Published online Nov 7, 2012. doi: 10.3748/wjg.v18.i41.5870
Revised: July 30, 2012
Accepted: August 4, 2012
Published online: November 7, 2012
AIM: To retrospectively evaluate the effectiveness of impedance monitoring for predicting popping during radiofrequency ablation (RFA) using internally cooled electrodes.
METHODS: We reviewed 140 patients (94 males, 46 females; age range 73.0 ± 11.1 year) who underwent RFA between February 2006 and November 2008 with a modified protocol using a limited power delivery rather than a conventional one to avoid popping. All the patients provided their written informed consent, and the study was approved by the institutional review board. Intraprocedural impedances were measured for the study subjects, and the tumors were classified into three types according to the characteristics of their impedance curves: increasing, flat, or decreasing. The tumors were further sorted into seven subtypes (A-G) depending on the curvature of the impedance curve’s increase or decrease. Relative popping rates were determined for the three types and seven subtypes. A chi-square test was performed to estimate statistical significance.
RESULTS: A total of 148 nodules treated by RFA were analyzed. The study samples included 132 nodules of hepatocellular carcinoma, 14 nodules of metastatic liver cancer, and two nodules of intrahepatic cholangiocarcinoma. The numbers of nodules with each impedance curve type were as follows: 37 increasing-type nodules, 43 flat-type nodules, and 68 decreasing-type nodules. Popping occurrence rates were 24.3%, 46.5% and 64.7%, respectively. Flat-type nodules exhibited a significantly higher rate of popping compared to increasing-type nodules (P = 0.039). Decreasing-type nodules exhibited a significantly higher rate of popping compared to increasing-type nodules (P < 0.0001). Notably, nodules that showed a sharp decrease in impedance in the latter ablation period (subtype E) exhibited a significantly higher rate of popping compared to other subtypes.
CONCLUSION: Intraprocedural impedance monitoring can be a useful tool to predict the occurrence of popping during liver tumor RFA performed with internally cooled electrodes.
- Citation: Iida H, Aihara T, Ikuta S, Yamanaka N. Effectiveness of impedance monitoring during radiofrequency ablation for predicting popping. World J Gastroenterol 2012; 18(41): 5870-5878
- URL: https://www.wjgnet.com/1007-9327/full/v18/i41/5870.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i41.5870
In addition to resection, radiofrequency ablation (RFA) is one of the most effective local treatments applied to liver tumors along with resection[1-10]. The procedure is effective for relatively small tumors or tumors that recur after resection[11-13]. In addition, it can be performed safely in elderly patients and cirrhotic patients[14,15]. The RFA process involves inserting an electrode into the tumor and exciting it with a radio frequency current. This leads to a temperature increase in, and subsequent coagulation necrosis of, the tissue surrounding the electrode. However, complications arising from liver tumor RFA have been reported in numerous cases. Among those complications are intraperitoneal bleeding, subcapsular hematoma, biliary tract damage, portal vein thrombosis, peritoneal dissemination and gastrointestinal tract damage[16-21].
There is a phenomenon called “popping” that refers to a form of explosive tissue disruption caused by a rapid elevation of intra-tissue pressure[22-26]. When intra-tissue fluid vaporizes due to elevated tissue temperature, the tissue volume expands to approximately 1700 times that of the initial volume. The mechanism underlying this degree of tissue volume expansion is as follows: when 1 mol (18 mL) of water turns into gas at a standard temperature, pressure and dry, its volume increases to 22.4 L, which is 1244.4 times the volume of water. Assuming that the steam temperature is 373 K, the volume can be estimated by accounting for the increase in volume due to temperature with the following formula: 1244.4 times × 373 K / 273 K = 1700 times. Protein coagulation occurs at a temperature of approximately 60 °C, while vaporization occurs above 100 °C. Popping that occurs close to the subcapsular or main vessel has been thought to raise the risk of complications such as bleeding and dissemination.
In RFA procedures, two types of devices with different kinds of tips, the “internally cooled electrode” needle and the “expandable electrode” needle, are commercially available for ablation. It has been reported that the probability of popping is higher when using the internally cooled electrode due to the likelihood of intra-tissue pressure increasing more rapidly[27]. According to the literature, there is a higher probability of scattered recurrence with the internally cool electrode[28]. The conventional RFA protocol involves power delivery starting from 40 W followed by a power increase of 10 W every minute when using a 2-cm exposed tip; power delivery is started at 60 W, then increased by 20 W every minute when using a 3-cm exposed tip. Power output is increased with no limitations until a break occurs. A modified protocol to address the concern of complications from RFA has been introduced in recent years, and the authors reported that the likelihood of popping during RFA may be reduced by limiting power delivery[24]. We have applied these findings to our RFA procedure, limiting power delivery for the treatment of nodules on the surface of the liver or close to main vessels; however, a challenge still remained with respect to implementation of the modified protocol: in our hospital, popping occurred during 73 out of 148 sessions.
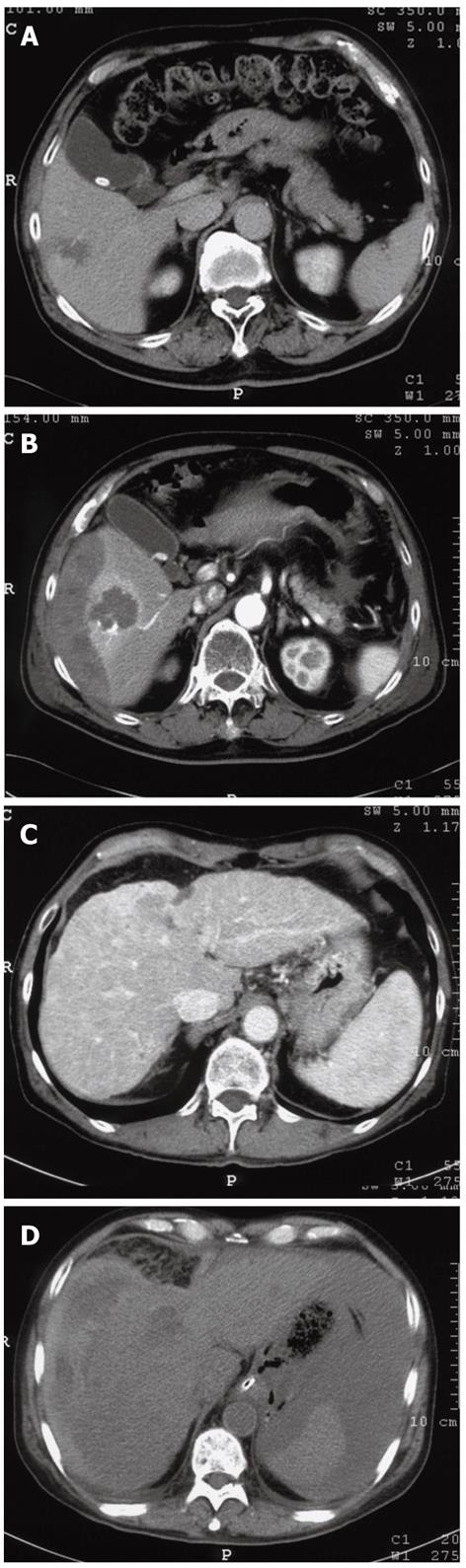
In our search for a safer RFA procedure, we reviewed 305 consecutive cases in which RFA was performed using the standard conventional protocol between June 2004 and January 2006. Of these 305 cases, major complications occurred in three cases (0.98%): a subcapsular hemorrhage, an intraperitoneal hemorrhage and a case of hemobilia (Figure 1). Steam popping had occurred in all three cases during RFA and it was therefore deemed as a potential contributor to the complications listed above.
In the present study, we retrospectively analyzed our RFA cases in which the modified protocol was applied between February 2006 and November 2008. The purpose of our study is to assess intraprocedural impedance monitoring to predict the likelihood of popping as hypothesized based on the aforementioned popping mechanism.
Between February 2006 and November 2008, 280 patients in our hospital underwent RFA using internally cooled electrodes (Covidien) according to the modified protocol with limited power delivery to prevent popping. Among these individuals, 140 patients (94 males, 46 females; age range 73.0 ± 11.1 year) and 148 sessions were retrospectively analyzed in this study, excluding the 54 patients who showed unstable impedance curves and the 86 patients who did not reach the break point during RFA. In our hospital, RFA is indicated for tumors that are 3 cm or less in the largest dimension, and for patients with no more than three tumors. Patients with impaired liver function could also be candidates for RFA if they are free of ascites. Among the 140 patients analyzed, 35 had undergone transcatheter arterial chemoembolization (TACE) prior to RFA. All patients provided their written informed consent before treatment, and the study was approved by the institutional review board.
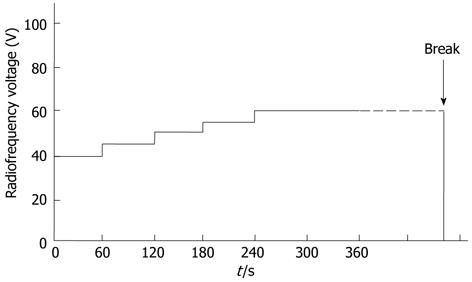
RFA was performed by three surgeons who specialize in liver surgery with 10, 20 and 25 year of experience, respectively. Midazolam (Dormicum; Astellas, Tokyo, Japan) was used for sedation at a dose of 0.03-0.06 mg/kg, and lidocaine (Xylocaine; Fujisawa, Tokyo, Japan) was used for local anesthesia. Cefazolin (Cefamezin; Astellas, Tokyo, Japan) was administered prophylactically against infection for 1-2 d following RFA. Abdominal ultrasound (Nemio; Toshiba, Tokyo, Japan) was used to place the radiofrequency (RF) electrode in the tumors. A 17-gauge internally cooled electrode with either a 2-cm or 3-cm exposed tip was used, depending on the tumor size as observed during the study period. For tumors smaller than 2 cm in diameter, a 2-cm exposed tip was used, while for tumors 2 cm or larger in diameter, a 3-cm exposed tip was used. Overlapping ablation was performed in three cases in which the tumors were greater than 3 cm in diameter. The electrodes were then connected to a generator (Series CC-1, Radionics: Covidien at present). Power was delivered using an impedance control mode to avoid popping. The RF voltage was initially 40 V and was increased by 5 V every minute to a maximum of 60 V, with no limitations on ablation time (Figure 2). This protocol was applied consistently whether using a 2-cm or 3-cm exposed tip.
The occurrence of a break was considered as one of the reasons for terminating ablation. During RFA, patients were monitored for popping before reaching the first break; the impedance curve reflects the data collected prior to popping. The time that elapsed prior to the break was recorded by the performing surgeon. When it was determined that coagulation necrosis was obtained after review of the echogram or by measuring the temperature in the ablated site after the first break, the procedure was terminated; otherwise, it continued even after reaching a break multiple times. The equipment was configured so that a break automatically occurred when the impedance increased to 25 Ω before the start of RFA. Thereafter the RF power was automatically returned to 0 W. The occurrence of popping is defined as the audible explosion sound confirmed by the rapidly expanding, highly echoic area.
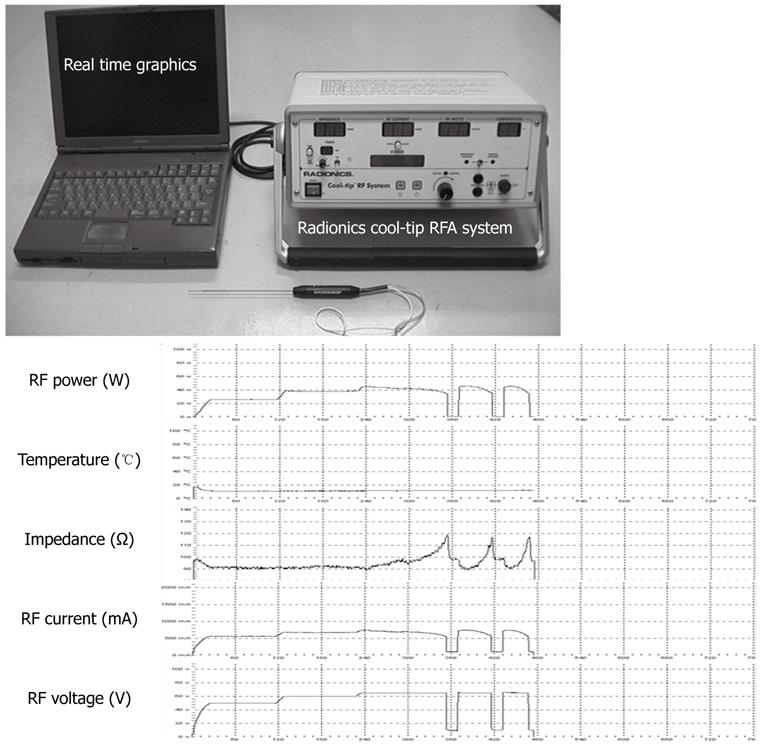
During each procedure, a computer with monitoring software was connected to the main unit of the generator to record the RF power (W), RF current (mA), RF voltage (V), temperature (°C), and impedance (Ω) simultaneously (Figure 3).
All statistical analyses were conducted using JMP 8.0.2 software (Macintosh; SAS institute Japan). A χ2 test was performed to identify any significant differences among the three types and among the seven subtypes. Differences were considered statistically significant at P < 0.05.
A total of 140 patients and 148 nodules treated by RFA were analyzed. The study samples included 132 nodules of hepatocellular carcinoma, 14 nodules of metastatic liver cancer, and two nodules of intrahepatic cholangiocarcinoma. Popping occurred in 73 out of the 148 RFA sessions (Table 1).
| Characteristics | Value |
| Male/female (n/n) | 94/46 |
| Age (yr) | 73.0 ± 11.1 |
| ICGR 15 (%) | 22.6 ± 14.4 |
| Maximum tumor diameters (mm) | 21.9 ± 7.1 |
| Hepatocellular carcinomas | 132 |
| Metastatic liver cancers | 14 |
| Intrahepatic cholangiocarcinomas | 2 |
| Tumor locations | |
| Segment 1 | 3 |
| Segment 2 | 11 |
| Segment 3 | 23 |
| Segment 4 | 13 |
| Segment 5 | 20 |
| Segment 6 | 22 |
| Segment 7 | 23 |
| Segment 8 | 33 |
| RFA needle lengths (2 cm/3 cm) | 79/69 |
| Ablation time (s) | 862 ± 613 |
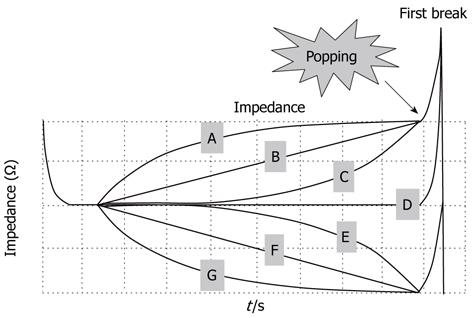
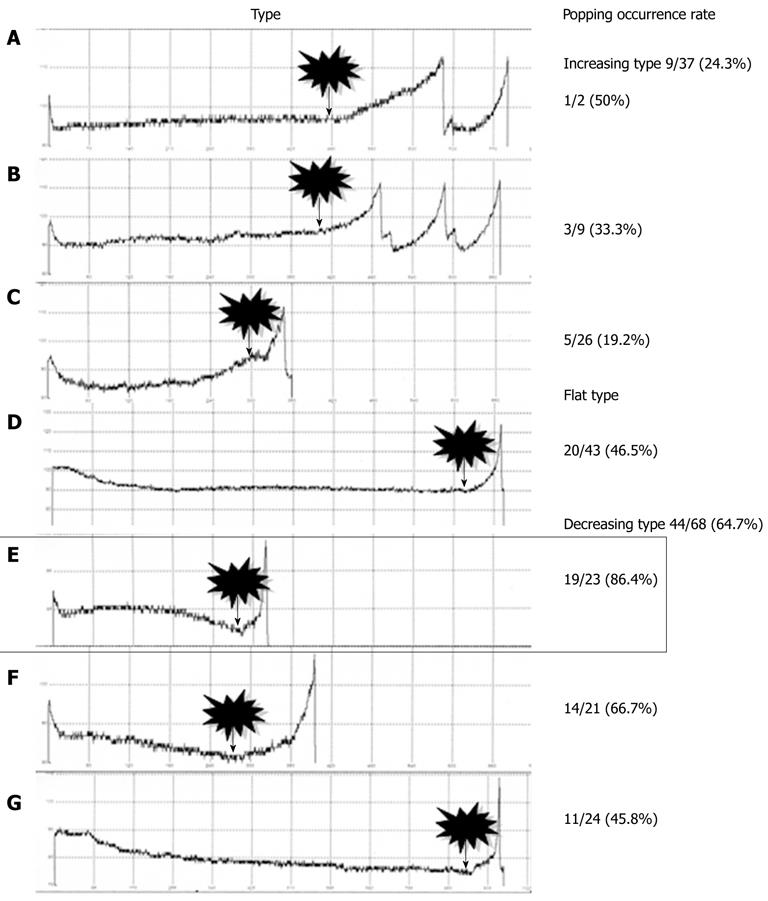
All 148 nodules were classified into three types according to the characteristics of their impedance curves up to the point where the first break took place (Figure 4): increasing-type nodules showed an increase in impedance; flat-type nodules showed a flat impedance curve; and decreasing-type nodules showed a decrease in impedance. Popping rates were determined for each type. The nodules were further sorted into seven subtypes, A to G, depending on the curvature of each impedance curve’s increase or decrease up to the first break point. Likewise, popping rates were determined for each subtype (Figure 4).
Though there was no significant difference among subtypes in terms of maximum tumor diameters, type B exhibited significantly higher ICGR 15 levels than type C (P = 0.028). There was no distinct difference among subtypes in terms of tumor location (Table 2).
| Impedance subtype | Maximum tumor diameter (mm)1 | ICGR 15 (%)1 | Tumor location(segments 1,2,3,4,5,6,7,8)2 |
| A | 20.3± 6.0 | 17.8 ± 13.9 | (0,1,0,0,0,0,0,1) |
| B | 20.4 ± 5.3 | 29.8 ± 10.7a | (1,1,1,1,1,2,1,1) |
| C | 21.2 ± 8.1 | 19.9 ± 11.1 | (0,3,3,3,5,3,3,6) |
| D | 22.7 ± 6.7 | 24.2 ± 17.9 | (0,2,8,4,8,6,4,11) |
| E | 22.7 ± 6.3 | 21.4 ± 10.2 | (1,2,1,2,1,3,8,5) |
| F | 22.3 ± 7.8 | 24.1 ± 15.9 | (1,1,4,1,3,5,3,3) |
| G | 20.9 ± 8.1 | 20.2 ± 14.0 | (0,1,5,2,2,4,4,6) |
The nodule distribution was as follows: 37, 43 and 68 for increasing, flat and decreasing types, respectively. Popping occurred with nine increasing-type nodules (24.3%), 20 flat-type nodules (46.5%), and 44 decreasing-type nodules (64.7%) (Figure 5). Flat-type nodules exhibited a significantly higher rate of popping compared to increasing-type nodules (P = 0.039). Decreasing-type nodules exhibited a significantly higher rate of popping compared to increasing-type nodules (P < 0.0001). Regarding the subtype analysis, the popping occurrence rates were 1/2 (50%) for nodule subtype A, 3/9 (33.3%) for B, 5/26 (19.2%) for C, 20/43 (46.5%) for D, 19/23 (86.4%) for E, 14/21 (66.7%) for F and 11/24 (45.8%) for G (Figure 5). Notably, subtype E showed a rapid decrease in impedance during the latter half of the ablation period and exhibited a significantly higher rate of popping compared to subtypes B (P = 0.006), C (P < 0.0001), D (P = 0.004) and G (P = 0.008). The results for subtype A could not be examined statistically because of the small number of samples.
With respect to the lengths of the needles that were used, popping occurred during 36 out of the 79 (45.6%) RFA sessions using a 2-cm exposed tip, and 37 out of the 69 (53.6%) RFAs using a 3-cm exposed tip (P = 0.328). The distribution of nodules treated by a 2-cm exposed tip was as follows: A (n = 2), B (n = 5), C (n = 14), D (n = 23), E (n = 11), F (n = 12) and G (n = 12). The distribution of those treated using a 3-cm exposed tip was as follows: A (n = 0), B (n = 4), C (n = 12), D (n = 20), E (n = 12), F (n = 9) and G (n = 12). Popping was most common in the subtype E nodules, which showed a rapid decrease in impedance during the latter half of ablation, regardless of the exposed tip length (2 cm: 9/11 = 81.8%; 3 cm: 10/12 = 83.3%). Among 14 metastatic liver cancer samples, popping occurred in seven cases with the highest occurrence in subtypes E (2/3 = 66.7%) and F (2/3 = 66.7%). Popping occurred in neither of the two intrahepatic cholangiocarcinomas. TACE was performed prior to RFA in 35 nodules; in these TACE cases, popping was found in subtypes C (2/6 = 33.3%), D (4/8 = 50.0%), E (3/3 = 100%), F (3/9 = 66.6%) and G (3/9 = 66.6%). No nodule of TACE was classified as subtype A or B. We also evaluated 48 nodules located on the surface of the liver and 100 nodules close to major vessels. Among 48 nodules that were on the surface of the liver, popping was observed in subtypes A (1/1 = 100%), B (1/3 = 33.3%), C (2/10 = 20.0%), D (4/10 = 40.0%), E (9/11 = 81.8%), F (4/7 = 57.1%) and G (4/6 = 66.7%). Among 100 nodules close to major vessels, popping occurred in subtypes A (0/1 = 0%), B (2/6 = 33.3%), C (3/16 = 18.8%), D (16/33 = 48.5%), E (10/12 = 83.3%), F (10/14 = 71.4%) and G (7/18 = 38.9%). Among the samples in which TACE was performed prior to RFA, popping was observed in subtype E most frequently, regardless of tumor location.
Although the rate of complications caused by RFA varies among hospitals, it is generally between 2.2% and 9.5%[29-33]. Peritoneal dissemination is one of the most serious complications that should be avoided and for which certain types of tumors are reportedly at a high risk, particularly subcapsular liver tumors and hepatocellular carcinomas that are poorly differentiated or that have high levels of alpha fetoprotein[34,35]. Several studies have also shown that tumors abutting the main portal vein are at a high risk of rapid intrahepatic dissemination[36,37]. Consequently, RFA should be performed with special caution when treating tumors that have developed close to the subcapsular or main vessel. In our hospital, because of these risks, liver tumors in these regions have been treated by RFA with limited power delivery to avoid complications.
Our results showed that decreases in impedance, particularly a rapid decrease in impedance during the latter half of the ablation period, strongly predict popping. We investigated tumor location, diameter, and hepatic function reserve in each subtype of impedance. We used an ICGR value of 15 min for the hepatic functional reserve test. The principle is that indocyanine green (ICG) is transported to the liver in association with lipoprotein in the blood and then ingested by hepatocytes. We calculated the retention rate of ICG after 15 min of injection. If hepatic functional reserve is impaired, the retention rate is high. The result achieved with an ICGR of 15 min showed that the rate of popping was slightly higher in subtype B than in subtype C (P = 0.028). There were no significant differences among subgroups. We did not find any relationship between the impedance curve and the hepatic function reserve. Tumor location and diameter were also similar among the subtypes.
The reason for the drop in impedance just before the occurrence of popping is that the elevation of intra-tissue temperature activates intra-tissue molecular movement, which results in higher electrical conductivity. Electrical conductivity is the amount of electricity that a substance can conduct, and it varies for different substances, e.g., 5% NaCl has an electrical conductivity of 67 mS/cm, and 5% HCl has an electrical conductivity of 395 mS/cm. The electrical conductivity of a substance increases as the temperature rises, and it can be estimated with the formula kT = k25 [1 + 0.02 (T - 25)], where kT is the electrical conductivity at temperature T (°C), and k25 is the electrical conductivity at 25 °C. Because tissue impedance is inversely related to electrical conductivity, tissue temperature increases as tissue impedance decreases. Thus, an abrupt decrease in impedance can be an indication of a rapid increase in intra-tissue temperature, possibly leading to steam popping following the vaporization of intra-tissue fluid.
The most favorable pattern of impedance curvature exhibited during the ablation period is the increasing type, which indicates that tissue coagulation occurs in parallel with an elevation in tissue temperature alongside a gradual increase in tissue impedance. If the temperature of intra-tissue fluid rises above 100 °C and starts to vaporize, the tissue impedance decreases, resulting in popping. As for the reason for the variation in impedance curves obtained with the same RFA protocol, we believe that fluid produced through coagulation may have played a part in some way.
Between June 2004 and January 2006, when we implemented RFA in the conventional protocol, major complications associated with RFA were observed in three out of 305 cases (0.98%). It was determined that popping had occurred during all three cases. Following this outcome, we opted for a lower power output when treating tumors on the surface of the liver or in close proximity to major vessels. Although no significant differences were found, this modified protocol may have contributed to a decrease in complications; hemobilia as a postoperative complication occurred in only one out of 473 cases (0.21%). The rates of local recurrence were equivalent in the conventional and modified protocols: 42 out of 305 cases (13.8%) between June 2004 and January 2006, and 48 out of 473 cases (10.1%) between February 2006 and November 2008. Based on the present study findings, we reduce the power delivery if we observe impedance curvatures that signal imminent popping. Between December 2008 and November 2011, we performed RFA on 731 nodules using the modified protocol; popping occurred for only 44 nodules (6.0%). No severe complication was observed in any of these cases.
There were several limitations on our analysis that have to be acknowledged. The first limitation concerns the patients excluded: for the 54 patients who showed unstable impedance curves, popping remained unpredictable; for the 86 patients who did not reach the break point during RFA (meaning that complete coagulation necrosis had not been achieved according to the present consensus on break points), there was a relatively higher risk of tumor recurrence. Second, due to a limitation resulting from the nature of our retrospective study design, a further prospective study will be necessary to confirm whether using the modified method actually improves the complication rate among patients whose tumors are close to major vessels or located subcapsularly.
In conclusion, by monitoring intraprocedural impedance during the RFA procedure, it is possible to predict popping in certain cases.
The authors express their sincere gratitude to Keita Oogake, Clinical Engineer at Meiwa Hospital and to Hidehiko Waki, a Clinical Technologist at Meiwa Hospital.
Radiofrequency ablation (RFA) is one of the most effective and safe treatments administered to patients with liver tumors. Certain complications may be induced in association with RFA, such as liver abscess, gastrointestinal tract injury, bleeding, subcapsular hematoma, biliary tract injury, portal vein thrombosis, and peritoneal seeding. A modified protocol used to address the concern of complications related to RFA has been introduced in recent years. This procedure reportedly decreased the frequency of popping by limiting power delivery.
Popping is a phenomenon of vapor explosion that can occur during RFA. Popping occurs if water vaporizes prior to tumor coagulation and may pose a risk of complications. RFA using low power delivery has been advocated to avoid this phenomenon. The authors applied this procedure for the treatment of tumors near Glisson’s capsule or the surface of the liver.
The authors monitored and analyzed the impedance curves to identify the characteristic warning signs that precede popping. It was found that popping was most frequent in nodules that exhibited a rapid decrease in impedance during the latter half of the ablation. This is the first such investigation.
By monitoring the intraprocedural impedance during RFA, it is possible in certain cases to predict popping. To avoid popping, power delivery should be limited when an impedance curve displays the characteristic warning signs.
Popping is a phenomenon that refers to a form of explosive tissue disruption caused by a rapid elevation of intra-tissue pressure. The occurrence of popping should be avoided during RFA due to the risk of complications. During RFA, tumor necrosis is caused by protein coagulation, which occurs at a temperature of approximately 60 °C. However, if the temperature rises above 100 °C prior to achieving protein coagulation, intra-tissue fluid vaporizes and the tissue volume expands to approximately 1700 times that of the initial volume. This is the mechanism underlying popping, which can be anticipated through the use of intraprocedural impedance monitoring.
The topic deals with the issue of RFA efficacy control, and the ideas presented seem to be of interest for those involved in liver tumor management. The manuscript is well structured. Materials and methods are appropriately characterized and results with tables and pictures provide evidence to draw conclusions.
Peer reviewers: Giedrius Barauskas, Professor, Department of Surgery, Kaunas University of Medicine, Eiveniu str. 2, Kaunas LT-50009, Lithuania; Manabu Morimoto, MD, Gastroenterological Center, Yokohama City University Medical Center, 4-57 Urafune-cho, Minami-ku, Yokohama City 232-0024, Japan
S- Editor Shi ZF L- Editor A E- Editor Zhang DN
| 1. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, Brock G, McMasters KM. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475-486. [PubMed] |
| 3. | Hiraoka A, Horiike N, Yamashita Y, Koizumi Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y. Efficacy of radiofrequency ablation therapy compared to surgical resection in 164 patients in Japan with single hepatocellular carcinoma smaller than 3 cm, along with report of complications. Hepatogastroenterology. 2008;55:2171-2174. [PubMed] |
| 4. | Kudo M. Radiofrequency ablation for hepatocellular carcinoma: updated review in 2010. Oncology. 2010;78 Suppl 1:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Helmberger T, Dogan S, Straub G, Schrader A, Jüngst C, Reiser M, Waggershauser T, Jakobs T, Hoffmann RT, Löhe F. Liver resection or combined chemoembolization and radiofrequency ablation improve survival in patients with hepatocellular carcinoma. Digestion. 2007;75:104-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, Watanabe T, Mine T. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: comparison with surgical resection. Cancer. 2010;116:3638-3644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 7. | Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 336] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Sutherland LM, Williams JA, Padbury RT, Gotley DC, Stokes B, Maddern GJ. Radiofrequency ablation of liver tumors: a systematic review. Arch Surg. 2006;141:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 825] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 10. | Minami Y, Kudo M. Radiofrequency ablation of hepatocellular carcinoma: a literature review. Int J Hepatol. 2011;2011:104685. [PubMed] |
| 11. | Hsu CY, Huang YH, Chiou YY, Su CW, Lin HC, Lee RC, Chiang JH, Huo TI, Lee FY, Lee SD. Comparison of radiofrequency ablation and transarterial chemoembolization for hepatocellular carcinoma within the Milan criteria: a propensity score analysis. Liver Transpl. 2011;17:556-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Choi D, Lim HK, Rhim H, Kim YS, Yoo BC, Paik SW, Joh JW, Park CK. Percutaneous radiofrequency ablation for recurrent hepatocellular carcinoma after hepatectomy: long-term results and prognostic factors. Ann Surg Oncol. 2007;14:2319-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Khan MR, Poon RT, Ng KK, Chan AC, Yuen J, Tung H, Tsang J, Fan ST. Comparison of percutaneous and surgical approaches for radiofrequency ablation of small and medium hepatocellular carcinoma. Arch Surg. 2007;142:1136-1143; discussion 1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Takahashi H, Mizuta T, Kawazoe S, Eguchi Y, Kawaguchi Y, Otuka T, Oeda S, Ario K, Iwane S, Akiyama T. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res. 2010;40:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Arch-Ferrer JE, Smith JK, Bynon S, Eckhoff DE, Sellers MT, Bland KI, Heslin MJ. Radio-frequency ablation in cirrhotic patients with hepatocellular carcinoma. Am Surg. 2003;69:1067-1071. [PubMed] |
| 16. | Kasugai H, Osaki Y, Oka H, Kudo M, Seki T. Severe complications of radiofrequency ablation therapy for hepatocellular carcinoma: an analysis of 3,891 ablations in 2,614 patients. Oncology. 2007;72 Suppl 1:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, De Wever I, Michel L. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002;89:1206-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 496] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 18. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 933] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 19. | Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, Scaife C, Raut C, Wolff R, Choi H. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 20. | Moumouh A, Hannequin J, Chagneau C, Rayeh F, Jeanny A, Weber-Holtzscherer A, Tasu JP. A tamponade leading to death after radiofrequency ablation of hepatocellular carcinoma. Eur Radiol. 2005;15:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Buscarini E, Savoia A, Brambilla G, Menozzi F, Reduzzi L, Strobel D, Hänsler J, Buscarini L, Gaiti L, Zambelli A. Radiofrequency thermal ablation of liver tumors. Eur Radiol. 2005;15:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 22. | Eick OJ, Gerritse B, Schumacher B. Popping phenomena in temperature-controlled radiofrequency ablation: when and why do they occur? Pacing Clin Electrophysiol. 2000;23:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Watanabe I, Masaki R, Min N, Oshikawa N, Okubo K, Sugimura H, Kojima T, Saito S, Ozawa Y, Kanmatsuse K. Cooled-tip ablation results in increased radiofrequency power delivery and lesion size in the canine heart: importance of catheter-tip temperature monitoring for prevention of popping and impedance rise. J Interv Card Electrophysiol. 2002;6:9-16. [PubMed] |
| 24. | Topp SA, McClurken M, Lipson D, Upadhya GA, Ritter JH, Linehan D, Strasberg SM. Saline-linked surface radiofrequency ablation: factors affecting steam popping and depth of injury in the pig liver. Ann Surg. 2004;239:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Fernandes ML, Lin CC, Lin CJ, Chen WT, Lin SM. Prospective study of a 'popping' sound during percutaneous radiofrequency ablation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:237-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Haines DE, Verow AF. Observations on electrode-tissue interface temperature and effect on electrical impedance during radiofrequency ablation of ventricular myocardium. Circulation. 1990;82:1034-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 207] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Kotoh K, Nakamuta M, Morizono S, Kohjima M, Arimura E, Fukushima M, Enjoji M, Sakai H, Nawata H. A multi-step, incremental expansion method for radio frequency ablation: optimization of the procedure to prevent increases in intra-tumor pressure and to reduce the ablation time. Liver Int. 2005;25:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Kotoh K, Enjoji M, Arimura E, Morizono S, Kohjima M, Sakai H, Nakamuta M. Scattered and rapid intrahepatic recurrences after radio frequency ablation for hepatocellular carcinoma. World J Gastroenterol. 2005;11:6828-6832. [PubMed] |
| 29. | Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, Vallone P, Mastro A, Fornage BD, Curley SA. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Chen MH, Yang W, Yan K, Gao W, Dai Y, Wang YB, Zhang XP, Yin SS. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005;11:6395-6401. [PubMed] |
| 31. | Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol. 2008;23:e445-e450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Kim JH, Kim PN, Won HJ, Shin YM. Percutaneous radiofrequency ablation using internally cooled wet electrodes for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 2012;198:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Sato M, Tateishi R, Yasunaga H, Horiguchi H, Yoshida H, Matsuda S, Koike K. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol. 2012;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 34. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 35. | Shirai K, Tamai H, Shingaki N, Mori Y, Moribata K, Enomoto S, Deguchi H, Ueda K, Maekita T, Inoue I. Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011;41:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Nicoli N, Casaril A, Abu Hilal M, Mangiante G, Marchiori L, Ciola M, Invernizzi L, Campagnaro T, Mansueto G. A case of rapid intrahepatic dissemination of hepatocellular carcinoma after radiofrequency thermal ablation. Am J Surg. 2004;188:165-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Ruzzenente A, Manzoni GD, Molfetta M, Pachera S, Genco B, Donataccio M, Guglielmi A. Rapid progression of hepatocellular carcinoma after Radiofrequency Ablation. World J Gastroenterol. 2004;10:1137-1140. [PubMed] |