilirubin 24.7 mg/dL, amylase 91 U/L and lipase level of 56 IU/L. Abdominal computerized tomography (CT) revealed an ill-defined enhancing mass-like lesion in the uncinate process of the pancreas measuring 5.7 cm × 3.2 cm with regional infiltrations and small amounts of fluid collections around the pancreas head (Figure 1). Magnetic resonance cholangiopancreatography showed moderate dilation of the intrahepatic duct and common bile duct (CBD) but the pancreatic duct was unremarkable (Figure 2A). Endoscopic retrograde cholangiopancreatography revealed a distal biliary stricture (Figure 2B), and brush cytology demonstrated no malignant cells. 7-Fr Amsterdam type inside stent was placed to decompress the biliary system. A subsequent endoscopic ultrasound (EUS) demonstrated a hypoechoic lesion located in the head of the pancreas with blurred delineated margins, which suggested inflammation rather than malignancy. EUS guided fine needle aspiration cytology revealed lymphocytes, neutrophils, and irregular sheets of bland ductal epithelial cells lacking atypia (Figure 3). Serum tumor markers were unremarkable; carbohydrate antigen 19.9 was 20.36 U/mL (normal < 37 U/mL), and carcino-embryonic antigen was 0.8 ng/mL (normal < 7.5 ng/mL). Serum immunoglobulin G level was slightly elevated to 1703 mg/dL (normal < 1600 mg/dL), but serum IgG4 level was 0.41 g/L (normal < 1.21 g/L). In consideration of autoimmune pancreatitis, we started with 40 mg/d prednisolone orally for 2 wk. After that, the patient’s symptoms and bilirubin level improved significantly. A follow-up CT scan showed a dramatic resolution of the pancreatic lesion. The daily dose of prednisolone was gradually tapered and 2.5 mg of prednisolone per day was maintained. The stent in the CBD was removed two months later. He remained well until six months later, when he was readmitted with acute jaundice after self-discontinuation of prednisolone for 3 wk. The total bilirubin level was elevated to 19.2 mg/dL. ALP and GGT were 222 IU/L and 447 IU/L, respectively. IgG4 level was also elevated to 2.51 g/L, which was two times above the upper limit of the normal range. The following CT scan showed a stricture at the proximal extrahepatic bile duct and dilation of the intrahepatic bile duct (Figure 4A). The common hepatic duct and proximal CBD showed marked concentric wall thickening and enhancement. However, there was no pancreatic aggravation (Figure 4B). Newly developed autoimmune cholangitis was strongly suspected. He was treated with 40 mg prednisolone/d for 2 wk, after which serum bilirubin, ALP, and GGT became normal and the following CT scan showed resolution of the thickening of the bile duct (Figure 5). After tapering of the prednisolone, he took 5 mg of prednisolone daily without aggravation of clinical findings.
AIP is a form of chronic pancreatitis of presumed autoimmune etiology[1]. It is frequently presented with obstructive jaundice and pancreatic swelling. AIP presents with lympho-plasmacytic infiltration and fibrosis in the pancreas and shows a dramatic response to steroids[4]. Lympho-plasmacytic infiltrate in AIP shows abundant IgG4-positive plasma cells on immunostaining[5]. Elevation of serum IgG4 is the most remarkable characteristic of this disease. Since AIP can mimic pancreatic cancer, its diagnosis is important to avoid unnecessary surgery. The classic appearance of AIP on abdominal CT is sausage-shaped enlargement of the pancreas with a capsule-like rim. However, AIP can be presented as a pancreatic mass-like lesion, similar to the present case. In such case, pancreatic cancer should be discriminated from AIP. Serum autoimmune markers are helpful for the diagnosis of mass forming AIP, but histological diagnosis is necessary to confirm AIP in many cases[1]. Because histological confirmation of AIP is difficult, ‘‘a 2-wk steroid trial and subsequent assessment of its response’’ is introduced as a diagnostic tool in patients whose clinical and laboratory findings are equivocal for AIP[6]. Our patient showed rapid resolution of the pancreatic mass after treatment with steroid despite the normal IgG4 level. Since serum IgG4 level was elevated afterward, our case was diagnosed as type I AIP by International Consensus Diagnostic Criteria for AIP[4].
AIP can be associated with sclerosing extrapancreatic lesions[7]. Because all tissues involved have characteristic infiltration of IgG4-positive cells, the term “IgG4-associated systemic disease” has been proposed. The most common sites of extrapancreatic involvement in AIP are the bile duct, followed by salivary glands, retroperitoneal fibrosis, orbital pseudotumors, lymphadenopathy, and renal parenchyma[5,7]. Although the stenosis of the CBD in the intrapancreatic area usually occurs with AIP, there are some debates about whether the involvement of only distal CBD should be included in the category of IgG4-associated cholangitis (IAC). Because the narrowing of the intrapancreatic CBD may merely be a secondary phenomenon from an extrinsic compression owing to the pancreatic enlargement of AIP, intrapancreatic CBD involvement is considered as a part of AIP rather than as a IAC[8]. Our patient had a beak shaped intrapancreatic CBD stenosis without proximal extrahepatic or intrahepatic biliary involvement at initial presentation. Therefore, IAC was not combined at first.
AIP responds well to steroids. The general initial recommended dose of oral prednisolone for the induction of remission is 0.6 mg/kg per day for 2-4 wk[9]. Pancreatic size usually normalizes within a few weeks, and biliary drainage becomes unnecessary within about 1 mo. Rapid response to the steroid confirms the diagnosis of AIP[10]. Remission of AIP is defined as the disappearance of clinical symptoms and the resolution of the pancreatic and/or extrapancreatic manifestations in the imaging studies. Our patient had a rapid response to the steroid treatment showing improvements in symptoms, hepatic biochemistry, biliary stricture, and pancreatic lesion.
Relapse of AIP is defined as reappearance of symptoms such as weight loss, jaundice, or abdominal discomfort and elevation of serum IgG4 concentrations with reappearance of pancreatic and/or extrapancreatic abnormalities in the bile duct, salivary gland, or retroperitoneum on imaging studies[3]. The relapse rate after remission of AIP is variable between 20% and 60%[2,10]. Relapse patterns regarding the pancreas or extrapancreatic lesions have not been established and it is not certain that the extrapancreatic involvements at the diagnosis is related to the relapse of AIP. Although initial extrapancreatic involvement was not defined, Kamisawa et al[9] reported that the relapse of AIP occurred in the pancreas (52%), bile duct (34%) and other lesions (n = 19). Moreover, Sandanayake et al[11] reported that all relapsed AIP patients have had extrapancreatic or proximal biliary strictures at the time of diagnosis. When defining relapse, authors generally do not distinguish between relapse of the pancreatic manifestation of IgG4-associated systemic disease, namely AIP, versus occurrence of the disease in another organ, either de novo or true relapse of a previously treated disease in that organ[3]. Newly developed proximal extrahepatic biliary involvement without pancreatic relapse is very rare. One case of hilar and proximal extrahepatic bile duct involvements with sustained pancreatic remission in a diffuse type AIP patient was reported briefly[12,13]. In our case, the focal enlargement type AIP relapsed as the proximal extrahepatic biliary stricture with marked wall thickening and no pancreatic aggravation. The feature of our case is that the serum IgG4 level was normal at initial diagnosis of AIP, but it was markedly elevated with the relapsed autoimmune cholangitis. Although it has been reported that the predictors for relapse of AIP is elevated serum IgG4 levels during remission[10], this case showed that seronegative AIP could relapse as seropositive autoimmune cholangitis, which means progression of the autoimmune disease.
In summary, autoimmune pancreatitis may relapse to other organs as IgG4-associated systemic disease without pancreatic aggravation, even if the organs were not involved and IgG4 level was normal at initial diagnosis. Therefore, clinicians should pay close attention to involvement of other organs during follow up of patients with AIP even with sustained pancreatic remission.
Peer reviewers: Pietro Invernizzi, MD, PhD, Division of Internal Medicine and Hepatobiliary Immunopathology Unit, IRCCS Istituto Clinico Humanitas, Via A Manzoni 113, 20089 Rozzano, Milan, Italy; Yasushi Matsuzaki, Associated Professor, Division of Gastroenterology and Hepatology, Graduate School of Comprehensive Human Sciences and University Hospital, 1-1-1, Tennodai, Tsukuba 305-8575, Japan
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670-2676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 304] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140-148; quiz 140-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 3. | Chari ST, Murray JA. Autoimmune pancreatitis, Part II: the relapse. Gastroenterology. 2008;134:625-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1055] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 5. | Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. Am J Surg Pathol. 2004;28:1114. [PubMed] |
| 6. | Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, Seo DW, Lee SK. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut. 2008;57:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Okamoto A. Autoimmune pancreatitis: proposal of IgG4-related sclerosing disease. J Gastroenterol. 2006;41:613-625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 341] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Park do H, Kim MH. Intrapancreatic common bile duct involvement of autoimmune pancreatitis: is it really IgG4-associated cholangitis? Gastroenterology. 2008;135:324-35; author reply 325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 10. | Kamisawa T, Takuma K, Hara S, Tabata T, Kuruma S, Inaba Y, Gopalakrishna R, Egawa N, Itokawa F, Itoi T. Management strategies for autoimmune pancreatitis. Expert Opin Pharmacother. 2011;12:2149-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Sandanayake NS, Church NI, Chapman MH, Johnson GJ, Dhar DK, Amin Z, Deheragoda MG, Novelli M, Winstanley A, Rodriguez-Justo M. Presentation and management of post-treatment relapse in autoimmune pancreatitis/immunoglobulin G4-associated cholangitis. Clin Gastroenterol Hepatol. 2009;7:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Moon SH, Kim MH, Park do H. Treatment and relapse of autoimmune pancreatitis. Gut Liver. 2008;2:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Park SJ, Kim MH, Moon SH, Han JH, Park do H, Lee SS, Seo DW, Lee SK. [Clinical characteristics, recurrence features, and treatment outcomes of 55 patients with autoimmune pancreatitis]. Korean J Gastroenterol. 2008;52:230-246. [PubMed] |