Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5889
Revised: July 3, 2012
Accepted: August 14, 2012
Published online: November 7, 2012
AIM: To characterize the dual effects of deslanoside on the contractility of jejunal smooth muscle.
METHODS: Eight pairs of different low and high contractile states of isolated jejunal smooth muscle fragment (JSMF) were established. Contractile amplitude of JSMF in different low and high contractile states was selected to determine the effects of deslanoside, and Western blotting analysis was performed to measure the effects of deslanoside on myosin phosphorylation of jejunal smooth muscle.
RESULTS: Stimulatory effects on the contractility of JSMF were induced (45.3% ± 4.0% vs 87.0% ± 7.8%, P < 0.01) by deslanoside in 8 low contractile states, and inhibitory effects were induced (180.6% ± 17.8% vs 109.9% ± 10.8%, P < 0.01) on the contractility of JSMF in 8 high contractile states. The effect of deslanoside on the phosphorylation of myosin light chain of JSMF in low (78.1% ± 4.1% vs 96.0% ± 8.1%, P < 0.01) and high contractile state (139.2% ± 8.5% vs 105.5 ± 7.34, P < 0.01) was also bidirectional. Bidirectional regulation (BR) was abolished in the presence of tetrodotoxin. Deslanoside did not affect jejunal contractility pretreated with the Ca2+ channel blocker verapamil or in a Ca2+-free assay condition. The stimulatory effect of deslanoside on JSMF in a low contractile state (low Ca2+ induced) was abolished by atropine. The inhibitory effect of deslanoside on jejunal contractility in a high contractile state (high Ca2+ induced) was blocked by phentolamine, propranolol and L-NG-nitro-arginine, respectively.
CONCLUSION: Deslanoside-induced BR is Ca2+ dependent and is related to cholinergic and adrenergic systems when JSMF is in low or high contractile states.
- Citation: Chen DP, Xiong YJ, Tang ZY, Yao QY, Ye DM, Liu SS, Lin Y. Characteristics of deslanoside-induced modulation on jejunal contractility. World J Gastroenterol 2012; 18(41): 5889-5896
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5889.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5889
More than 200 naturally occurring cardiotonic glycosides (CGs, cardiac glycosides) have been identified to date[1]. CGs have long been and will continue to be used in the treatment of congestive heart failure and have entered the clinical trial phase for treating cancer[2-5]. CGs enhance the myocardial contraction by increasing intra-cellular Ca2+via inhibiting the activities of Na+/K+-ATPase[6-8]. CGs ouabain has been found to induce excitation on colonic smooth muscle[9]. Toxic effects of CGs are observed in clinics, e.g., atrioventricular block, bradycardia, and gastrointestinal irritation[1]. Probably due to the fact that no therapeutic applications are yet known, the characteristics of CGs on the intestinal motility have rarely been investigated.
Intestinal motility is mainly modulated by neurotransmitters and hormones; the neuronal regulation of intestinal motility involves intrinsic, e.g., enteric nervous system (ENS), as well as extrinsic nerves, e.g., the sympathetic and parasympathetic nervous system (SPNS)[10]. The central nervous system is able to modulate, but not entirely control, the motor activity by sending instructions via SPNS, and ENS modulates the motility of intestinal smooth muscle even when isolated from the body to fulfill pivotal functions[10,11]. In this study, we proposed a hypothesis that inducible bidirectional regulation (BR) is the major autonomous control of intestinal motility in the absence of CNS control, and that both low and high contractile states of intestinal smooth muscle can be regulated back toward normal contractile state by a single CGs deslanoside-induced BR. To test the hypothesis, different low and high contractile states of intestinal smooth muscle were established. Considering both colon and small intestine are sites of “abnormal” motility in intestinal smooth muscle disorders, e.g., irritable bowel syndrome (IBS)[12-14], and that the jejunum is a “typical” region of the small intestine, we chose to investigate the contractility of isolated jejunal smooth muscle fragment (JSMF) and its underlying mechanisms involved in deslanoside-induced BR.
The animal protocol was approved by Dalian Medical University Animal Care and Ethics Committee, and all experimental procedures described were carried out in accordance with the Declaration of Helsinki. Sprague-Dawley rats (200-250 g) were used in the assay. Constipation-predominant (CP) rats were established by daily gavage with cool water (0 °C-4 °C) for 14 d, and the control rats were prepared by daily gavage with water at room temperature[15,16]. Diarrhea-predominant (DP) rats were established by intracolonic instillation of acetic acid and restraint stress, and control rats received intracolonic instillation with saline[17-19]. The granule number and the moisture content of the feces from the control group and the model group were measured daily, and the body mass was recorded once every 3 d.
Tissue fragments from the intact tubular jejunum were prepared according to the methods described previously[20,21]. Jejunum was isolated from normal, CP and DP rats. Jejunal fragments were cut into approximately 2 cm in length (tubes). One end of the jejunal fragment in longitudinal direction was fixed to the wall of a tissue bath chamber (20 mL volume), and the other end was connected to a force-displacement transducer. This montage measured the contractile response of JSMF.
The organ bath was maintained at 37 °C, and the resting tension was set optimally at 1.0 g. Preliminary experiments showed that this load stretched tissues to their optimal length for force development during contraction. JSMF was allowed to equilibrate in aerated Krebs buffer for 50 min and the bath solution was replaced every 10 min. Contractile amplitude of JSMF was measured from the baseline to the peak and was expressed as a percentage of normal contractile amplitude. Contractile amplitude was recorded and identical time-interval of each assay with the same start and stop time was chosen to compare the amplitude before and after drug treatment in different assay conditions. The mean amplitude was calculated from six independent assays.
The contractility of JSMF was measured in Krebs buffer (118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 4.2 mmol/L NaHCO3, 2.5 mmol/L CaCl2, 10 mmol/L glucose; pH 7.4) and selected as the normal contractile state (NCS). The jejunal contractility measured in modified low Ca2+ (1.25 mmol/L) and high Ca2+ (5.0 mmol/L) Krebs buffer was selected as the representative low contractile state (RLCS) and representative high contractile state (RHCS), respectively, since spontaneous contractions of intestinal smooth muscle were paralleled to intracellular Ca2+ concentration[22,23]. One pair of low-high contractile states was established from jejunal smooth muscle isolated from CP and DP rats. The other six pairs of low-high contractile states were generated by incubating JSMF in modified low K+ (2.5 mmol/L)-high K+ (10.0 mmol/L) Krebs buffer, low Na+ (100 mmol/L)-high Na+ (150 mmol/L) Krebs buffer, high Mg2+ (3.0 mmol/L)-low Mg2+(1.0 mmol/L) Krebs buffer, adrenaline (5.0 μmol/L)-ACh (5.0 μmol/L) Krebs buffer, quercetin (10.0 μmol/L)-capsaicin (10.0 μmol/L) and nitric oxide (NO) donor sodium nitroprusside (SNP) (5 μmol/L)-erythromycin (10 μmol/L) Krebs buffer[24,25]. After the stable contractile state of jejunal contraction was obtained, deslanoside was added to the bath to make a final concentration of 20 μmol/L, unless otherwise indicated.
The phosphorylation of myosin light chain (PMLC) in jejunum was examined by Western blotting as described previously[26,27]. JSMF was immediately treated with low Ca2+ or high Ca2+ Krebs buffer for 1 min in the absence or presence of 20 μmol/L deslanoside, and then were frozen and stored in liquid nitrogen. Ground product was incubated for 30 min in ice-cold homogenization buffer. The blots on nitrocellulose filter membrane were probed with phosphor-myosin light chain 2 (Ser 19) antibody (1:1000) [No. 3671, Cell Signaling Technology, Inc (CST), United States] and myosin light chain 2 (total myosin light chain) antibody (1:1000) (No. 3672, CST, United States) at 4 °C with gentle shaking overnight. Anti-rabbit IgG secondary antibodies were used at 1:2500 for 60 min at room temperature and bands were detected and quantified using Multispectral imaging system (UVP, United States).
Injectable deslanoside was obtained from Sine Pharmaceutical (Shanghai, China). Capsaicin and quercetin was purchased from Chengdu Biopurify Phytochemicals Co. Ltd, China. Tetrodotoxin (TTX) was a product of Aladdin Chemistry Co. Ltd (Shanghai, China). Unless otherwise indicated, chemicals were obtained from Sigma (United States).
Student’s t test was used to compare statistical differences between two groups, and P < 0.05 was considered statistically significant.
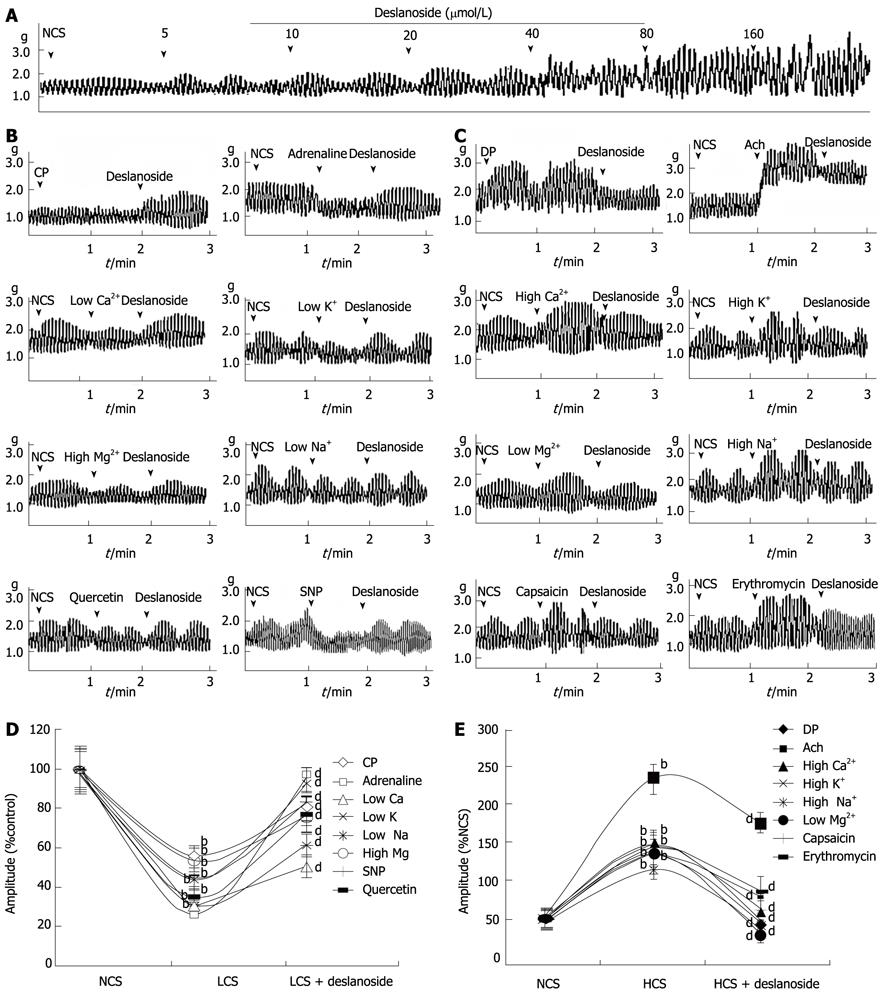
Deslanoside exerted stimulatory effects on JSMF in NCS in a dose range of 5-160 µmol/L (Figure 1A).
Eight low and 8 high contractile states of jejunal smooth muscle were established as described in Materials and Methods. The contractility of JSMF in both low and high contractile states was statistically different from that of normal control (Figure 1). Deslanoside (20 µmol/L) was used in all the assays based on the fact that deslanoside-induced BR on jejunal contractility was observed in a dose range of 10-40 µmol/L. Deslanoside produced significant stimulatory effects (45.3% ± 4.0% vs 87.0% ± 7.8%, P < 0.01) on the contractility of JSMF in all 8 low contractile states (Figure 1B), and produced significant inhibitory effects (180.6% ± 17.8% vs 109.9% ± 10.8%, P < 0.01) on the contractility of JSMF in all 8 high contractile states (Figure 1C).
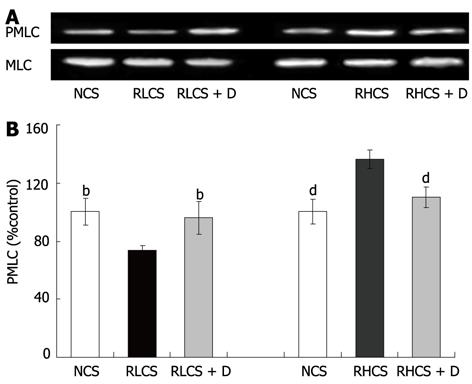
The PMLC in jejunum was significantly decreased in RLCS in comparison with that in NCS (100.0% ± 9.4% vs 78.1% ± 4.1%, P < 0.01), and was significantly increased at RHCS in comparison with that in NCS (100.0% ± 6.7% vs 139.2% ± 8.5%, P < 0.01) (Figure 2). Deslanoside significantly increased the PMLC in RLCS (78.1% ± 4.1% vs 96.0% ± 8.1%, P < 0.01), and significantly decreased the PMLC in RHCS (139.2% ± 8.5% vs 105.5 ± 7.34, P < 0.01).
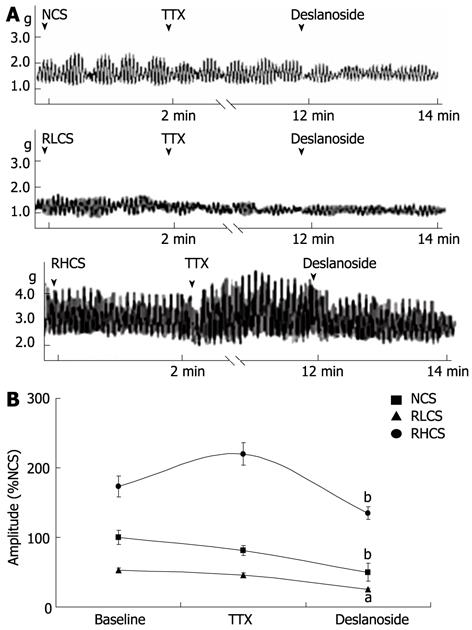
In the presence of TTX, BR was not observed when deslanoside was tested on the contractility of JSMF in RLCS and RHCS (Figure 3).
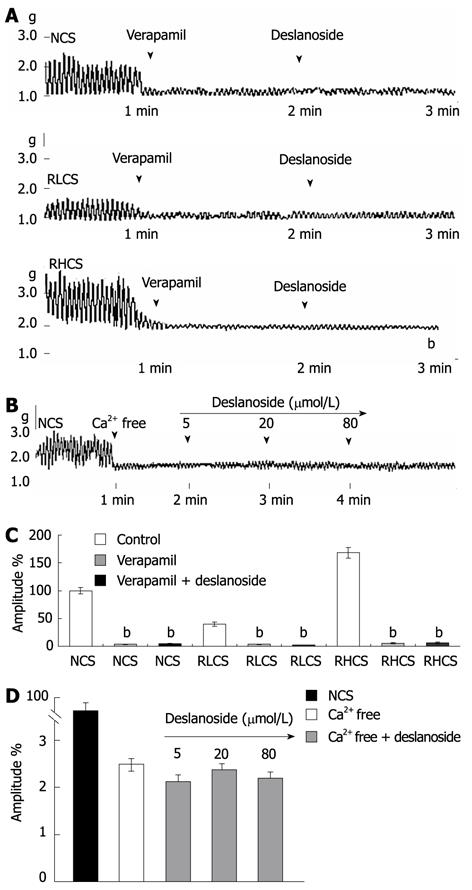
Deslanoside, at bath concentrations of 5 µmol/L, 20 µmol/L and 80 µmol/L, did not affect jejunal contractility in a Ca2+-free assay condition, and 20 µmol/L deslanoside did not stimulate the contractility of JSMF pre-incubated with the Ca2+channel blocker verapamil at normal, low and high contractile states (Figure 4).
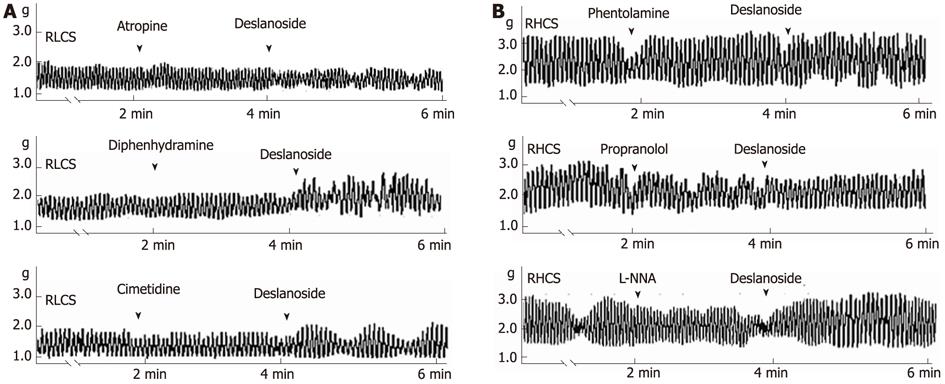
The underlying mechanisms involved in deslanoside-induced BR were investigated. Muscarinic receptor antagonist atropine abolished the stimulatory effect of deslanoside on the contractility of JSMF in RLCS (Table 1; Figure 5A). Neither histamine H1-receptor antagonist diphenhydramine nor histamine H2-receptor antagonist cimetidine blocked deslanoside-induced stimulatory effects on the contractility of JSMF in RLCS (Table 1; Figure 5A). α-adrenergic receptor antagonist phentolamine, β-adrenergic receptor antagonist propranolol and NO synthase inhibitor L-NNA abolished deslanoside-induced inhibitory effect on the contractility of JSMF in RHCS (Table 1; Figure 5B).
| Agents | Normal contractile state | Low contractile state | High contractile state | |||
| Pre-deslanoside | Post-deslanoside | Pre-deslanoside | Post-deslanoside | Pre-deslanoside | Post-deslanoside | |
| Krebs buffer | 100.0 ± 12.1 | 149.0 ± 13.0b | 39.1 ± 2.8 | 89.1 ± 5.1b | 177.7 ± 16.0 | 109.3 ± 11.9b |
| Atropin | 93.3 ± 6.3 | 155.0 ± 15.1b | 29.1 ± 1.1 | 32.4 ± 3.3 | 149.7 ± 11.0 | 100.3 ± 11.2b |
| Diphenhydramine | 109.0 ± 14.3 | 145.2 ± 13.1b | 46.5 ± 3.8 | 94.0 ± 4.9b | 169.6 ± 15. 2 | 108.5 ± 11.6b |
| Cimetidine | 100.0 ± 11.8 | 153.4 ± 13.0b | 42.9 ± 3.9 | 93.4 ± 8.1b | 180.1 ± 17.4 | 113.3 ± 12.1b |
| Phentolamine | 92.5 ± 6.5 | 160.6 ± 16.2b | 55.4 ± 5.5 | 90.6 ± 5.2b | 189.3 ± 19.2 | 184.5 ± 17.2 |
| Propranolol | 89.4 ± 9.8 | 145.5 ± 14.2b | 33.2 ± 2.6 | 95.6 ± 7.8b | 163.1 ± 16.1 | 161.7 ± 17.3 |
| L-NNA | 103.0 ± 11.3 | 150.0 ± 13.2b | 51.3 ± 4.6 | 119.6 ± 12.5b | 190.5 ± 18.2 | 195.1 ± 19.1 |
Eight pairs of low-high contractile states were established to imitate intestinal hyper- and hypomotility and to evaluate the characteristics of deslanoside-induced BR and potential clinical implication. IBS is known as one of the major functional gastrointestinal disorders, affecting approximately 10% of all adults worldwide[28]. IBS is usually categorized into three subclasses: IBS with constipation (hypo-motility), IBS with diarrhea (hyper-motility), and IBS with alternating symptoms of both constipation and diarrhea (IBS-A)[13,14,29]. None of the currently available drugs are globally effective in treating all IBS symptoms[30], and developing treatment strategies for patients with IBS has been difficult because of the lack of pharmacological targets and the wide range of symptomatology[31]. Considering that the precise cause of IBS is unknown and it is unlikely that one single factor could explain all instances of IBS[32], we established various assay conditions to mimic the possible intestinal hyper- and hypo-motility. These low and high contractile states of isolated intestinal smooth muscle were established (1) by changing ionic concentration in assay buffers; (2) using inhibitory and stimulatory neurotransmitters, or using exogenous inhibitors and stimulators in the assay buffers; and (3) using isolated intestinal smooth muscle obtained from constipation-prominent rat model and diarrhea-prominent rat model.
In this study, we tried to evaluate the possibility that whether deslanoside-induced adverse gastrointestinal irritation could be beneficialized as a potential therapeutic effect on the intestinal smooth muscle dysfunction, and characterized deslanoside-induced BR on the contractility of JSMF. Deslanoside was found to induce stimulatory effects on JSMF in all eight low contractile states and induced inhibitory effects on JSMF in all eight high contractile states. In accordance with deslanoside-induced BR on the contractility of JSMF, the effects deslanoside on myosin phosphorylation of JMSF were also bidirectional.
Activation of muscarinic receptor increases the intestinal motility and stimulation of α and β-adrenoceptors inhibits intestinal motility. Inhibition of intestinal motility is also mediated by NO, a nonadrenergic, noncholinergic neurotransmitter, producing its effect by directly acting on smooth muscle and by indirectly inhibiting acetylcholine and substance P releasing[33,34]. Based on the aforementioned mechanisms involved in the modulation of intestinal contractility, our results have the following implications. The evidence that atropine blocked the stimulatory effects of deslanoside on JSMF in RLCS implies that stimulatory effects of deslanoside on JSMF in low contractile state are correlated with M receptor linked stimulation; and the evidence that phentolamine, propranolol and L-NNA abolished the inhibitory effects of deslanoside on JSMF in RHCS suggests that the inhibitory effects of deslanoside are correlated with adrenergic α, β receptor, as well as NO synthase linked relaxing mechanisms. Deslanoside-induced BR is Ca2+-dependent, since it neither affected jejunal contractility in a Ca2+-free assay condition, nor stimulated jejunal contractility pre-incubated with the Ca2+ channel blocker verapamil in normal, low and high contractile states (Figure 4). The evidence that deslanoside-induced BR is not observed in the presence of TTX implies that deslanoside-induced BR is based on the presence of ENS.
Compared with controls (85.3 ± 37.3 min), the transit times (a measurement of bowel movement) obtained in constipation-predominant (67.4 ± 19.6 min) and diarrhea-predominant patients with IBS (108.4 ± 34.3 min) were decreased and increased, respectively (P < 0.05)[35]. The results implicate that deslanoside-induced BR on jejunum is informative for preclinical investigation of a drug with potential value for the modulation of both abnormally low and high contractility of intestinal smooth muscle. To relieve the symptoms of functional bowel disorders, such as alternating-type IBS, BR-inducer deslanoside could be considered for the potential future clinical application.
It is known that ENS is highly interconnected and responsible for secreting at least 50 different modulators, regulating intestinal motility and other functions[36]. We are still not clear about the diverse mechanisms for BR induction, including how dozens of neurotransmitters in intestinal smooth muscle are interrelated in normal contractile state, and how they correlate with BR in both the low and high contractile states. Although we have partially revealed the characteristics of deslanoside-induced BR, further study is still required to identify the detailed mechanisms.
The authors wish to thank Zhi Lin and Fan Yuan for their comments.
Irritable bowel syndrome (IBS) is known as one of major functional gastrointestinal disorders, contracting approximately 10% of all adults world wide. Cardiotonic glycosides (CGs) have long been and continue to be used in the treatment of congestive heart failure and have entered clinical trials for treating cancer. Gastrointestinal irritation of CGs has been reported, however, the characteristics of CGs on intestinal motility remain unknown.
Developing treatment strategies for patients with IBS has been difficult because of the lack of pharmacological targets and the wide range of symptomatology, especially in the alternating-type IBS (IBS-A) which is a functional gastrointestinal disorder with alternating symptoms of both constipation and diarrhea.
The present study established 8 pairs of low-high contractile states to mimic the possible intestinal smooth muscle disorders. These different low and high contractile states of isolated intestinal smooth muscle were established by changing ionic concentration in assay buffers; using inhibitory and stimulatory neurotransmitters; exogenous inhibitors and stimulators, respectively in the assays; and isolated intestinal smooth muscle obtained from both constipation-prominent rat model and diarrhea-prominent rat model. The results indicate that the contractile state determines deslanoside-induced effects to be stimulatory or inhibitory, namely, stimulatory effects on the contractility of intestinal fragment were induced by deslanoside in all low contractile states, and inhibitory effects were induced on the contractility of jejunal smooth muscle fragment (JSMF) in all high contractile states. The present study indicates that deslanoside-induced Bidirectional regulation (BR) requires the presence of enteric nervous system and is Ca2+ dependent. The possible mechanism of deslanoside-induced BR is related to cholinergic system when jejunal smooth muscle is in a low contractile state, and related to adrenergic system and nitric oxide relaxing mechanism when in a high contractile state.
The results implicate that deslanoside-induced BR on jejunum is informative for preclinical investigation of a drug with potential value for the modulation of both abnormally low and high contractility of intestinal smooth muscles. To relieve the symptoms of functional bowel disorders, such as IBS-A, BR-inducer deslanoside could be considered for the potential future clinical application.
IBS is usually classified into three subclasses: IBS with constipation (hypo-motility), IBS with diarrhea (hyper-motility), and IBS with alternating symptoms of both constipation and diarrhea.
This is a well done study that provides interesting insight into the action of deslanoside. The study is complete, well-written and suitable for publication.
Peer reviewers: Dr. John R Grider, PhD, Professor, Department of Physiology and Biophysics, Virginia Commonwealth University, PO Box 980551, Richmond, VA 23298, United States; Angelo A Izzo, Professor, Department of Experimental Pharmacology, University of Naples Federico II, Via D Montesano 49, 80131 Naples, Italy; Oliver Grundmann, PhD, Clinical Assistant Professor, Department of Medicinal Chemistry, College of Pharmacy, University of Florida, 1600 SW Archer RD, Room P6-20, Gainesville, FL 32610-0484, United States
S- Editor Shi ZF L- Editor Ma JY E- Editor Lu YJ
| 1. | Froberg B, Ibrahim D, Furbee RB. Plant poisoning. Emerg Med Clin North Am. 2007;25:375-433; abstract ix. [PubMed] |
| 2. | Mijatovic T, Van Quaquebeke E, Delest B, Debeir O, Darro F, Kiss R. Cardiotonic steroids on the road to anti-cancer therapy. Biochim Biophys Acta. 2007;1776:32-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Prassas I, Diamandis EP. Novel therapeutic applications of cardiac glycosides. Nat Rev Drug Discov. 2008;7:926-935. [PubMed] |
| 4. | Winnicka K, Bielawski K, Bielawska A. Cardiac glycosides in cancer research and cancer therapy. Acta Pol Pharm. 2006;63:109-115. [PubMed] |
| 5. | Yang P, Menter DG, Cartwright C, Chan D, Dixon S, Suraokar M, Mendoza G, Llansa N, Newman RA. Oleandrin-mediated inhibition of human tumor cell proliferation: importance of Na,K-ATPase alpha subunits as drug targets. Mol Cancer Ther. 2009;8:2319-2328. [PubMed] |
| 6. | Arispe N, Diaz JC, Simakova O, Pollard HB. Heart failure drug digitoxin induces calcium uptake into cells by forming transmembrane calcium channels. Proc Natl Acad Sci USA. 2008;105:2610-2615. [PubMed] |
| 7. | Liu J, Tian J, Haas M, Shapiro JI, Askari A, Xie Z. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838-27844. [PubMed] |
| 8. | Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem. 2004;271:1054-1062. [PubMed] |
| 9. | Barajas-López C, Huizinga JD. Ouabain-induced excitation of colonic smooth muscle due to block of K+ conductance by intracellular Na+ ions. Eur J Pharmacol. 1992;221:51-58. [PubMed] |
| 10. | Hansen MB. Neurohumoral control of gastrointestinal motility. Physiol Res. 2003;52:1-30. [PubMed] |
| 11. | Schemann M. Control of gastrointestinal motility by the "gut brain"--the enteric nervous system. J Pediatr Gastroenterol Nutr. 2005;41 Suppl 1:S4-S6. [PubMed] |
| 12. | Pimentel M. The Treatment of Patients With Irritable Bowel Syndrome: Review of the Latest Data From the 2010 DDW Meeting. Gastroenterol Hepatol (. N Y). 2010;6:1-15. [PubMed] |
| 13. | Ringel Y, Sperber AD, Drossman DA. Irritable bowel syndrome. Annu Rev Med. 2001;52:319-338. [PubMed] |
| 14. | Tack J, Fried M, Houghton LA, Spicak J, Fisher G. Systematic review: the efficacy of treatments for irritable bowel syndrome--a European perspective. Aliment Pharmacol Ther. 2006;24:183-205. [PubMed] |
| 15. | Zou BC, Dong L, Wang Y, Wang SH, Cao MB. Expression and role of 5-HT7 receptor in brain and intestine in rats with irritable bowel syndrome. Chin Med J (. Engl). 2007;120:2069-2074. [PubMed] |
| 16. | Peng LH, Yang YS, Sun G, Wang WF. A new model of Constipation-predominant irritable bowel syndrome in rats. Shijie Huaren Xiaohua Zazhi. 2004;12:112-116. |
| 17. | La JH, Kim TW, Sung TS, Kang JW, Kim HJ, Yang IS. Visceral hypersensitivity and altered colonic motility after subsidence of inflammation in a rat model of colitis. World J Gastroenterol. 2003;9:2791-2795. [PubMed] |
| 18. | Mozaffari S, Esmaily H, Rahimi R, Baeeri M, Sanei Y, Asadi-Shahmirzadi A, Salehi-Surmaghi MH, Abdollahi M. Effects of Hypericum perforatum extract on rat irritable bowel syndrome. Pharmacogn Mag. 2011;7:213-223. [PubMed] |
| 19. | La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Increase in neurokinin-1 receptor-mediated colonic motor response in a rat model of irritable bowel syndrome. World J Gastroenterol. 2005;11:237-241. [PubMed] |
| 20. | Mathison R, Shaffer E. Increased cholinergic contractions of jejunal smooth muscle caused by a high cholesterol diet are prevented by the 5-HT4 agonist--tegaserod. BMC Gastroenterol. 2006;6:8. |
| 21. | Zhu J, Chen L, Xia H, Luo HS. Mechanisms mediating CCK-8S-induced contraction of proximal colon in guinea pigs. World J Gastroenterol. 2010;16:1076-1085. [PubMed] |
| 22. | Frings M, Haschke G, Heinke B, Schäfer KH, Diener M. Spontaneous contractions of intestinal smooth muscle re-aggregates from the new-born rat triggered by thromboxane A2. J Vet Med A Physiol Pathol Clin Med. 2000;47:469-475. [PubMed] |
| 23. | Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, Miyamoto S, Nakazawa H, Won KJ, Sato K. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157-230. [PubMed] |
| 24. | Scarpignato C, Pelosini I. Management of irritable bowel syndrome: novel approaches to the pharmacology of gut motility. Can J Gastroenterol. 1999;13 Suppl A:50A-65A. [PubMed] |
| 25. | Chen D, Xiong Y, Wang L, Lv B, Lin Y. Characteristics of emodin on modulating the contractility of jejunal smooth muscle. Can J Physiol Pharmacol. 2012;90:455-462. [PubMed] |
| 26. | Kobayashi K, Murata T, Hori M, Ozaki H. Prostaglandin E2-prostanoid EP3 signal induces vascular contraction via nPKC and ROCK activation in rat mesenteric artery. Eur J Pharmacol. 2011;660:375-380. [PubMed] |
| 27. | Iwabu A, Smith K, Allen FD, Lauffenburger DA, Wells A. Epidermal growth factor induces fibroblast contractility and motility via a protein kinase C delta-dependent pathway. J Biol Chem. 2004;279:14551-14560. [PubMed] |
| 28. | Quigley EM. Prebiotics for irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2009;3:487-492. [PubMed] |
| 29. | Galligan JJ. Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br J Pharmacol. 2004;141:1294-1302. [PubMed] |
| 30. | Chang FY, Lu CL. Treatment of irritable bowel syndrome using complementary and alternative medicine. J Chin Med Assoc. 2009;72:294-300. [PubMed] |
| 31. | Dunphy RC, Verne GN. Drug treatment options for irritable bowel syndrome: managing for success. Drugs Aging. 2001;18:201-211. [PubMed] |
| 32. | Kraneveld AD, Rijnierse A, Nijkamp FP, Garssen J. Neuro-immune interactions in inflammatory bowel disease and irritable bowel syndrome: future therapeutic targets. Eur J Pharmacol. 2008;585:361-374. [PubMed] |
| 33. | Mang CF, Truempler S, Erbelding D, Kilbinger H. Modulation by NO of acetylcholine release in the ileum of wild-type and NOS gene knockout mice. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1132-G1138. [PubMed] |
| 34. | Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G544-G552. [PubMed] |
| 35. | Lu CL, Chen CY, Chang FY, Lee SD. Characteristics of small bowel motility in patients with irritable bowel syndrome and normal humans: an Oriental study. Clin Sci (. Lond). 1998;95:165-169. [PubMed] |
| 36. | Goldstein AM, Nagy N. A bird's eye view of enteric nervous system development: lessons from the avian embryo. Pediatr Res. 2008;64:326-333. [PubMed] |