Published online Oct 28, 2012. doi: 10.3748/wjg.v18.i40.5688
Revised: April 6, 2012
Accepted: April 12, 2012
Published online: October 28, 2012
Regulatory T cells (Tregs) are key elements in immunological self-tolerance. The number of Tregs may alter in both peripheral blood and in colonic mucosa during pathological circumstances. The local cellular, microbiological and cytokine milieu affect immunophenotype and function of Tregs. Forkhead box P3+ Tregs function shows altered properties in inflammatory bowel diseases (IBDs). This alteration of Tregs function can furthermore be observed between Crohn’s disease and ulcerative colitis, which may have both clinical and therapeutical consequences. Chronic mucosal inflammation may also influence Tregs function, which together with the intestinal bacterial flora seem to have a supporting role in colitis-associated colorectal carcinogenesis. Tregs have a crucial role in the immunoevasion of cancer cells in sporadic colorectal cancer. Furthermore, their number and phenotype correlate closely with the clinical outcome of the disease, even if their contribution to carcinogenesis has previously been controversial. Despite knowledge of the clinical relationship between IBD and colitis-associated colon cancer, and the growing number of immunological aspects encompassing sporadic colorectal carcinogenesis, the molecular and cellular links amongst Tregs, regulation of the inflammation, and cancer development are still not well understood. In this paper, we aimed to review the current data surrounding the role of Tregs in the pathogenesis of IBD, colitis-associated colon cancer and sporadic colorectal cancer.
- Citation: Műzes G, Molnár B, Sipos F. Regulatory T cells in inflammatory bowel diseases and colorectal cancer. World J Gastroenterol 2012; 18(40): 5688-5694
- URL: https://www.wjgnet.com/1007-9327/full/v18/i40/5688.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i40.5688
Immunological self-tolerance is crucial for preventing autoimmune diseases and maintaining immune homeostasis[1]. Regulatory T cells (Tregs) are a subpopulation of T cells including CD4+CD25+ forkhead box P3 (Foxp3)+ T cells, Tr1, Th3, and CD8+ Tregs. They serve to suppress the immune system and maintain self-tolerance. Inappropriate T cell response to intestinal microflora has a role in the pathological process of inflammatory bowel diseases (IBD)[2]. Accumulating evidence indicates that the imbalance of immunity, including a discrepancy between the number of Tregs in peripheral blood and in inflamed colonic mucosa, an excess of pro-inflammatory stimuli and an altered function of immunoregulatory cells has a central role in the pathogenesis of IBD[3].
Risk for developing cancer rises substantially as a result of poorly regulated inflammatory responses to pathogenic bacteria. Tregs function to restore immune homeostasis during chronic inflammation, and therefore it seems logical that Tregs would reduce the risk of inflammation-associated colon cancer by down-regulating inflammation. Alteration of T cell phenotype could also have an effect not only on the regulation of the chronic mucosal inflammation[3], but on colitis-associated carcinogenesis as well[4,5].
On the other hand, it is also widely believed, that Tregs in cancer primarily suppress protective anticancer immune responses. This alteration of Tregs function, together with the tumor-helping chemokine milieu produced by the cancer tissue could support cancer progression[4,5]. Tregs role in the carcinogenesis of inflammation-associated colon cancer and sporadic colorectal cancer is paradoxical due to conflicting results.
The bone marrow progenitors of all T cells become committed to their lineage in the thymus. The double negative T cells (CD4-CD8-) will rearrange their T cell receptor (TCR) genes to form a unique, functional molecule, which they test against cells in the thymic cortex for a minimal level of interaction with self-major histocompatibility complex (MHC). Upon receiving these signals, they begin to proliferate and express both CD4 and CD8. Then, the double-positive cells are selected by their interaction with thymic cells, begin the transcription of Foxp3, becoming Tregs. Foxp3 expression begins at the single-positive stage, at which point they are functional Tregs[6].
Foxp3 is a member of the forkhead and winged helix family of transcriptional regulators, and plays a critical role in the development and function of CD4+ Tregs[6]. In previous studies, CD4 and CD25 were selected as the markers for Tregs, but in recent studies Foxp3 has been proposed as a more reliable and superior biomarker[7,8].
The exact process of Treg cell selection is not well known. It appears to be a process determined by the affinity of interaction with the self-peptide MHC complex[9]. Characteristic features of CD4+CD25+Foxp3+ Tregs are their anergic state, and their ability to actively inhibit CD4+CD25- T cells, CD8+ T cells, dendritic cells, natural killer cells, natural killer T cells, and B cells in a cell-to-cell contact and dose-dependent manner[10].
Recent results[3-5] suggest that Tregs could have an important, but controversial regulatory role in both the pathogenesis of chronic colonic inflammation and colorectal carcinogenesis. Despite the clinical relationship between IBD and IBD-associated colon cancer, and the growing number of immunological aspects encompassing sporadic colon cancer, the molecular and cellular links between infiltrating immune cells, regulation of the inflammation, and cancer development are not well understood. Therefore, we aimed to review the current information available on the role of Tregs in the pathogenesis of IBD, colitis-associated colon cancer and sporadic colorectal cancer.
Tregs are able to suppress the expansion of effector T cells by interleukin (IL)-10 and transforming growth factor (TGF)-β secretion and cytotoxic T lymphocyte-associated antigen expression[11]. The frequency of circulating Tregs was shown to be lower in patients with relapsing than in remitting autoimmune diseases[12,13], therefore leading to the speculation that insufficient levels of Tregs in peripheral blood are critical in inducing a relapse of autoimmune disease.
Maul et al[14] reported that CD4+CD25+ cells were decreased in the peripheral blood of IBD patients. Moreover, Wang et al[3] found the percentage of CD4+Foxp3+ Tregs in peripheral blood to be significantly lower in IBD (both ulcerative colitis and Crohn’s disease) patients compared with healthy controls. However, at the mucosal level, Maul et al[14] and Wang et al[3] found the frequency of Tregs higher in active and inactive IBD samples compared to healthy controls. Their results and the results of Holmén et al[15] indicated that the increased frequency of CD25+ T cells in inflamed mucosa might be associated with the decreased number of circulating CD4+CD25+ T cells. During active inflammation, circulating Tregs migrate into the lamina propria to maintain homeostasis. As direct cell-to-cell contact is critical for Tregs to function, a low number of Tregs may result in a disturbance in the inhibition of the inflammatory reaction[15,16]. The results of Wang et al[3] suggest that an insufficient number of Tregs in the peripheral blood may be associated with the recurrence of IBD.
While Wang et al[3] did not find any alteration in the frequency of mucosal CD4+Foxp3+ Tregs in ulcerative colitis and Crohn’s disease, Hovhannisyan et al[17] showed that the inflammatory environment in the colonic mucosa of Crohn’s disease patients (but not in that of ulcerative colitis patients) contributes to the generation of a distinct population of Tregs that are Foxp3+ and produce IL-17. These Foxp3+ IL-17-producing cells also produced large amounts of interferon (IFN)-γ, another effector cytokine. On the other hand, Kryczek et al[18] observed mucosal Foxp3+IL17+CD4+ Tregs in ulcerative colitis that induce proinflammatory cytokines and suppress effector T cell function, exhibiting their dual inflammatory and regulatory function.
The balance between these subpopulations of mucosal Treg cells, which may be regulated by the inflammatory milieu, seems to be crucial in the pathogenesis of IBD.
The IL-17-producing CD4+ T (Th17) cells constitute a subset of helper T cells that has been linked to the pathogenesis of IBD[19]. Mucosal Th17 cells are thought to protect the host from infection.
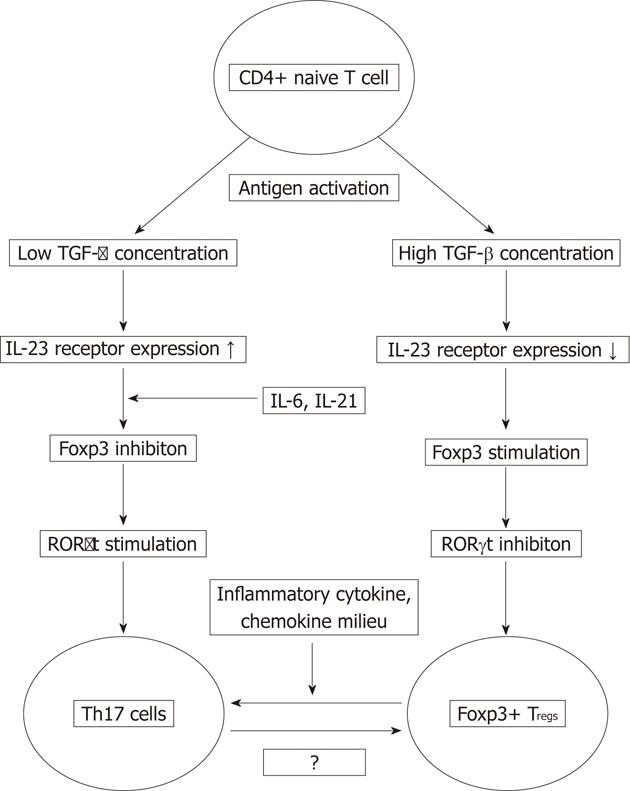
The development of Th17 cells and Tregs requires TGF-β which suggests that both cell types may be related and may arise from the same precursor based on the distinct cytokine milieus[20-23]. TGF-β exposure of antigen-activated naive CD4+ T cells results in upregulation of Foxp3 and retinoic acid-related orphan receptor (RORγt), two transcription factors that regulate Treg and Th17 cell differentiation[24]. Zhou et al[25] showed that at low concentrations, TGF-β synergizes with IL-6 and IL-21 to promote IL-23 receptor (IL23R) expression, favouring Th17 cell differentiation, while in high concentrations it represses IL23R expression and favours Foxp3+ Tregs differentiation. In vitro, TGF-β-induced Foxp3 inhibits RORγt function, at least in part through their interaction. Consequently, lamina propria Foxp3+RORγt+T cells produce less IL-17 than those that express RORγt alone. IL-6, IL-21 and IL-23 inhibit Foxp3-mediated inhibition of RORγt, therefore promoting Th17 cell differentiation. Based on these data it seems, that the decision of antigen-stimulated cells to differentiate into either Th17 or Treg cell depends on the cytokine-regulated balance of Foxp3 and RORγt[25].
Hovhannisyan et al[17] identified such a Foxp3+ IL-17-producing CD4+ Treg cell population in the lamina propria of Crohn’s disease patients that shared phenotypic characteristics of both Th17 and Treg cells, and showed potent suppressor activity in vitro. Their analysis of the TCR repertoire revealed a similar TCR β-chain variable region usage by Foxp3+IL-17+ and Foxp3+CD4+ T cells, suggesting that Foxp3+ IL-17-producing cells may come from Foxp3+ Tregs when exposed to unique inflammatory signals present in Crohn’s disease (Figure 1).
Increased mucosal TGF-β and IL-6 expression have been detected in Crohn’s disease[26,27]. Similar results were not found in ulcerative colitis associated mucosa[26,27]. These cytokines are supposed to promote the conversion of Tregs into effector T cells, while retaining their suppressive properties[17,20]. Despite the presence of cytokines involved in Th17 and Treg cell differentiation, the generation of Foxp3+IL-17+ Tregs from normal lamina proprial or peripheral blood CD4+ T cells failed[17]. Therefore it is speculated that additional, presumably local, factors are also required for the induction of these cells in vivo. Kryczek et al[18] found that these inflammatory Tregs are induced from memory chemokine (CC motif) receptor 6+ T cells and Tregs. Furthermore, TGF-β, IL-2 and the presence of antigen presenting cells are also essential for their induction.
The better understanding of the origin and function of Tregs, and the interaction between effector and regulatory sites of mucosal immune cells in IBD may lead to new therapeutic possibilities.
In mice, Faubion et al[28] showed that chronic inflammation arising from the bowel may induce thymic involution and Treg cell suppression. This was suggested to lead to the enhancement of inflammation-mediated events that worsen IBD. Restoration of homeostasis through suppression of tumor necrosis factor (TNF)-α production and fortification of Tregs were proposed for human IBD patients[4]. These data on IBD may support to the concept that uncontrolled inflammation weakens the Treg-mediated inhibition and increases the risk for inflammation-associated carcinogenesis.
In colitis-associated colon cancer, a high frequency of Foxp3+IL-17+CD4+ Tregs was found in the tumor, but not in the adjacent mucosa[18]. Though human tumor-infiltrating Tregs express limited effector cytokines[29], colitic Foxp3+IL-17+CD4+ Tregs express a moderate amount of IFN-γ, TNF-α and IL-2, and shares similar cytokine profiles to blood Foxp3+IL-17+ Tregs[18]. This cytokine profile reveals a phenotype for polyfunctional effector T cells similar to that observed in infectious diseases[30,31] and tumor-infiltrating Th17 cells in multiple human cancers[32]. These data indicate that Foxp3+IL-17+ Tregs express effector cytokines and are distinct from Tregs within the same inflammatory milieu.
Moreover, in suppressive assays, Foxp3+IL-17+ T cells from colitis-associated colon cancer were able to suppress T cell proliferation and IFN-γ production in a dose-dependent manner[18], which suggests that Foxp3+IL-17+ T cells belong to a functional Treg cell population.
Kryczek et al[18] also found that Tregs induce the production of IL-1 and IL-6 in ulcerative colitis. IL-17 derived from Foxp3+IL-17+CD4+ T cells may also induce IL-6 expression[33,34]. As IL-1 and IL-6 play a crucial role in ulcerative colitis[35,36], it is possible that IL-17-producing Foxp3+CD4+ T cells together with Th17 cells may potentially contribute to the early tumorigenesis seen in chronic ulcerative colitis.
The role of pathogenic microbial infection in colitis-associated carcinogenesis is also known[4]. After pathogenic microbial challenges, a pro-inflammatory response mediated by effector T cells serves to eliminate pathogenic bacteria. Educated CD4+CD45RBlow Tregs then subsequently suppress inflammation, offering protection from immunological self-destruction. It was shown that IL-10-dependent Tregs protect from malignancy[37,38]. Deficiency in IL-10 increases susceptibility to IBD-associated colorectal cancer (CRC) with poor prognosis[39]. Mice lacking IL-10 were shown to be susceptible to colitis-associated CRC after Helicobacter hepaticus (H. hepaticus) infection, while their wild type counterparts infected with H. hepaticus had only minimal bowel alterations[37,38,40,41]. The lack of IL-10 leads to elevated levels of TNF-α, IL-6 and IL-17, therefore allowing chronic inflammation to persist[42]. After H. hepaticus infection, IL-10 insufficient CD4+ T cells preferentially recruit to a Th-17 phenotype in the case of a pro-inflammatory challenge[4-45]. It was also shown in mice that bacteria-triggered inflammation mobilizes neutrophils bearing TGF-β1 and IL-6, which may inhibit Tregs and promote Th-17 response, a phenomenon that has been linked to carcinogenesis[40]. Based on these results, one may conclude that colonic bacterial infections supposed to trigger protective IL-10-dependent Tregs, while, under hygienic conditions, individuals with insufficiently educated Tregs are predisposed to sustained inflammatory reactions and may subsequently developed cancers[4].
Acting downstream of TGF-β1 signaling, Runx proteins are the interacting and functional partners of R-Smad proteins[46]. Among Runx proteins, Runx3 is involved in the differentiation of immune cells[47], and acts as a homeostasis maintainer and tumor suppressor in the gastrointestinal epithelium[48,49]. In mice, Sugai et al[47] found that the loss of Runx3 in T cells resulted in suppression of Treg cell function which lead to the development of colitis and colonic tumors (associated with luminal microorganisms) in Runx3-/- animals.
Further studies are needed to reveal the interaction between the commensal intestinal bacterial flora and Tregs for the better understanding of colitis-associated colorectal carcinogenesis.
Cancer cells have evolved to develop strategies of immunoevasion to escape control by the immune system[50]. There are quite numerous possible immunoevasion mechanisms that appear to affect diverse steps in cancer-specific lymphocyte priming, activation, and effector function. These mechanisms may also operate simultaneously[51,52]. Evidence is accumulating that CD4+CD25+Foxp3+ Tregs are crucially involved in cancer immunobiology[10]. The number of Foxp3+ Tregs was found to be significantly increased in the peripheral blood, lymph nodes and surrounding tumors of patients with colorectal cancer compared to that of healthy controls[20,53]. Several data show that increased frequencies of Tregs prognosticate a worse outcome for colon cancer patients, as this may reflect the inability of the immune system to adequately respond to cancer[54-56]. Moreover, studies aimed at reducing Treg cell frequencies in experimental models have resulted in successful control of cancer[57].
On the contrary, others showed that an increased intratumoral Foxp3+ Treg cell number correlates with a favourable clinical outcome[58]. This can be the result of mast cell-Treg cell interaction, which induces Tregs to switch function and escalate inflammation in CRC without losing T-cell-suppressive properties in an IL6 and IL17 independent manner[59].
Szczepanik et al[60] found that the absolute number of Tregs in the peripheral blood of gastric cancer patients, especially in those with lymph node metastases was significantly decreased in comparison to that of healthy controls. This phenomenon was not observed in CRC patients. Their results suggest that the population of Tregs in peripheral blood does not simply mimic intratumoral Tregs.
Based on this data, one can conclude that when inflammatory cells that promote tumor progression dominate the immune response, Tregs may be beneficial in suppressing carcinogenesis. However, when T cells dominate the immune response, Tregs may promote disease progression by suppressing their anti-tumoral immunological effects. The discrepancy between the peripheral blood and local, intratumoral number phenotype of Tregs in sporadic CRC may have an effect on this theory, and should be further investigated.
The colonic adenoma-dysplasia-carcinoma sequence is associated less clearly with inflammation then it is with colitis-associated colon cancer. C57BL/6 mice heterozygous for a mutation in the Apc gene (ApcMin/+) are genetically at risk for intestinal polyps and mimic early stages of sporadic CRC in humans[61]. It was observed, that ApcMin/+ mice have higher serum and intestinal levels of TNF-α, IL-6 and IL-17 than matched wild type animals, which may indicate a subclinical inflammatory condition[4]. Elevated inflammatory cytokine levels were found in human colorectal cancer as well[62].
Using Treg cell titration assay it was also shown, that Tregs from H. hepaticus infected donor ApcMin/+ mice consistently provided complete protection from cancer and more effectively suppressed TNF-α, IL-6 and IL-17 cytokine levels in tumor-prone tissues than Tregs from uninfected donor mice[4,63]. It is thought that the protection from cancer is attributable to Treg cell functions, and is more dependent upon the former conditions of Tregs than that of the recipient mice.
In humans, Ma et al[64] revealed that CD4+CD25+Foxp3+IL-17+ T cells in CRC tissue express TGF-β and IL-10, the functional molecules of Tregs. This subset of T cells suppresses anti-tumoral peripheral CD8+ T cells in a tumor antigen-specific manner, therefore contributing to cancer development. If the effector CD8+ T cells could disengage from the suppression of CD4+CD25+Foxp3+IL-17+ Tregs, they might again proliferate and fight against tumor development.
It has been shown that chemokine (CC motif) ligand (CCL) 5 is highly expressed in colon tumor tissues from early-stage CRC compared with paired normal tissues[65]. CCL5 promotes tumor growth and metastasis by inducing tumor cell proliferation, angiogenesis, and expression of matrix metalloproteinases[66,67]. It can also diminish anti-tumoral immune responses by increasing the presence of tumor-infiltrating macrophages and Tregs[67]. Chang et al[68] have recently found that CCL5 expression could not only promote migration of Tregs to tumors, but also enhance their killing ability on CD8+ T cells. Furthermore, this enhanced killing ability was associated with an increase in production of TGF-β by Tregs. Based on these results, it seems that the production of CCL5 by cancer cells is a part of CRC’s immunoevasion repertoire.
Further studies may help us to better understand the interaction between tumor cells and Tregs and to identify an unrevealed mechanism by which tumor cells induce immune tolerance in CRC.
Targeting Tregs may offer an important therapeutic strategy as an adjunct to treatment of patients.
A better understanding of the microenvironmental cues that influence Treg cell commitment toward a given lineage and their balance between regulation and inflammation may result in new therapeutic targets in IBD and colitis-associated cancer.
Tregs and IL-10 producing Tr1 cells have the potential to prevent or cure colitis in graft-versus-host disease, a finding supported by a favourable safety profile was demonstrated in phase I clinical trials[69]. Although it must be confirmed in further clinical trials, these results emphasize that Tregs may also be promising tools for therapeutic applications in IBD[70]. Changing the distribution of peripheral and mucosal Tregs may also have therapeutic potential in colonic inflammations.
We presently lack sufficient data to determine the clinical effect of an immunotherapy targeting Tregs in patients with sporadic CRC. The unexpected finding[58] that tumor-infiltrating Foxp3+ T cells could be associated with a favourable prognosis in CRC, suggests the potential in targeting these cells in immune-based anti-cancer therapies. On the other hand, eliminating CD4+CD25+Foxp3+IL-17+ Tregs may help the effector CD8+ T cells to fight against cancer cells. Therefore this subset of T cells may offer a novel therapeutic target in the treatment of CRC[64]. In conclusion, these conflicting results should be appreciated with some discretion, as the mechanisms linking tumor-infiltrating Foxp3+ Treg cell density with a favourable outcome remain equivocal.
We thank Tiana M Germann for her assistance in English language editing.
Peer reviewer: Dr. Pingchang Yang, Pathology and Molecular Medicine, McMaster University, BBI-T3330, 50 Charlton Ave East, Hamilton L8N 4A6, Canada
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303-1310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 839] [Cited by in RCA: 849] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 2. | Blumberg RS, Strober W. Prospects for research in inflammatory bowel disease. JAMA. 2001;285:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Wang Y, Liu XP, Zhao ZB, Chen JH, Yu CG. Expression of CD4+ forkhead box P3 (FOXP3)+ regulatory T cells in inflammatory bowel disease. J Dig Dis. 2011;12:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Erdman SE, Poutahidis T. Roles for inflammation and regulatory T cells in colon cancer. Toxicol Pathol. 2010;38:76-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Nishikawa H, Kato T, Tawara I, Takemitsu T, Saito K, Wang L, Ikarashi Y, Wakasugi H, Nakayama T, Taniguchi M. Accelerated chemically induced tumor development mediated by CD4+CD25+ regulatory T cells in wild-type hosts. Proc Natl Acad Sci USA. 2005;102:9253-9257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 752] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 7. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6391] [Article Influence: 290.5] [Reference Citation Analysis (0)] |
| 8. | Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1729] [Cited by in RCA: 1832] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 9. | Loser K, Beissert S. Regulatory T cells: banned cells for decades. J Invest Dermatol. 2012;132:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 11. | Levings MK, Bacchetta R, Schulz U, Roncarolo MG. The role of IL-10 and TGF-beta in the differentiation and effector function of T regulatory cells. Int Arch Allergy Immunol. 2002;129:263-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 305] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Frisullo G, Nociti V, Iorio R, Patanella AK, Caggiula M, Marti A, Sancricca C, Angelucci F, Mirabella M, Tonali PA. Regulatory T cells fail to suppress CD4T+-bet+ T cells in relapsing multiple sclerosis patients. Immunology. 2009;127:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Suen JL, Li HT, Jong YJ, Chiang BL, Yen JH. Altered homeostasis of CD4(+) FoxP3(+) regulatory T-cell subpopulations in systemic lupus erythematosus. Immunology. 2009;127:196-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 497] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 15. | Holmén N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjövall H, Ohman L. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12:447-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 16. | Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn's disease. Clin Immunol. 2007;125:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 17. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 18. | Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388-4395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 19. | Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali F. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849-1861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1399] [Cited by in RCA: 1480] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 20. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5475] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 21. | Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2489] [Article Influence: 131.0] [Reference Citation Analysis (0)] |
| 22. | Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 755] [Cited by in RCA: 808] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 23. | Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340-2352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 612] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 24. | Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1569] [Cited by in RCA: 1541] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 25. | Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1566] [Cited by in RCA: 1702] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 26. | Del Zotto B, Mumolo G, Pronio AM, Montesani C, Tersigni R, Boirivant M. TGF-beta1 production in inflammatory bowel disease: differing production patterns in Crohn's disease and ulcerative colitis. Clin Exp Immunol. 2003;134:120-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | León AJ, Gómez E, Garrote JA, Bernardo D, Barrera A, Marcos JL, Fernández-Salazar L, Velayos B, Blanco-Quirós A, Arranz E. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Faubion WA, De Jong YP, Molina AA, Ji H, Clarke K, Wang B, Mizoguchi E, Simpson SJ, Bhan AK, Terhorst C. Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in the periphery. Gastroenterology. 2004;126:1759-1770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3582] [Cited by in RCA: 3882] [Article Influence: 184.9] [Reference Citation Analysis (0)] |
| 30. | Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405-1416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 31. | Almeida JR, Price DA, Papagno L, Arkoub ZA, Sauce D, Bornstein E, Asher TE, Samri A, Schnuriger A, Theodorou I. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473-2485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 600] [Cited by in RCA: 578] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 32. | Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 33. | Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1035-G1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 172] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 34. | Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 670] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 35. | Gotteland M, Lopez M, Muñoz C, Saez R, Altshiller H, Llorens P, Brunser O. Local and systemic liberation of proinflammatory cytokines in ulcerative colitis. Dig Dis Sci. 1999;44:830-835. [PubMed] |
| 36. | Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1511] [Article Influence: 83.9] [Reference Citation Analysis (2)] |
| 37. | Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 38. | Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042-6050. [PubMed] |
| 39. | Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 399] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 40. | Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN. Nitric oxide and TNF-alpha trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009;106:1027-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 162] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 41. | Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, Powrie F, Cheever AW, Maloy KJ. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485-2494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 475] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 43. | Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 678] [Cited by in RCA: 654] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 44. | Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 480] [Cited by in RCA: 466] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 45. | Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin Immunol. 2007;19:362-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 334] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 46. | Zhang Y, Derynck R. Transcriptional regulation of the transforming growth factor-beta -inducible mouse germ line Ig alpha constant region gene by functional cooperation of Smad, CREB, and AML family members. J Biol Chem. 2000;275:16979-16985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Sugai M, Aoki K, Osato M, Nambu Y, Ito K, Taketo MM, Shimizu A. Runx3 is required for full activation of regulatory T cells to prevent colitis-associated tumor formation. J Immunol. 2011;186:6515-6520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 48. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 835] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 49. | Ito K, Lim AC, Salto-Tellez M, Motoda L, Osato M, Chuang LS, Lee CW, Voon DC, Koo JK, Wang H. RUNX3 attenuates beta-catenin/T cell factors in intestinal tumorigenesis. Cancer Cell. 2008;14:226-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 50. | Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565-1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4947] [Cited by in RCA: 4542] [Article Influence: 324.4] [Reference Citation Analysis (0)] |
| 51. | Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1353] [Cited by in RCA: 1297] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 52. | Yaguchi T, Sumimoto H, Kudo-Saito C, Tsukamoto N, Ueda R, Iwata-Kajihara T, Nishio H, Kawamura N, Kawakami Y. The mechanisms of cancer immunoescape and development of overcoming strategies. Int J Hematol. 2011;93:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-Loebenstein B. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606-612. [PubMed] |
| 54. | Ling KL, Pratap SE, Bates GJ, Singh B, Mortensen NJ, George BD, Warren BF, Piris J, Roncador G, Fox SB. Increased frequency of regulatory T cells in peripheral blood and tumour infiltrating lymphocytes in colorectal cancer patients. Cancer Immun. 2007;7:7. [PubMed] |
| 55. | Sellitto A, Galizia G, De Fanis U, Lieto E, Zamboli A, Orditura M, De Vita F, Giunta R, Lucivero G, Romano C. Behavior of circulating CD4+CD25+Foxp3+ regulatory T cells in colon cancer patients undergoing surgery. J Clin Immunol. 2011;31:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Sinicrope FA, Rego RL, Ansell SM, Knutson KL, Foster NR, Sargent DJ. Intraepithelial effector (CD3+)/regulatory (FoxP3+) T-cell ratio predicts a clinical outcome of human colon carcinoma. Gastroenterology. 2009;137:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 263] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 57. | Schabowsky RH, Madireddi S, Sharma R, Yolcu ES, Shirwan H. Targeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapy. Curr Opin Investig Drugs. 2007;8:1002-1008. [PubMed] |
| 58. | Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 805] [Article Influence: 47.4] [Reference Citation Analysis (0)] |
| 59. | Blatner NR, Bonertz A, Beckhove P, Cheon EC, Krantz SB, Strouch M, Weitz J, Koch M, Halverson AL, Bentrem DJ. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc Natl Acad Sci USA. 2010;107:6430-6435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 60. | Szczepanik AM, Siedlar M, Sierzega M, Goroszeniuk D, Bukowska-Strakova K, Czupryna A, Kulig J. T-regulatory lymphocytes in peripheral blood of gastric and colorectal cancer patients. World J Gastroenterol. 2011;17:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1131] [Cited by in RCA: 1161] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 62. | Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 445] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 63. | Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395-7400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Ma C, Dong X. Colorectal cancer-derived Foxp3(+) IL-17(+) T cells suppress tumour-specific CD8+ T cells. Scand J Immunol. 2011;74:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Erreni M, Bianchi P, Laghi L, Mirolo M, Fabbri M, Locati M, Mantovani A, Allavena P. Expression of chemokines and chemokine receptors in human colon cancer. Methods Enzymol. 2009;460:105-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Sugasawa H, Ichikura T, Kinoshita M, Ono S, Majima T, Tsujimoto H, Chochi K, Hiroi S, Takayama E, Saitoh D. Gastric cancer cells exploit CD4+ cell-derived CCL5 for their growth and prevention of CD8+ cell-involved tumor elimination. Int J Cancer. 2008;122:2535-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 67. | Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 442] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 68. | Chang LY, Lin YC, Mahalingam J, Huang CT, Chen TW, Kang CW, Peng HM, Chu YY, Chiang JM, Dutta A. Tumor-derived chemokine CCL5 enhances TGF-β-mediated killing of CD8(+) T cells in colon cancer by T-regulatory cells. Cancer Res. 2012;72:1092-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 69. | Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 536] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 70. | van der Marel S, Majowicz A, van Deventer S, Petry H, Hommes DW, Ferreira V. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol. 2011;2:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (2)] |