Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5925
Revised: February 28, 2012
Accepted: March 20, 2012
Published online: November 7, 2012
AIM: To study the effects of live and dead Lactobacillus rhamnosus GG (GG) on rotavirus infection in a neonatal rat model.
METHODS: At the age of 2 d, suckling Lewis rat pups were supplemented with either live or dead GG and the treatment was continued daily throughout the experiment. At the age of 5 and 6 d the pups received oral rotavirus (RV) SA-11 strain. The pups were sacrificed at the age of 7 or 8 d by decapitation. The gastrointestinal tract was removed and macroscopic observations were done. The consistency of feces in the colon was classified using a four-tier system. RV was detected from the plasma, small intestine, colon and feces by real-time quantitative polymerase chain reaction (PCR).
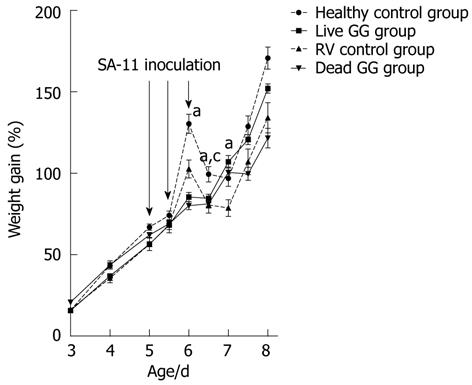
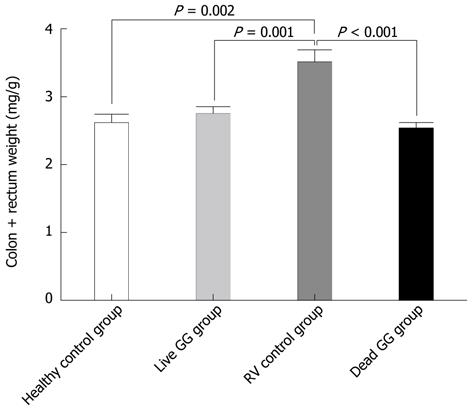
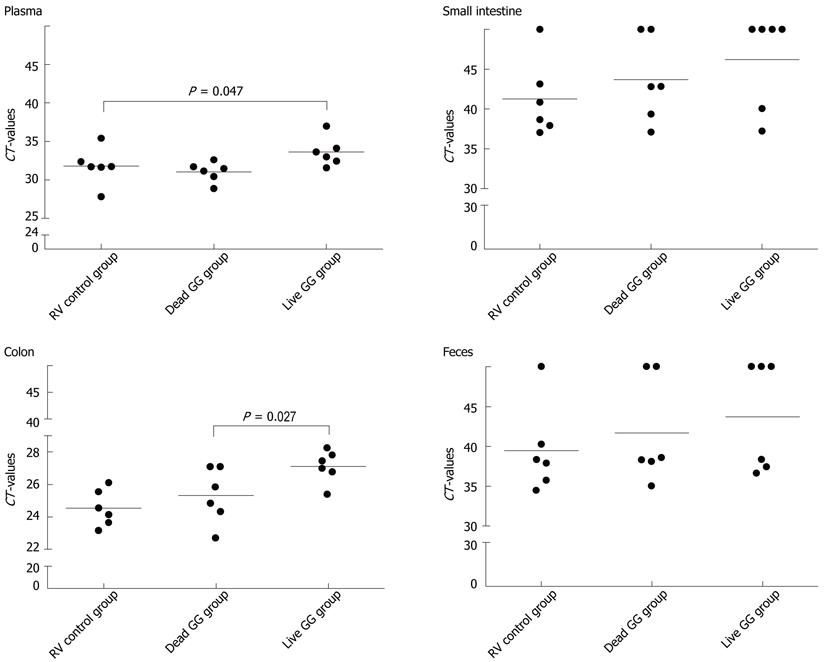
RESULTS: In this neonatal rat model, RV induced a mild-to-moderate diarrhea in all except one pup of the RV-inoculated rats. RV moderately reduced body weight development from day 6 onwards. On day 7, after 2 d of RV infection, live and dead GG groups gained significantly more weight than the RV group without probiotics [36% (P = 0.001) and 28% (P = 0.031), respectively]. In addition, when compared with the RV control group, both live and dead GG reduced the weight ratio of colon/animal body weight to the same level as in the healthy control group, with reductions of 22% (P = 0.002) and 28% (P < 0.001), respectively. Diarrhea increased moderately in both GG groups. However, the diarrhea incidence and severity in the GG groups were not statistically significantly different as compared with the RV control group. Moreover, observed diarrhea did not provoke weight loss or death. The RV control group had the largest amount of RV PCR-positive samples among the RV-infected groups, and the live GG group had the smallest amount. Rats receiving live GG had significantly less RV in the colon (P = 0.027) when compared with the RV control group. Live GG was also more effective over dead GG in reducing the quantity of RV from plasma (P = 0.047).
CONCLUSION: Both live and dead GG have beneficial effects in RV infection. GG may increase RV clearance from the body and reduce colon swelling.
-
Citation: Ventola H, Lehtoranta L, Madetoja M, Simonen-Tikka ML, Maunula L, Roivainen M, Korpela R, Holma R. Effects of the viability of
Lactobacillus rhamnosus GG on rotavirus infection in neonatal rats. World J Gastroenterol 2012; 18(41): 5925-5931 - URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5925
Group A rotaviruses are the leading cause of acute gastroenteritis in children < 2 years of age and account annually for nearly 600 000 child deaths worldwide[1]. Rotavirus-induced diarrhea causes severe dehydration and vomiting which can be fatal for malnourished babies. In developed countries rotavirus gastroenteritis causes a large economic burden with a substantial number of hospitalizations, doctoral visits, and medical costs. Two vaccines are available to prevent RV infection, but their use may be limited by financial constraints especially in low-income countries.
The use of probiotic bacteria has gained considerable attention as a safe and accessible form of treatment for gastrointestinal diseases[2,3]. Lactobacillus rhamnosus GG (GG), in particular, has been effective in reducing both duration[3-6], and severity[6,7] of rotavirus-induced diarrhea. The therapeutic capacity of GG against rotavirus gastroenteritis might be due to its ability to adhere to intestinal epithelial cells and compete for binding with the pathogens[8,9], or displace bound pathogens[10], stabilize and reinforce the mucosal barrier[11-13], and stimulate the local antigen specific and nonspecific immune responses[5,12,14]. However, the effect mechanisms of GG in rotavirus diarrhea are not completely understood.
Only a few studies have addressed the effects of inactivated probiotics in rotavirus diarrhea[5,15]. When studying the effects of inactivated and live GG on acute RV diarrhea in children, both product forms equally promoted clinical recovery from diarrhea, but only live GG enhanced an IgA antibody response to RV[5]. In mice, in contrast to live GG, heat-killed GG failed to protect animals from duration or severity of RV diarrhea[15]. However, these studies did not include an untreated control group to allow comparison between the effects of product forms and RV.
In order to gain more understanding of the effect mechanisms of live and dead GG in RV-induced diarrhea, we compared their effects in the suckling rat RV SA-11 infection model with regard to parameters of infection severity such as weight gain, colon weight, consistency of the feces, and also measured the amount of rotavirus in plasma, intestine, and feces.
This study was approved by the Animal Care and Use Committee of the State Provincial Office of Southern Finland (license number ESAVI-2010-06221_Ym-23). Pregnant-specific pathogen-free Lewis rats were obtained from Harlan (Horst, The Netherlands) and they were allowed to give birth naturally in the test facility. The birth time of the pups was monitored twice a day. All pups were born on the same day within 12 h. Prior to all experiments, each litter was adjusted to 6 pups per dam to avoid biological variation due to litter size. The litters were randomly assigned to 4 experimental groups (4 dams with 6 pups each, n = 24): rats infected with RV SA-11 alone (RV control group); dead GG treated + RV SA-11 infected rats (dead GG group); live GG treated + RV SA-11 infected rats (live GG group); and minimum essential medium (MEM) control animals (healthy control group). The rat pups remained with their dams throughout the study. Control and inoculated groups were housed in the same individually ventilated Scantainer (Scanburg, Denmark), and each RV-infected group in its own Scantainer, in a normal rat cage (Makrolon III) with Aspen chips bedding (Tapvei Oy, Kaavi, Finland) and nest material (Aspen chips PM90L/R). The temperature was 22 ± 2 °C with relative humidity 50%-95%. Lighting was artificial, 12 h light and 12 h dark (18:00-06:00). Food (TEKLAD T.2916 IRR*; Irradiated Global 16%, Rodent Diet for mice and rats, Harlan) and deionized water were autoclaved and provided ad libitum from the day of the rats arrival until the completion of the experiments. The animals were identified individually by dorsal numbering.
Viability was determined by plating the GG preparation after inactivation and no colony forming units (cfu) were detected. Both live and dead GG (ATCC 53103) were obtained from Valio (Valio Ltd, Helsinki, Finland). Prior use live GG stock (1011 cfu/mL) was aliquoted in de Man, Rogosa and Sharpe culture medium broth and stored at -20 °C. Powdered dead GG (killed at Valio Ltd, trade secret) at an equivalent cfu number of 1011 cfu/g of viable GG was maintained at room temperature. For the experiments live GG was thawed and dead GG weighed, and both were prepared daily in PBS at a concentration of 3 × 109cfu/mL.
Simian RV SA-11 strain was grown in a continuous cell line of rhesus monkey kidney cells, MA-104. The cells were cultivated in MEM containing 10% heat-inactivated fetal bovine serum supplemented with 2 mmol L-glutamine, penicillin and streptomycin in roller flasks in roller apparatus at 37 °C. When the cells had a confluency of 70%-80%, they were inoculated from a stock containing 108 pfu/mL of plaque-purified rotaviruses. Before inoculation, RV stock was treated with 10-20 μg/mL (final concentration) of trypsin (Sigma, St Louis, United States) for 30 min at 37 °C. RV stock in dilution 10-4 was added to each roller bottle. After incubation for 1 h, 30 mL of serum-free MEM with 1 μg/mL of trypsin was added, and the cultivation was continued for 48 h at 37 °C. RV were harvested by freeze-thawing of cells for 3 times, cell debris was removed by low-speed centrifugation, supernatant was collected, divided into aliquots, and stored at -70 °C until use. RV titre was determined as 1.4 × 108 pfu/mL.
The pups were weighed at fixed times daily before, and twice a day after, RV infection. At the age of 2 d, pups received a single daily 0.05 mL dose of either dead or live GG supplementation (1.5 × 108 cfu/pup). RV SA-11 was inoculated by plastic feeding tube in 3 separate doses to achieve the total amount of 108 pfu/pup as follows: at the age of 5 d pups received 2 RV doses (0.3 mL each), and the third dose at the age of 6 d (0.12 mL) in order to boost the RV infection. MEM containing 100 × glutamine, penicillin 100 IU/mL, streptomycin 100 μg/mL was used as a healthy control. After inoculations pups were returned to their dams and allowed to suckle.
The pups were randomized to be exsanguinated from either 2 d or 3 d post-infection at the age of 7 d and 8 d. The blood samples were collected from all animals by decapitation into EDTA tubes (Venosafe™), and the plasma was obtained by centrifugation (10 min, 4000 rpm), and frozen at -20 °C within 1 h from sampling. The gastrointestinal tract was removed for macroscopic observations and specimen collection immediately after blood sampling. Small intestine was collected and weighed; colon tissue was collected and removed from its content by gently pushing along the tissue length by a spatula after which it was weighed. The feces were collected by carefully emptying the colon and rectum. Specimens were stored in dried ice until storing them at -80 °C. Consistency of feces was classified from 0-3 using a four-tier system: [0 = normal feces, 1 = slight diarrhea (feces is pale but solid); 2 = moderate diarrhea (feces is pale and semi-solid); 3 = strong diarrhea (feces is clearly wet)].
Plasma samples were thawed and viral RNA was extracted from 0.1 mL of sample with BioSprint® 96 One-For-All Vet-kit (Qiagen GmbH, Hilden, Germany), using the automated KingFisher mL purification system (Thermo Fisher Scientific, Vantaa, Finland) according to the manufacturer’s instructions.
Frozen small intestine, colon, and feces were homogenized for nucleic acid extraction. Feces were processed on ice in 0.2 mL of 10% protease-inhibitor solution containing 1% bovine serum albumin, 10 mmol pefabloc (Roche Applied Science, Mannheim, Germany), 100 μg/mL aprotinin (Sigma-Aldrich, St. Louis, MO, United States) 100 μg/mL leupeptin (Sigma-Aldrich) in Eagle minimum essential medium I (Gibco, Carlsbad, CA) supplemented with 5% fetal calf serum, and 20 mmol Hepes (pH 7.4). Suspensions were vortexed with sterile glass beads, centrifuged (10 min, 5000 rpm), and viral RNA was extracted from supernatants with E.Z.N.A.® Total RNA Kit (Omega Bio-Tek, Doraville, GA, United States) according to manufacturer’s instructions.
Colon and the entire small intestine with its contents were homogenized with sterile glass rods, and 30 mg of homogenized tissue was added into 0.6 mL of RLT buffer (Qiagen) and incubated at 37 °C for 10 min in a water bath. The lysate was centrifuged with QIAshredder (Qiagen) (2 min, 12 000 ×g), and RNA was extracted with RNeasy Mini Kit (Qiagen) or BioSprint® 96 One-For-All Vet-kit (Qiagen) using the automated KingFisher mL purification system as above.
A total of 10 μL of the viral RNA was reverse transcribed into cDNA with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States) in a 20 μL reaction volume according to the manufacturer’s instructions. real-time (RT) reaction was performed as described with few modifications[16]. Depending on the sample material, RNA was first denatured for 5 min at 95 °C, and RT was performed by incubating the reaction mixture for 10 min at 25 °C, 120 min at 37 °C, and 5 min at 85 °C. The quantitative polymerase chain reaction (qPCR) protocol and the primers which target the VP7 gene of RV were designed according to the primers described by Li et al[17]. Briefly, the qPCR reaction was carried out in 25 μL reaction mixtures consisting of 12.5 μL of 2 × SYBR Premix Ex Taq™ (Takara, Dalian, China), 0.5 μL of each primer (20 μmol/L final concentrations), 0.5 μL ROX Ref Dye II (50 ×), 4 μL of RV cDNA template, and 7 μL of sterile water (Sigma-Aldrich). The thermocycling profile included initial denaturation at 95 °C for 30 s, followed by 45 cycles of 95 °C for 5 s, 58 °C for 20 s, and 72 °C for 30 s. Finally, the melting curve analysis was performed at 95 °C for 1 min, 55 °C for 30 s, and 95 °C for 30 s. RNA isolated from cultured RV SA-11 samples was used as a positive control to establish the standard curve, and sterile water (Sigma-Aldrich) as a negative control. The samples were regarded RV SA-11 positive if the melting peak temperature was 83 ± 1.5 °C. The results were analyzed by comparing the cycle threshold (CT) values, which were inversely correlated with the amount of RV VP7 gene in the sample, i.e., the lower the CT value, the greater the amount of gene in sample.
Analysis of variance was applied to compare the groups with respect to weight gain and colon weight, and the results are given as means with standard error of the mean ± SE. In cases of significant global P-values, multiple comparisons were performed and the P-values were Bonferroni corrected. RV diarrhea occurrence and severity between the study groups were analyzed using logistic regression analysis. Statistical differences in the CT-values between RV-infected groups were analyzed using Kruskal-Wallis test (global test) and Mann-Whitney U-test (pair-wise comparisons). P-values < 0.05 were considered statistically significant. The data were analyzed using PASW version 18.0 (SPSS Inc. Chicago, IL, United States).
Weight gain: The pups were weighed 1-2 times daily during the experiment. There were no significant differences in body weight development before the virus inoculation between the study groups. RV moderately reduced body weight development from day 6 onwards (1 d after the infection) when compared with the pups receiving only MEM (Figure 1). RV did not severely compromise the condition of the pups. One pup died from the healthy group due to technical difficulties in dosing. The groups pre-colonized with live or dead GG had gained significantly more weight on day 7 than the RV group without probiotics [36% (P = 0.001) and 28% (P = 0.031), respectively].
Colon weight: Tissue samples were blindly collected and weighed at necropsy. In the large intestine, RV increased the weight of colon. Results between groups were compared by measuring the ratio of colon weight/body weight (Figure 2). When compared with the RV control group, both live and dead GG reduced the weight ratio of the colon to the same level as seen in the healthy control group, with reductions of 22% (P = 0.002) and 28% (P < 0.001), respectively.
Diarrhea: At the necropsy, diarrhea was determined in a blinded fashion by scoring the consistency of feces using the four-tier system from 0-3. RV induced a mild-to-moderate diarrhea in all except one of the RV-inoculated rats when compared with the healthy control group. In live and dead GG groups, diarrhea seemed to be moderately increased. However, the diarrhea incidence or severity in the groups was not statistically significant (P > 0.05) as compared with the RV control group (Table 1).
The number of RV PCR-positive samples in the study groups is shown in Table 2. In the healthy control group, RV was detected from none of the samples. Overall, the RV control group had the largest amount of RV PCR-positive samples among the 3 groups, and live GG group the smallest amount. By comparing the CT values between the groups, we found that rats receiving live GG had significantly less RV VP7 gene in the colon (P = 0.027) when compared with the RV control group (Figure 3). Live GG was also more effective than dead GG in reducing the quantity of RV from plasma (P = 0.047).
| Group | RV PCR-positive samples | |||
| Plasma | Small intestine | Colon | Feces | |
| Healthy control group | 0/5 | 0/5 | 0/5 | 0/5 |
| RV control group | 6/6 | 5/6 | 6/6 | 5/6 |
| Dead GG group | 6/6 | 4/6 | 6/6 | 4/6 |
| Live GG group | 6/6 | 2/6 | 6/6 | 3/6 |
In the present study conducted in a neonatal rat model, we characterized the effects of live and dead probiotic strain Lactobacillus rhamnosus GG on RV-induced diarrhea. We found that both groups receiving GG had smaller amounts of RV in the intestinal tissues and feces over the RV control group. In particular, live GG was effective in reducing the number of RV in the colon. Similar studies exist in mice, where live GG supplementation in combination with antibodies reduced rhesus RV load in the small intestine[15]. This increased RV clearance could be one of the effect mechanisms of GG in RV diarrhea, as it could shorten the duration of disease, as seen in clinical studies[5,18,19]. Since RV is also capable of spreading systemically, and infecting extraintestinal tissues such as liver, kidney, and central nervous system[20-23], the other potentially beneficial effect mechanism of GG against RV diarrhea might be its ability to inhibit the virus entering the blood circulation. Here, live GG appeared to reduce the quantity of RV in plasma.
Similar to other studies, RV SA-11 was effective in inducing diarrhea in the rat pups[24,25]. Interestingly, we found that both live and dead GG seemed to slightly, though not significantly, increase diarrhea. Lactobacillus species in general seem to have an anti-diarrheal effect in clinical and in vivo studies[5,6,26,27]. Especially in neonatal rats, Lactobacillus casei (L. casei) DN-114 001 strain in fermented milk decreased clinical signs of RV SA-11-induced diarrhea, reduced the number of RV antigens from the small intestine 48 h after infection, and reduced RV antigen load in the feces[24]. Our results may be explained by the fact that nitric oxide (NO), which may stimulate the enteric nervous system and induce water secretion into a luminal space further causing diarrhea[28], is released by RV-infected enterocytes[29], and GG also induces NO in macrophages[30]. Enhanced diarrhea in the GG groups may further lead to the increased clearance of RV from the intestinal tissues by inhibiting adherence of RV, and “flushing” the virus from the body. However, we did not include a group receiving only GG, and cannot confirm whether the amount of GG could have an effect on the consistency of feces. On the other hand, another study did not report any changes in the feces in groups receiving probiotic supplements alone[24].
Although the pups suffered from diarrhea, both groups receiving either live or dead GG gained more weight than the RV control group after RV inoculation. However, another probiotic strain, L. casei DN-114 001, failed to induce weight gain in a similar RV SA-11 rat model[24], suggesting that the effect is strain specific. Interestingly, after day 6, the percentage weight gain was also reduced in the uninfected healthy control group. It is possible that a relatively large number of dosings during a short period of time partly inhibited rat pups to suckle milk from their dams.
RV induces inflammation and promotes tissue swelling by activating cytokine response of intestinal epithelial cells[31]. RV-induced tissue swelling could this way increase weight of the colon. Interestingly, we found that both live and dead GG reduced colon weight. In the GG group this reduction may result from the GG’s ability to stimulate the production of anti-inflammatory cytokines[32]. These results further support the idea that GG might shorten the duration, and enhance the recovery from RV diarrhea.
The question of whether unviable and killed bacteria could have similar beneficial effects as live probiotic strains is contradicted. In clinical studies, heat-inactivated GG in children was unable to elicit local or systemic effects in rotavirus diarrhea[5]. In addition, a heat-inactivated probiotic mixture including GG was ineffective against antibiotic-associated diarrhea when compared to equivalent live strains[33]. In children with milk allergy, heat-inactivated GG treatment was associated with diarrhea[34]. However, animal experiments conducted with unviable GG showed that the unviable form has beneficial effects against several inflammatory conditions such as arthritis and Escherichia coli lipopolysaccharide-induced inflammation in the lungs and liver of rats[32,35]. This finding was possibly seen in our study as reduced colon weight. Nevertheless, live GG seemed to be more effective over dead GG in increasing the weight gain of rat pups after RV infection, and was more efficient in reducing the number of RV from plasma. The effects of dead bacteria, however, might depend on the method of inactivation. For instance, inactivation by heat or irradiation might disrupt the surface protein conformation of the bacteria, inhibiting the probiotic’s ability to adhere to epithelial cell[36]. In case the anti-diarrheal effects are due to secreted bioactive or antimicrobial peptides[13,36,37], GG needs to be viable.
In conclusion, only live GG decreased the number of RV in the colon of infected rat pups. However, dead GG had also some potential to alleviate RV infection possibly by reducing tissue swelling. The results provide new insights into aspects of the bacterial strain’s viability, offering new possibilities to develop novel functional food matrices.
Group A rotaviruses are responsible for most cases of gastroenteritis in children under 2 years of age worldwide. Probiotics have gained an important role as adjuvant therapy in the treatment of acute diarrhea. Probiotic strain Lactobacillus rhamnosus GG (GG) in particular is known to reduce the duration of rotavirus-induced diarrhea in young children. However, it is unknown whether the viability of the strain plays a critical role in the probiotic’s beneficial effects on diarrhea.
The potential of unviable/inactivated/killed bacteria to relieve rotavirus (RV) gastroenteritis is not known. In this preliminary study, the authors explored the effects of both live and dead GG in RV infection in a neonatal rat model.
Recent clinical and animal studies have shown that GG relieves RV infection by shortening the duration of diarrhea, and reduces the amount of RV in intestinal tissues. In the present study the authors found only live GG reduced the amount of RV in intestinal tissues. However, the dead product form was found to have a potential to decrease RV diarrhea-induced weight reduction, and inhibit RV-induced colon swelling.
The results of this study indicate that the viable and dead forms of the bacterium have different favorable effects on RV infection. Dead product forms would have a great potential in the food industry by providing new product applications, increasing product shelf life, and reducing storage costs.
Probiotic bacteria are defined as live microorganisms that have beneficial effects on human health. However, data regarding whether dead bacteria could have similar favorable health effects to live probiotic strains is limited.
This study has investigated in experimental animals the effects of probiotics on rotavirus-induced diarrhea using Lactobacillus strain. They have used killed and live Lactobacillus strain in animals in which diarrhea was induced by rotaviruses. The findings are supportive of early observations of similar nature and are of clinical significance.
Peer reviewers: Marco Silano, Department of Human Nutrition and Health, Istituto Superiore di Sanità, Viale Regina Elena 299, 00161 Roma, Italy; Gopal Nath, Professor, Microbiology, Institute of Medical Sciences, BHU, Varanasi 221005, India
S- Editor Gou SX L- Editor Logan S E- Editor Zhang DN
| 1. | Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1282] [Cited by in RCA: 1258] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 2. | Bibiloni R, Simon MA, Albright C, Sartor B, Tannock GW. Analysis of the large bowel microbiota of colitic mice using PCR/DGGE. Lett Appl Microbiol. 2005;41:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Isolauri E, Juntunen M, Rautanen T, Sillanaukee P, Koivula T. A human Lactobacillus strain (Lactobacillus casei sp strain GG) promotes recovery from acute diarrhea in children. Pediatrics. 1991;88:90-97. [PubMed] |
| 4. | Grandy G, Medina M, Soria R, Terán CG, Araya M. Probiotics in the treatment of acute rotavirus diarrhoea. A randomized, double-blind, controlled trial using two different probiotic preparations in Bolivian children. BMC Infect Dis. 2010;10:253. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Kaila M, Isolauri E, Saxelin M, Arvilommi H, Vesikari T. Viable versus inactivated lactobacillus strain GG in acute rotavirus diarrhoea. Arch Dis Child. 1995;72:51-53. [PubMed] |
| 6. | Van Niel CW, Feudtner C, Garrison MM, Christakis DA. Lactobacillus therapy for acute infectious diarrhea in children: a meta-analysis. Pediatrics. 2002;109:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 330] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 7. | Canani RB, Cirillo P, Terrin G, Cesarano L, Spagnuolo MI, De Vincenzo A, Albano F, Passariello A, De Marco G, Manguso F. Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations. BMJ. 2007;335:340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Elo S, Saxelin M, Salminen S. Attachment of Lactobacillus casei strain GG to human colon carcinoma cell line Caco-2: comparison with other dairy strains. Lett Appl Microbiol. 1991;13:154-156. [RCA] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 86] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Kankainen M, Paulin L, Tynkkynen S, von Ossowski I, Reunanen J, Partanen P, Satokari R, Vesterlund S, Hendrickx AP, Lebeer S. Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human- mucus binding protein. Proc Natl Acad Sci USA. 2009;106:17193-17198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 10. | Lee YK, Puong KY, Ouwehand AC, Salminen S. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J Med Microbiol. 2003;52:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int J Food Microbiol. 2010;141 Suppl 1:S91-S97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Schiffrin EJ, Blum S. Interactions between the microbiota and the intestinal mucosa. Eur J Clin Nutr. 2002;56 Suppl 3:S60-S64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 590] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 14. | Zhang L, Xu YQ, Liu HY, Lai T, Ma JL, Wang JF, Zhu YH. Evaluation of Lactobacillus rhamnosus GG using an Escherichia coli K88 model of piglet diarrhoea: Effects on diarrhoea incidence, faecal microflora and immune responses. Vet Microbiol. 2010;141:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Pant N, Marcotte H, Brüssow H, Svensson L, Hammarström L. Effective prophylaxis against rotavirus diarrhea using a combination of Lactobacillus rhamnosus GG and antibodies. BMC Microbiol. 2007;7:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Gutiérrez-Aguirre I, Steyer A, Boben J, Gruden K, Poljsak-Prijatelj M, Ravnikar M. Sensitive detection of multiple rotavirus genotypes with a single reverse transcription-real-time quantitative PCR assay. J Clin Microbiol. 2008;46:2547-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Li D, Gu AZ, Yang W, He M, Hu XH, Shi HC. An integrated cell culture and reverse transcription quantitative PCR assay for detection of infectious rotaviruses in environmental waters. J Microbiol Methods. 2010;82:59-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Guarino A, Canani RB, Spagnuolo MI, Albano F, Di Benedetto L. Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea. J Pediatr Gastroenterol Nutr. 1997;25:516-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 178] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 19. | Majamaa H, Isolauri E, Saxelin M, Vesikari T. Lactic acid bacteria in the treatment of acute rotavirus gastroenteritis. J Pediatr Gastroenterol Nutr. 1995;20:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 296] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 20. | Crawford SE, Patel DG, Cheng E, Berkova Z, Hyser JM, Ciarlet M, Finegold MJ, Conner ME, Estes MK. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J Virol. 2006;80:4820-4832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | de Villiers FP, Steele AD, Driessen M. Central nervous system involvement in neonatal rotavirus infection. Ann Trop Paediatr. 2003;23:309-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Mossel EC, Ramig RF. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J Virol. 2002;76:6502-6509. [PubMed] |
| 23. | Ray P, Fenaux M, Sharma S, Malik J, Subodh S, Bhatnagar S, Greenberg H, Glass RI, Gentsch J, Bhan MK. Quantitative evaluation of rotaviral antigenemia in children with acute rotaviral diarrhea. J Infect Dis. 2006;194:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Guérin-Danan C, Meslin JC, Chambard A, Charpilienne A, Relano P, Bouley C, Cohen J, Andrieux C. Food supplementation with milk fermented by Lactobacillus casei DN-114 001 protects suckling rats from rotavirus-associated diarrhea. J Nutr. 2001;131:111-117. [PubMed] |
| 25. | Pérez-Cano FJ, Castell M, Castellote C, Franch A. Characterization of clinical and immune response in a rotavirus diarrhea model in suckling Lewis rats. Pediatr Res. 2007;62:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Guandalini S, Pensabene L, Zikri MA, Dias JA, Casali LG, Hoekstra H, Kolacek S, Massar K, Micetic-Turk D, Papadopoulou A. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr. 2000;30:54-60. [PubMed] |
| 27. | Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute diarrhoea in children. Aliment Pharmacol Ther. 2007;25:871-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Izzo AA, Mascolo N, Capasso F. Nitric oxide as a modulator of intestinal water and electrolyte transport. Dig Dis Sci. 1998;43:1605-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 29. | Rodríguez-Díaz J, Banasaz M, Istrate C, Buesa J, Lundgren O, Espinoza F, Sundqvist T, Rottenberg M, Svensson L. Role of nitric oxide during rotavirus infection. J Med Virol. 2006;78:979-985. [PubMed] |
| 30. | Korhonen R, Korpela R, Saxelin M, Mäki M, Kankaanranta H, Moilanen E. Induction of nitric oxide synthesis by probiotic Lactobacillus rhamnosus GG in J774 macrophages and human T84 intestinal epithelial cells. Inflammation. 2001;25:223-232. [PubMed] |
| 31. | Rollo EE, Kumar KP, Reich NC, Cohen J, Angel J, Greenberg HB, Sheth R, Anderson J, Oh B, Hempson SJ. The epithelial cell response to rotavirus infection. J Immunol. 1999;163:4442-4452. [PubMed] |
| 32. | Li N, Russell WM, Douglas-escobar M, Hauser N, Lopez M, Neu J. Live and heat-killed Lactobacillus rhamnosus GG: effects on proinflammatory and anti-inflammatory cytokines/chemokines in gastrostomy-fed infant rats. Pediatr Res. 2009;66:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Wenus C, Goll R, Loken EB, Biong AS, Halvorsen DS, Florholmen J. Prevention of antibiotic-associated diarrhoea by a fermented probiotic milk drink. Eur J Clin Nutr. 2008;62:299-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr. 2003;36:223-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Baharav E, Mor F, Halpern M, Weinberger A. Lactobacillus GG bacteria ameliorate arthritis in Lewis rats. J Nutr. 2004;134:1964-1969. [PubMed] |
| 36. | Ouwehand AC, Tölkkö S, Kulmala J, Salminen S, Salminen E. Adhesion of inactivated probiotic strains to intestinal mucus. Lett Appl Microbiol. 2000;31:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Lu R, Fasano S, Madayiputhiya N, Morin NP, Nataro J, Fasano A. Isolation, identification, and characterization of small bioactive peptides from Lactobacillus GG conditional media that exert both anti-Gram-negative and Gram-positive bactericidal activity. J Pediatr Gastroenterol Nutr. 2009;49:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |