Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5897
Revised: August 23, 2012
Accepted: August 25, 2012
Published online: November 7, 2012
AIM: To investigate the characteristics and diagnostic value of annexin A2 (ANXA2) expression in cancerous tissues and sera of patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC).
METHODS: Levels of liver ANXA2 gene transcription or protein expression were analyzed in HCC-, their self-controlled precancerous-, and distant cancerous- tissues from 30 HCC. Serum levels of ANXA2 expression in 115 patients with HCC, 25 with metastatic liver cancer, 35 with chronic hepatitis, 28 with acute hepatitis, 38 with cirrhosis, and 30 healthy controls were determined. Clinicopathological characteristics of circulating ANXA2 expression were analyzed, and its diagnostic efficiency and clinical values in HCC were evaluated.
RESULTS: ANXA2 expression was localized in both cell membrane and cytoplasm in HCC tissue, mainly in the cytoplasm of matched adjacent cancerous tissue, and there was almost no positive staining in matched distant cancerous tissue. Abnormal expression of liver ANXA2 was present in HCC tissues compared with self-controlled adjacent- and distant-cancerous tissues at protein or mRNA level. Circulating ANXA2 in HCC patients was significantly higher than that of other liver diseases (P < 0.01) except metastatic liver cancer. If the diagnostic cutoff value of ANXA2 level was more than 18 ng/mL, the incidence of serum ANXA2 was 86.96% in the HCC group, 80% in the metastatic liver cancer group, 31.58% in the liver cirrhosis group, none in the chronic hepatitis or acute hepatitis or normal control group, respectively. Serum ANXA2 expression in HCC patients was correlated with HBV infection (27.38 ± 5.67 ng/mL vs 18.58 ± 7.83 ng/mL, P < 0.01), extrahepatic metastasis (26.11 ± 5.43 ng/mL vs 22.79 ± 5.64 ng/mL, P < 0.01), and portal vein thrombus (26.03 ± 5.99 ng/mL vs 23.06 ± 5.03 ng/mL, P < 0.01), and was significantly higher (P < 0.01) in the moderately- (26.19 ± 5.34 ng/mL) or the poorly- differentiated group (27.05 ± 5.13 ng/mL) than in the well differentiated group (20.43 ± 4.97 ng/mL), and in the tumor node metastasis stages III-IV (P < 0.01) than in stages I-II. ANXA2 was not correlated with patient sex, age, size or α-fetoprotein (AFP) level. Area under the receiver operating characteristic curve for the whole range of sensitivities and specificities was 0.796 for ANXA2 and 0.782 for AFP. Combining detection of serum ANXA2 and AFP substantially improved the diagnostic efficiency (96.52%) and the negative predictive value (96.61%) for HCC.
CONCLUSION: The characteristics and distribution of ANXA2 expression has good diagnostic potential for HCC diagnosis.
- Citation: Zhang HJ, Yao DF, Yao M, Huang H, Wu W, Yan MJ, Yan XD, Chen J. Expression characteristics and diagnostic value of annexin A2 in hepatocellular carcinoma. World J Gastroenterol 2012; 18(41): 5897-5904
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5897.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5897
Hepatocellular carcinoma (HCC), as one of the most malignant tumors, is the third leading cause of cancer-related death, especially in the inshore area of the Yangtze River[1,2]. Surgical resection is not suitable for a considerable number of HCC cases because of metastasis, and the long-term survival of postoperative HCC patients is not satisfactory[3,4]. Its early detection and treatment is an effective way to improve patient survival. Detection of circulating markers is the most effective method because it is simple, accurate and low cost, but no ideal biomarker has been found so far[5,6]. Recent studies showed that annexin A2 (ANXA2) plays an important role in hepatocyte malignant transformation and HCC development[7-9]. ANXA2, as the best characterized of the Annexin family, is a calcium-dependent phospholipid-binding protein that plays a key role in the regulation of cellular growth and signal transduction pathways[10]. It is reported that ANXA2 expression is upregulated in HCC compared with benign liver disease. Furthermore, its phosphorylation at residues of Tyr23 by c-Src is also increased[11,12], and overexpression and tyrosine phosphorylation of ANXA2 may be of functional relevance.
Evaluation of the diagnostic value of ANXA2 in highly differentiated liver tumors suggests that adding sinusoidal ANXA2 expression to the marker panel [glypican 3, hepatoma-specific gamma-glutamyl transferase (HS-GGT), and heat shock protein 70] increases the reliability and objectivity of HCC diagnosis[13]. In addition, serum ANXA2 levels in HCC patients are elevated by a quantitative sandwich enzyme linked immunosorbent assay (ELISA) method[14]. It may be a serological marker for HCC to enable early diagnosis, as well as monitoring of aggressiveness, treatment responsiveness, recurrence and survival. However, the clinicopathologic characteristics of hepatic ANXA2 expression and the evaluation of its diagnostic value for hepatitis B virus (HBV)-related HCC have not been reported up to now. In this study, the expression of hepatic and circulating ANXA2 was investigated in HCC patients and compared with expression in benign liver diseases to evaluate the pathologic characteristics and efficiency in HCC diagnosis.
We evaluated 115 HCC patients (88 men and 27 women) who were treated at the Affiliated Hospital of Nantong University, Nantong, China. Patient age ranged from 25 to 81 years (median, 48.3 years). Other cases studied included 35 with chronic hepatitis, 28 with acute hepatitis, 38 with cirrhosis, and 25 with metastatic liver cancer (liver metastasis of lung cancer, 6; gastric cancer, 6; acute myeloid leukemia, 3; breast cancer, 3; colorectal cancer, 3; cervical cancer, 2; and pancreatic cancer, 2) and samples from 30 healthy people with hepatitis viral markers [HBV-DNA, HBV surface antigen, and anti-hepatitis C virus (HCV)] and a normal alanine aminotransferase level obtained from the Nantong Central Blood Bank as controls. All cases were diagnosed by biochemical tests, viral histology, and B-ultrasonic examination. Blood samples (5 mL) were collected with heparin in the morning and sera separated immediately. α-fetoprotein (AFP) level was detected by a radiological method[15].
The cancerous-, the self-matched adjacent cancerous- (more than 3 cm to cancer focus), and the distant cancerous- (more than 5 cm) specimens after surgical operation were respectively taken from 30 HCC patients who were treated at the Affiliated Hospital of Nantong University, Nantong, China. One portion of each specimen was immediately frozen in liquid nitrogen for total RNA extraction [ANXA2 mRNA by real time quantitative polymerase chain reaction (qPCR)], an extract was used to determine liver ANXA2 by Western blotting, and the remaining sample was fixed with 10% (vol/vol) formalin for ANXA2 immunohistochemistry. The diagnosis of HCC and viral hepatitis was based on the criteria proposed by the Chinese National Collaborative Cancer Research Group[16] and at the Chinese National Viral Hepatitis Meeting[17], respectively. Prior written informed consent was obtained from all patients according to the World Medical Association Declaration of Helsinki, and the study received ethics board approval from the Affiliated Hospital of Nantong University, Jiangsu Province, China.
The level of serum ANXA2 was detected by using a human ANXA2 ELISA kit (Uscn Life Science Inc., Wuhan, China) according to the manufacturer’s instructions. To each well was added 100 μL of serum sample or standard separately, and then 100 μL of detection reagent A was added and incubated for 1 h at 37 °C. Subsequently, 100 μL of detection reagent B was added and incubated for 0.5 h at 37 °C. Then, 90 μL of substrate solution was added and incubated for 25 min at 37 °C. Finally, 50 μL of stop solution was added to each well, and absorbance was read at 450 nm. During the procedure, washing the plate was according to the ELISA routine method.
Total RNA was isolated from 50 mg of liver tissue, using Trizol reagent (Invitrogen, United States) according to the manufacturer’s instructions. The integrity of the total RNA was examined by 1% agarose gel electrophoresis, the quantity was determined based on absorbance at 260 nm (A260), and the purity was analyzed based on the absorbance ratio at 260 nm and 280 nm (A260/280) (Bio-RAD smartspecTM plus, United States). The ANXA2 cDNA was synthesized from 1 μg of total RNA using First Strand cDNA Synthesis Kit (Fermentas, Canada) according to the manufacturer’s instructions.
The qPCR was run on an Applied Biosystems StepOneTM real-time PCR system according to the manufacturer’s recommendations. The reaction solution contained 25 μL 2 × SYBR Premix Ex Taq (TaKaRa, Japan), 2 μL primer mix, 1 μL 50 × ROX Reference Dye I, 4 μL cDNA, and 18 μL deionized water to make a total volume of 50 μL. ANXA2 primers were as follows: forward, 5’-TGAGCGGGATGCTTTGAAC-3’, and; reverse, 5’-ATCCTGTCTC TGTGCATTGCTG-3’; β-actin primers were as follows: forward, 5’-ATTGCC GACAGGATGCAGA-3’, and reverse, 5’-GAGTACTTGCGCTCAGGAGGA-3’ used as an internal control[18], while no template control (H2O) was included in each reaction run. The optimized PCR conditions were as follows: 1 cycle at 95 °C for 2 min; 40 cycles at 95 °C for 10 s, 62 °C for 1 min and final extension at 60 °C for 15 s. The relative quantitative analysis was performed by comparison of the 2-∆∆Ct values.
Liver tissues were homogenized in an ice-cold homogenization buffer containing 50 mmol/L 3-(N-Morpholino) propanesulfonic acid buffer (pH 7.4), 100 mmol/L KCl, 320 mmol/L sucrose, 50 mmol/L NaF, 0.5 mmol/L MgCl2, 0.2 mmol/L dithiothreitol, 1 mmol/L EDTA, 1 mmol/L EGTA, 1 mmol/L Na3VO4, 20 mmol/L sodium pyrophosphate, 20 mmol/L β-phosphoglycerol, 1 mmol/L p-nitrophenyl phosphate, 1 mmol/L benzamidine, 1 mmol/L phenylmethylsulfonyl fluoride, and 5 μg/mL each of leupeptin, aprotinin, and pepstatin A. The homogenates were centrifuged at 800 g for 10 min at 4 °C. The supernatants were collected, and total protein concentrations were determined by an enhanced bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology, China). A total of 20 mg of protein of each sample was run on a 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were then transferred onto polyvinylidene fluoride membranes and blocked with 5% bovine serum albumin in tris-buffered saline, pH 7.5 (100 mmol/L NaCl, 50 mmol/L Tris, and 0.1% Tween-20). Membranes were immunoblotted overnight at 4˚C with the anti-ANXA2 and anti-β-actin antibodies (Santa Cruz Biotechnology, United States), followed by respective horseradish peroxidase-conjugated secondary antibodies. The bands were subsequently visualized by a chemiluminescence detection system (Millipore, United States), and density analysis was performed by an image analyzer. The ANXA2 level was expressed with the relative ratio (RR), which was calculated by the following formula using signal intensity (SI) of ANXA2 and β-actin. RR = SI ANXA2/SIβ-actin.
The 3 μm thick sections were prepared from formalin-fixed, paraffin-embedded tissue blocks. Sections were deparaffinized in xylene twice for 10 min, then dehydrated through graded ethanol to distilled water for 5 min. Deparaffinized 5 µm thick liver sections were washed three times with phosphate buffered solution (PBS) (pH 7.4), incubated in endogenous peroxidase blocking solution (Immunostain EliVision Kit, Maxim Biotech, United States), and then treated with 0.01 mol/L citrate buffer pH 6.0 for 10 min in a microwave oven at 650 W. Non-specific-antibody binding was blocked by pretreatment with PBS containing 0.5% bovine serum albumin (fraction V powder, Sigma, United States). Sections were then rinsed in PBS and incubated overnight at 4 °C with diluted anti-human ANXA2 antibody (1:500, Santa Cruz Biotechnology, United States) followed by three washes in PBS containing 0.05% Tween-20. The steps were performed using Immunostain EliVision kit according to the manufacturer’s instructions. Sections were stained with 3,3’-diamino-benzidine tetrahydrochloride as a chromogen. The slide was rinsed with distilled water, counterstained with hematoxylin, dehydrated, air dried, and mounted. The negative control slides were treated with nonspecific mouse IgG. The sections were examined under light microscopy. ANXA2 staining was assessed using the immunoreactive score. In detail, the percentage of positive cells was assessed semiquantitatively and classified as follows: diffuse positive staining (+++) of more than 50% of total cells; moderate staining (++), 16%-50%; weak staining (+), 5%-15%; and negative staining (-), < 5%[19]. The results of staining were evaluated by two independent pathologists without knowledge of the clinicopathologic features, and any difference in interpretation was resolved by consensus. Duplicate tissue cores for each tumor showed high levels of homogeneity for staining intensity and percentage of positive cells. The higher score was taken as the final score in cases of a difference between duplicate tissue cores.
The data are expressed as mean ± SD. Differences between different groups were evaluated by using a Student t test, a χ2 test or a rank-sum test. P < 0.05 was considered significant. Receiver operating characteristic (ROC) curves were constructed by calculating the sensitivities and specificities at several cutoff points[15]. Efficiency evaluation was calculated according to sensitivity, specificity, accuracy, positive predictive value, and negative predictive value.
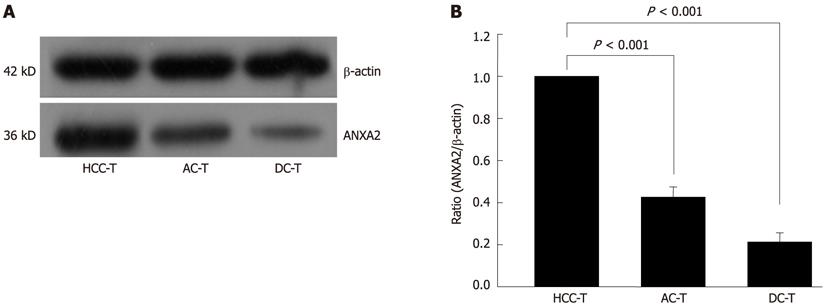
ANXA2 protein and mRNA levels were detected in 30 self-controlled HCC tissues, and their matched adjacent- and distant-cancerous specimens by Western blotting and real-time PCR, respectively. As shown in Figure 1, the ANXA2 protein level was obviously higher in HCC tissues than in the self-controlled adjacent- and distant-cancerous specimens (F = 498.221, P < 0.001). The relative qPCR analysis (Table 1) indicated that the level of ANXA2 mRNA expression in the HCC tissues (2-∆∆Ct = 1.00) was significantly higher (F = 7908.11, P < 0.001) than in the matched adjacent cancerous tissues (2-∆∆Ct = 0.43 ± 0.10) or the distant cancerous tissues (2-∆∆Ct = 0.23 ± 0.07). In short, ANXA2 was overexpressed in HCC tissues compared with the self-controlled adjacent- and distant-cancerous tissues, whether protein level or mRNA level.
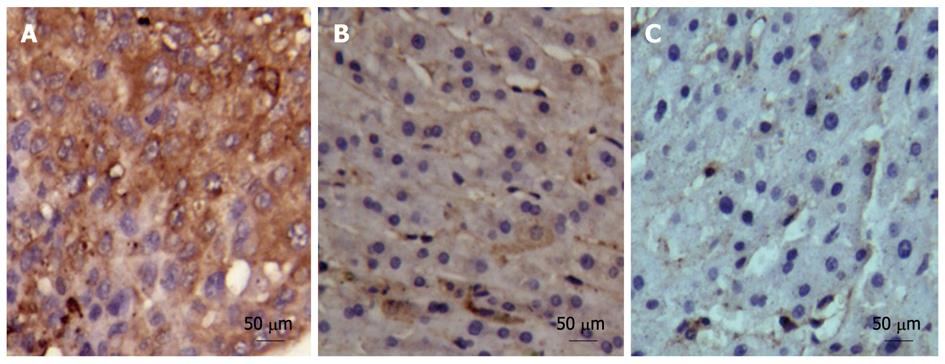
The expression and distribution of hepatic ANXA2 in 30 self-controlled HCC tissues, their matched adjacent- and distant-cancerous specimens are shown in Figure 2. The positive ANXA2 protein was localized in both cell membrane and cytoplasm (Figure 2A) in HCC tissue (30 of 30, 100%), mainly in the cytoplasm (Figure 2B) in matched adjacent cancerous tissue (27 of 30, 90%), and there was almost no positive staining (Figure 2C) in their matched distant cancerous tissue (0 of 30, 0%). The intensity and comparative analysis of ANXA2 expression in different liver tissues are shown in Table 1. Although no significant difference in the positive rate of ANXA2 expression (χ2 = 3.518, P = 0.070) was found between the HCC group and the adjacent cancerous group, the intensity of ANXA2 expression in the HCC group was significantly higher than that in the adjacent cancerous group (Z = 6.113, P < 0.001) or the distant cancerous group (Z = 7.328, P < 0.001).
The levels of circulating ANXA2 and AFP expression in 241 patients with liver diseases are shown in Table 2. The mean level of serum ANXA2 expression in HCC patients was significantly higher than in the cases with liver cirrhosis, chronic hepatitis, or acute hepatitis, or in control subjects (P < 0.001), but not compared with the metastatic liver cancer group. If the diagnostic cutoff value was more than 18 ng/mL, the incidence of circulating ANXA2 was 86.96% in the HCC group, 80% in the metastatic liver cancer group, 31.58% in the liver cirrhosis, and zero in the chronic hepatitis group, the acute hepatitis group or the normal control group. The incidence of serum AFP in the HCC patients (81 of 115, 70.44%) was significantly higher than in the metastatic liver cancer group (12 of 25, 48%, P < 0.05), the liver cirrhosis group (6 of 38, 15.79%), the chronic hepatitis group (5 of 35, 14.29%), the acute hepatitis group (4 of 28, 14.29%), and was zero in the normal control group.
| Group | n | (ng/mL) | q | P value | Pos. (%) | χ2 | P value |
| ANXA2 | |||||||
| HCC | 115 | 24.60 ± 7.60 | 100 (86.96)1 | ||||
| MLC | 25 | 24.22 ± 9.153 | 0.482 | 0.803 | 20 (80.00)3 | 0.812 | 0.368 |
| LC | 38 | 16.35 ± 8.863 | 11.621 | < 0.001 | 12 (31.58)3 | 44.652 | < 0.001 |
| CH | 35 | 6.85 ± 1.563 | 22.566 | < 0.001 | 0 (0.00)3 | 91.304 | < 0.001 |
| AH | 28 | 6.92 ± 1.413 | 20.948 | < 0.001 | 0 (0.00)3 | 80.971 | < 0.001 |
| NC | 30 | 6.16 ± 1.273 | 22.757 | < 0.001 | 0 (0.00)3 | 84.058 | < 0.001 |
| AFP | |||||||
| HCC | 115 | 1446.76 ± 1573.46 | 81 (70.44)2 | ||||
| MLC | 25 | 1241.76 ± 1349.143 | 1.087 | 0.442 | 12 (48.00)3 | 4.635 | 0.031 |
| LC | 38 | 73.73 ± 168.033 | 7.969 | < 0.001 | 6 (15.79)3 | 34.771 | < 0.001 |
| CH | 35 | 69.05 ± 106.733 | 6.761 | < 0.001 | 5 (14.29)3 | 34.583 | < 0.001 |
| AH | 28 | 70.54 ± 107.113 | 7.306 | < 0.001 | 4 (14.29)3 | 29.446 | < 0.001 |
| NC | 30 | 6.06 ± 1.633 | 7.506 | < 0.001 | 0 (0.00)3 | 47.874 | < 0.001 |
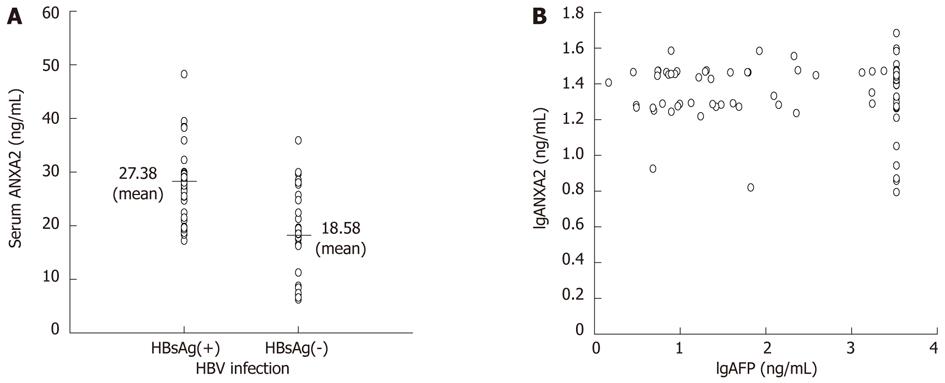
The clinicopathologic features of circulating ANXA2 expression in 115 HCC patients are shown in Table 3. The higher level of serum ANXA2 expression was correlated with HCC patients with HBV infection (Figure 3A, t = 6.820, P < 0.001), extrahepatic metastasis (t = 3.191, P = 0.002), or portal vein thrombus (t = 2.859, P= 0.005). Serum ANXA2 expression was graded, with 20.43 ± 4.97 ng/mL in the well differentiated group and significantly lower (P < 0.001) in the moderately- (26.19 ± 5.34 ng/mL) or the poorly- differentiated (27.05 ± 5.13 ng/mL) group, and levels of ANXA2 expression were obviously higher (P < 0.001) in tumor node metastasis (TNM) stage III or IV than in TNM stage I or II. ANXA2 expression was not correlated with patient sex, age, tumor size or serum AFP level (Figure 3B). Both circulating ANXA2 and AFP are useful biomarkers for HCC diagnosis.
| Group | n | (ng/mL) | t | P value | Pos. (%) > 18 ng/mL | χ2 | P value | |
| Sex | Male | 88 | 24.56 ± 5.84 | 0.150 | 0.881 | 78 (88.64) | 0.933 | 0.334 |
| Female | 27 | 24.79 ± 5.33 | 22 (81.48) | |||||
| Age (yr) | ≥ 50 | 87 | 24.84 ± 6.07 | 0.798 | 0.427 | 76 (87.36) | 0.050 | 0.822 |
| < 50 | 28 | 23.83 ± 4.56 | 24 (85.71) | |||||
| Tumor size (cm) | ≥ 5.0 | 75 | 24.38 ± 5.68 | 0.850 | 0.397 | 66 (88.00) | 0.207 | 0.649 |
| < 5.0 | 40 | 25.35 ± 5.95 | 34 (85.00) | |||||
| α-fetoprotein (ng/mL) | ≥ 400 | 53 | 24.91 ± 5.52 | 0.531 | 0.596 | 45 (84.91) | 0.365 | 0.546 |
| < 400 | 62 | 24.34 ± 5.96 | 55 (88.71) | |||||
| HBsAg | Positive | 79 | 27.38 ± 5.67 | 6.820 | < 0.001 | 73 (92.41) | 6.605 | 0.010 |
| Negative | 36 | 18.58 ± 7.83 | 27 (75.00) | |||||
| Differentiated grading | Well | 37 | 20.43 ± 4.97 | 25 (67.57) | ||||
| Moderate | 43 | 26.19 ± 5.34 | 4.9661 | < 0.0011 | 41 (95.35) | 10.6311 | 0.0011 | |
| Poor | 35 | 27.05 ± 5.13 | 5.5611 | < 0.0011 | 34 (97.14) | 10.6331 | 0.0011 | |
| TNM staging | Stages I-II | 52 | 21.16 ± 5.97 | 38 (71.43) | ||||
| Stages III-IV | 63 | 27.44 ± 6.01 | 5.5942 | < 0.0012 | 62 (97.06) | 16.1222 | < 0.0012 | |
| Extrahepatic metastasis | With | 62 | 26.11 ± 5.43 | 3.191 | 0.002 | 58 (93.55) | 5.514 | 0.023 |
| Without | 53 | 22.79 ± 5.64 | 42 (79.25) | |||||
| Portal vein thrombus | With | 60 | 26.03 ± 5.99 | 2.859 | 0.005 | 56 (93.33) | 4.498 | 0.034 |
| Without | 55 | 23.06 ± 5.03 | 44 (80.00) |
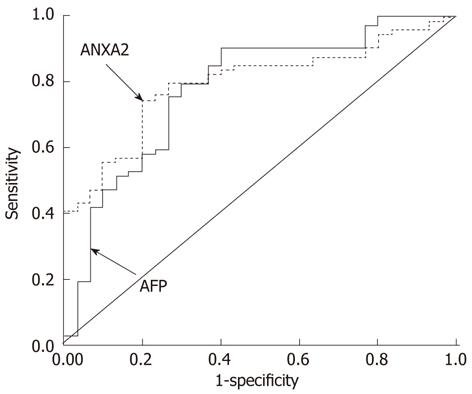
The evaluation of serum ANXA2 and AFP levels for HCC diagnosis is shown in Figure 4. The comparative analysis of two markers for the whole range of sensitivities and specificities was 0.796 in ANXA2 and 0.782 in AFP according to under the area under the ROC curve. The clinical evaluation of serum ANXA2 or/and AFP levels for HCC diagnosis is shown in Table 4. The sensitivity of serum ANXA2 only was 86.96%, while a combination of ANXA2 with AFP could increase the rate of HCC diagnosis (96.52%), and the negative predictive value was improved to 96.61%.
| Project of evaluation | ANXA2 (%) | AFP (%) | Both (%) |
| Sensitivity | 86.96 | 70.43 | 96.52 |
| Specificity | 66.67 | 73.08 | 68.67 |
| Accuracy | 75.28 | 71.96 | 80.07 |
| Positive predictive value | 65.79 | 65.85 | 68.10 |
| Negative predictive value | 87.39 | 77.03 | 96.61 |
HCC prognosis is poor, and early detection is of the utmost importance. Although serum AFP is a useful biomarker for the detection and monitoring of HCC, the false-negative rate using the AFP level alone may be as high as 40% for HCC patients with small size tumors. A previous report implied that ANXA2 expression was upregulated in HCC and it could be a useful molecular marker for HCC[10-12]. In this study, the expression of hepatic and circulating ANXA2 was investigated in HCC patients and compared with that in cases of benign liver diseases to explore the clinicopathological characteristics and diagnostic value in HCC.
The expression of hepatic ANXA2 was associated with hepatocyte malignant transformation. Hepatic ANXA2 was overexpressed in HCC tissues compared with their matched adjacent- and distant tissue, not only at protein level (Figure 1) but also at mRNA level (Table 1). Although no significant difference in the positive rate of ANXA2 expression was found between the HCC group and the adjacent cancerous group, the intensity of ANXA2 expression in the HCC group was significantly higher than in the adjacent cancerous group or the distant cancerous group (Table 1). There was a consistent overexpression of ANXA2 protein level and ANXA2 mRNA level. It is reported that adding ANXA2 to the established marker panel for the detection of early and well-differentiated HCC should increase the diagnostic reliability and objectivity, which may particularly improve the accuracy of HCC diagnosis in minute tissue samples[13]. It is worth mentioning that ANXA2 levels in HCC adjacent tissue present a very good intermediate state at protein or mRNA level, which might result from the change in the tumor microenvironment and the transfer of tumor cells, because a previous report suggested that ANXA2 binds with plasminogen and tissue plasminogen activator on the cell surface and promotes tumor metastasis by inducing the conversion of plasminogen to plasmin, which leads to activation of matrix metalloproteinases and degradation of extra-cellular matrix components[7-9].
Although the mechanisms of hepatocarcinogenesis have not been elucidated, a long-lasting inflammation induced by hepatitis virus infection is a definite risk for neoplastic degeneration and the accumulation of genetic alterations. The diagnosis and monitoring of small size tumors have been always difficult due to the lack of effective biomarkers that can characterize the formation and progression of HCC development[20,21]. The ability of several biomarkers, such as lens cularis agglutinin-reactive AFP-L3[22], HS-GGT, and glypican-3[23], to detect early HCC has been examined, but the sensitivity and specificity are still not satisfactory. In this study, the circulating ANXA2 level was investigated in HCC patients and cases with benign liver diseases (Table 1). If the cutoff value of ANXA2 abnormality is more than 18 ng/mL, its incidence in patients with HCC (86.96%) or metastatic liver cancer (80%) was significantly higher than in cases with liver cirrhosis (31.58%), chronic hepatitis (0%), acute hepatitis (0%), or controls (0%). The value of ANXA2 in patients with cirrhosis is intermediate, and it may be considered as an early marker during malignant transformation of liver cells. Serum AFP is a useful serological marker for HCC diagnosis; however, a high false negative rate has been found in patients with benign liver diseases, and our study suggests that serum ANXA2 is superior to AFP and a relatively distinct marker for HCC diagnosis.
The ANXA2 gene is upregulated in HBV- and/or HCV-associated HCC[24]. ANXA2 induces cell migration and neoangiogenesis via tissue plasminogen activator-dependent plasmin generation[25], represents metastatic potential[18], and promotes invasion and migration of HCC in vitro via its interaction with HAb18G/CD147[7]. Moreover, Tyr23 phosphorylation-dependent cell-surface localization of ANXA2 is required for invasion and metastases[12]. The clinicopathologic features of circulating ANXA2 expression in HCC patients (Table 3) demonstrated that there is a very close relationship between ANXA2 level and invasion and metastasis, as well as HBV infection. The higher level of ANXA2 expression in HCC patients was correlated with HBV infection (Figure 3A), extra-hepatic metastasis, portal vein thrombus, differentiated grading, and TNM staging. However, no significant correlation was found between serum ANXA2 level and tumor size, or AFP level (Figure 3B). It deserves to be mentioned that there was no significant difference between the moderately differentiated group and the poorly differentiated group, or TNM staging III-IV. The efficiency evaluation of serum ANXA2 or/and AFP level for HCC diagnosis (Table 4, Figure 4) indicated that serum ANXA2 detection has higher sensitivity, accuracy, negative predictive value and complementary diagnostic value in combination with AFP for HCC diagnosis.
In conclusion, hepatic ANXA2 expression is associated with hepatocyte malignant transformation and plays an important role in hepatic active metabolism, development, microenvironment, and prognosis of HCC. The higher intensity of ANXA2 expression in HCC tissues and circulating ANXA2 was correlated with HBV infection, extrahepatic metastasis and portal vein thrombus. Therefore, it could be developed as an effective diagnostic marker for HCC by a series of further independent and prospective studies, and become a research hotspot to reveal the mechanism of metastasis resulting from ANXA2 in the near future.
Hepatocellular carcinoma (HCC), as one of the most malignant tumors, is the third leading cause of cancer-related death. The development of biomarkers for early diagnosis and accurate prognosis of HCC is important for improving patients’ survival. Annexin A2 (ANXA2) could be used as a new marker for HCC in the future.
It is reported that ANXA2 expression and its phosphorylation is upregulated in HCC compared with benign liver disease. Moreover, the dose-response relationship between ANXA2 and optical density was linear in the range of 0-10 μg/mL. However, little research on the about expression characteristics and diagnostic value of ANXA2 in HCC have been reported to date. In this study, the authors analyzed the expression characteristics and specific distribution of ANXA2 as well as its diagnostic value in HCC.
This is the first report on the expression characteristics and specific distribution of ANXA2 as well as its diagnostic value in HCC. ANXA2 overexpression in HCC patients was correlated with hepatitis B virus infection, extrahepatic metastasis, portal vein thrombus, differentiated grading and tumor node metastasis staging, but not with patient sex, age, size or α-fetoprotein (AFP) level. Joint diagnosis using serum ANXA2 and AFP substantially improved the diagnostic efficiency.
The expression characteristics and specific distribution of ANXA2 have good diagnostic potential for HCC, and could be developed into an effective diagnostic marker for HCC by a series of further independent and prospective studies.
ANXA2 as a member of the Annexin family is a calcium-dependent phospholipid-binding protein and is involved in the regulation of cellular growth and signal transduction pathways. Its expression is upregulated in HCC with increased molecular phosphorylation at residues of Tyr23 by c-Src.
The evaluated manuscript reports ANXA2 as biomarker of HCC. The study has been well conducted and provide further validation.
Peer reviewer: Fernando J Corrales, Associate Professor of Biochemistry, Division of Hepatology and Gene Therapy, Proteomics Laboratory, CIMA, University of Navarra, Avd. Pío XII, 55, 31008 Pamplona, Spain
S- Editor Gou SX L- Editor Cant MR E- Editor Zhang DN
| 1. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 2. | DuBray BJ, Chapman WC, Anderson CD. Hepatocellular carcinoma: a review of the surgical approaches to management. Mo Med. 2011;108:195-198. [PubMed] |
| 3. | Petruzelka L. [Targeted biological treatment of solid tumours]. Vnitr Lek. 2011;57:740-744. [PubMed] |
| 4. | Portolani N, Baiocchi GL, Coniglio A, Tiberio GA, Prestini K, Gheza F, Benetti A, Maria Giulini S. Limited liver resection: a good indication for the treatment of hepatocellular carcinoma in elderly patients. Jpn J Clin Oncol. 2011;41:1358-1365. [PubMed] |
| 5. | Gonzalez SA, Keeffe EB. Diagnosis of hepatocellular carcinoma: role of tumor markers and liver biopsy. Clin Liver Dis. 2011;15:297-306, vii-x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | van Malenstein H, van Pelt J, Verslype C. Molecular classification of hepatocellular carcinoma anno 2011. Eur J Cancer. 2011;47:1789-1797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Zhao P, Zhang W, Tang J, Ma XK, Dai JY, Li Y, Jiang JL, Zhang SH, Chen ZN. Annexin II promotes invasion and migration of human hepatocellular carcinoma cells in vitro via its interaction with HAb18G/CD147. Cancer Sci. 2010;101:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Lokman NA, Ween MP, Oehler MK, Ricciardelli C. The role of annexin A2 in tumorigenesis and cancer progression. Cancer Microenviron. 2011;4:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Madureira PA, Surette AP, Phipps KD, Taboski MA, Miller VA, Waisman DM. The role of the annexin A2 heterotetramer in vascular fibrinolysis. Blood. 2011;118:4789-4797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331-371. [PubMed] |
| 11. | Mohammad HS, Kurokohchi K, Yoneyama H, Tokuda M, Morishita A, Jian G, Shi L, Murota M, Tani J, Kato K. Annexin A2 expression and phosphorylation are up-regulated in hepatocellular carcinoma. Int J Oncol. 2008;33:1157-1163. [PubMed] |
| 12. | Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Longerich T, Haller MT, Mogler C, Aulmann S, Lohmann V, Schirmacher P, Brand K. Annexin A2 as a differential diagnostic marker of hepatocellular tumors. Pathol Res Pract. 2011;207:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Ji NY, Park MY, Kang YH, Lee CI, Kim DG, Yeom YI, Jang YJ, Myung PK, Kim JW, Lee HG. Evaluation of annexin II as a potential serum marker for hepatocellular carcinoma using a developed sandwich ELISA method. Int J Mol Med. 2009;24:765-771. [PubMed] |
| 15. | Qian J, Yao D, Dong Z, Wu W, Qiu L, Yao N, Li S, Bian Y, Wang Z, Shi G. Characteristics of hepatic igf-ii expression and monitored levels of circulating igf-ii mRNA in metastasis of hepatocellular carcinoma. Am J Clin Pathol. 2010;134:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | The Liver Cancer Committee of Chinese Anticancer Association. Diagnostic criteria of primary hepatocellular carcinoma. Zhonghua Ganzangbing Zazhi. 2000;8:135. |
| 17. | The Group of Viral Hepatitis Research. The prevention and cure scheme of viral hepatitis. Zhonghua Ganzangbing Zazhi. 2000;8:324-329. |
| 18. | Ohno Y, Izumi M, Kawamura T, Nishimura T, Mukai K, Tachibana M. Annexin II represents metastatic potential in clear-cell renal cell carcinoma. Br J Cancer. 2009;101:287-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Nan KJ, Guo H, Ruan ZP, Jing Z, Liu SX. Expression of p57(kip2) and its relationship with clinicopathology, PCNA and p53 in primary hepatocellular carcinoma. World J Gastroenterol. 2005;11:1237-1240. [PubMed] |
| 20. | Malaguarnera G, Giordano M, Paladina I, Berretta M, Cappellani A, Malaguarnera M. Serum markers of hepatocellular carcinoma. Dig Dis Sci. 2010;55:2744-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 22. | Kobayashi M, Hosaka T, Ikeda K, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki F, Suzuki Y, Saitoh S. Highly sensitive AFP-L3% assay is useful for predicting recurrence of hepatocellular carcinoma after curative treatment pre- and postoperatively. Hepatol Res. 2011;41:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Lee YL, Ahn BC, Lee Y, Lee SW, Cho JY, Lee J. Targeting of hepatocellular carcinoma with glypican-3-targeting peptide ligand. J Pept Sci. 2011;17:763-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Yoon SY, Kim JM, Oh JH, Jeon YJ, Lee DS, Kim JH, Choi JY, Ahn BM, Kim S, Yoo HS. Gene expression profiling of human HBV- and/or HCV-associated hepatocellular carcinoma cells using expressed sequence tags. Int J Oncol. 2006;29:315-327. [PubMed] |
| 25. | Sharma M, Ownbey RT, Sharma MC. Breast cancer cell surface annexin II induces cell migration and neoangiogenesis via tPA dependent plasmin generation. Exp Mol Pathol. 2010;88:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |