Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.356
Revised: July 18, 2011
Accepted: July 25, 2011
Published online: January 28, 2012
AIM: To further investigate the important role of store-operated calcium channels (SOCs) in rat hepatocytes and to explore the effects of SOC blockers on hepatic ischemia-reperfusion injury (HIRI).
METHODS: Using freshly isolated hepatocytes from a rat model of HIRI (and controls), we measured cytosolic free Ca2+ concentration (by calcium imaging), net Ca2+ fluxes (by a non-invasive micro-test technique), the SOC current (ISOC; by whole-cell patch-clamp recording), and taurocholate secretion [by high-performance liquid chromatography and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays].
RESULTS: Ca2+ oscillations and net Ca2+ fluxes mediated by Ca2+ entry via SOCs were observed in rat hepatocytes. ISOC was significantly higher in HIRI groups than in controls (57.0 ± 7.5 pA vs 31.6 ± 2.7 pA, P < 0.05) and was inhibited by La3+. Taurocholate secretion by hepatocytes into culture supernatant was distinctly lower in HIRI hepatocytes than in controls, an effect reversed by SOC blockers.
CONCLUSION: SOCs are pivotal in HIRI. SOC blockers protected against HIRI and assisted the recovery of secretory function in hepatocytes. Thus, they are likely to become a novel class of effective drugs for prevention or therapy of HIRI patients in the future.
- Citation: Pan LJ, Zhang ZC, Zhang ZY, Wang WJ, Xu Y, Zhang ZM. Effects and mechanisms of store-operated calcium channel blockade on hepatic ischemia-reperfusion injury in rats. World J Gastroenterol 2012; 18(4): 356-367
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.356
Hepatic ischemia-reperfusion injury (HIRI) can occur in the liver in response to a wide variety of clinical and operative situations, including hemorrhagic shock, severe hepatic trauma, major hepatic resection/biliary tract operation with temporary clamping of hepatoduodenal ligament, and liver transplantation. HIRI can lead to liver dysfunction (or even loss of function) and thus represents a major therapeutic challenge.
The pathogenesis of HIRI is multifactorial, involving hepatocellular Ca2+ overload[1-5], release of excessive oxygen-derived free radicals[6,7], inflammatory cytokines[8], Kupffer cell activation[9,10], impairment of microvessels[11], apoptosis and nuclear factor kappa B[12]. Ca2+ are an important second messenger, and variation of intracellular Ca2+ concentration ([Ca2+]i) has been shown to play an important role in regulating a variety of physiological processes in both excitable and non-excitable cells. [Ca2+]i is a key factor in HIRI because it is integral to the activation of calcium-dependent phospholipases, nucleases and proteases, as well as oxidative phosphorylation. [Ca2+]i may be increased by the release of Ca2+ from the endoplasmic reticulum (ER) (or sarcoplasmic reticulum) or by the stimulation of Ca2+ entry from the extracellular space through calcium channels that are voltage-dependent (VDCC), receptor-operated or store-operated[13,14]. Hepatocytes are not known to express VDCC[15-18] but do express store-operated calcium channels (SOCs)[19-21]. The concept of a “store-operated calcium current” was proposed in 1986[22] and has been developed more fully during the past 20 years, including the identification of putative SOC proteins[14]. However, whether SOCs are the dominant Ca2+ channel in hepatocytes is uncertain. Studies have found that hepatocellular Ca2+ overload plays a crucial role in HIRI, although the exact mechanism of this overload remains unknown, as does the role of SOCs. Thus, no effective drugs currently exist for the prevention or therapy of HIRI.
In the present study, we aim to (1) further verify the importance of SOCs in rat hepatocytes, (2) explore the effects of SOCs in HIRI, and (3) investigate the potential use of SOC blockers for protecting against HIRI.
Male Sprague-Dawley rats weighing 180-200 g were obtained from the Beijing Laboratory Animal Research Center (Beijing, China). RPMI 1640 medium, penicillin, streptomycin, Fluo-4-acetoxymethyl (Fluo-4/AM), and poly-D-lysine were purchased from Invitrogen (United States). Collagenase IV, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), adenosine-triphosphate (ATP), CsOH, Taurocholic acid sodium salt, and SKF-96365 were obtained from Sigma (United States). Ethylene glycol tetraacetic acid (EGTA) was obtained from Solarbio (Beijing, China). Vasopressin, noradrenalin, thapsigargin (TG), and 2-aminoethoxydiphenyl borate (2-APB) were obtained from Merck KcaA (Darmstadt, Germany). Methanol and acetonitrile were of chromatographic grade; all other reagents were of reagent grade.
Rat hepatocytes were enzymatically isolated using a modification of the method originally reported by Seglen[23]. Briefly, rats were anesthetized with chloral hydrate (320 mg/kg body weight) and heparinized (1.5 U/g body weight) via intraperitoneal injection. A midline laparotomy was performed, and portal vein and the inferior vena cava were cannulated. The liver was initially perfused for 10 min at a constant flow rate of 25-30 mL/min with a modified oxygenated Ca2+-/Mg2+-free Hanks solution containing (in mmol/L) NaCl, 120; KCl, 5; Na2HPO4, 0.2; KH2PO4, 0.2; NaHCO3, 25; EGTA, 0.5; and glucose, 5 (pH 7.4), followed by perfusion with Type IV collagenase (0.2 g/L) in RPMI 1640 medium for 10 min. The solution was gassed with 100% O2 and warmed to 37 °C. After the perfusions, the three large cephalad lobes of the liver were excised and minced in a Ca2+-/Mg2+-free Hanks solution at 0 °C, before being filtered through a 74-μm nylon mesh and washed three times by centrifugation at 50 ×g for 2 min. The isolated hepatocytes (1 × 105/mL; 85%-95% viability assessed by trypan blue exclusion) were incubated in RPMI 1640 medium containing 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37 °C in a 95% air-5% CO2 incubator for 1 h.
The rat model of HIRI was established according to our previously reported procedure[10]. Briefly, under anesthesia and heparinization (as above), a midline laparotomy was performed in each rat, and an atraumatic clip was used to interrupt the arterial and portal venous blood supply to the three cephalad lobes. After 20 min of hepatic ischemia, the clip was removed, and the liver was reperfused for a further 40 min. Sham-operated control animals were treated in an identical manner, with the omission of vascular occlusion.
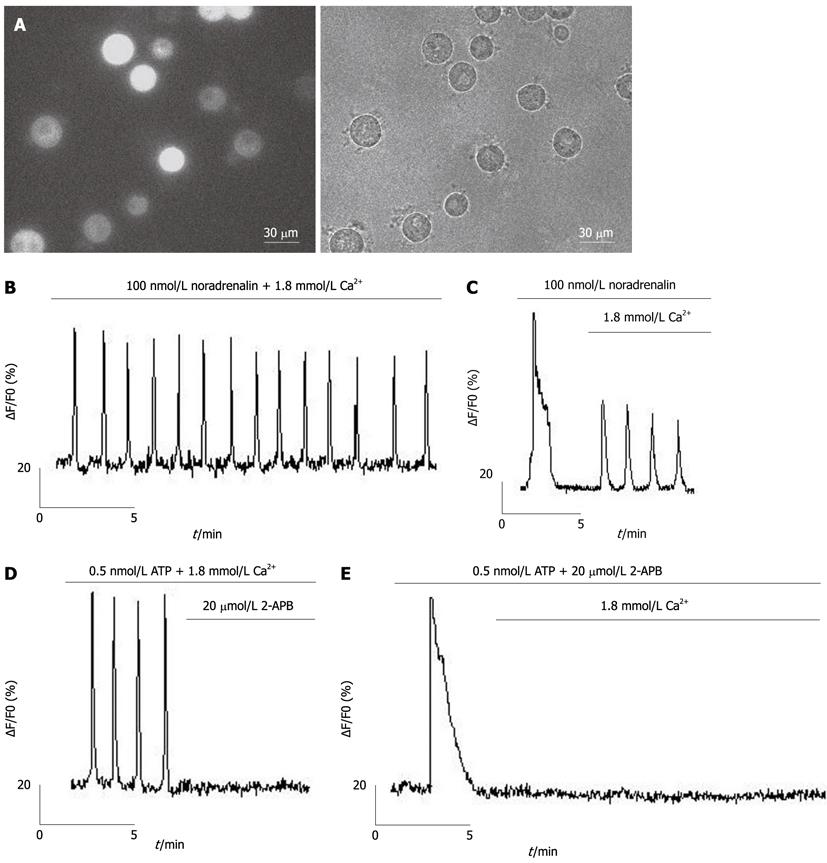
Isolated hepatocytes were seeded in 35-mm glass-bottomed dishes (MatTek, Ashland, MA, United States), pretreated with poly-D-lysine (500 μg/mL in borate buffer) for 2 h, and loaded with 6.7 μmol/L fluo-4/AM (Figure 1A) in a recording solution containing (in mmol/L) NaCl, 116; KCl, 5.4; CaCl2, 1.8; MgCl2, 0.8; glucose, 5.05; and HEPES, 10 (pH 7.4) for 30 min at 37 °C, followed by three washes in PBS and a 15-min incubation to allow de-esterification of fluo-4/AM before imaging. A Lambda DG-4 high-speed wavelength switcher (Sutter Instruments, Novato, CA, United States) was used for fluo-4 excitation at 480 nm, and a cooled charge-coupled device (CCD) camera (CoolSnap FX, Roper Scientific, Princeton, NJ, United States) was used for image acquisition. Meta Fluor imaging software (Universal Imaging, Downingtown, PA, United States) was used for hardware control, image acquisition and image analysis. Typically, time-lapse recording of Ca2+ signals in hepatocytes was performed for a 2-min control period before and for a 10-min period after the application of the different agonists. The CCD camera was used with a sampling rate of one frame per 2 s, a typical exposure time of 50-350 ms, and a 4 × 4 binning. Quantitative measurements of changes of [Ca2+]i were obtained by subtracting the average background intensity (measured in cell-free regions) from the average cellular fluo-4 fluorescence intensity values. Changes in [Ca2+]i for each hepatocyte were then represented by the changes in relative fluo-4 fluorescence (∆F/F0), where F0 was the baseline intensity obtained from the 2-min control period.
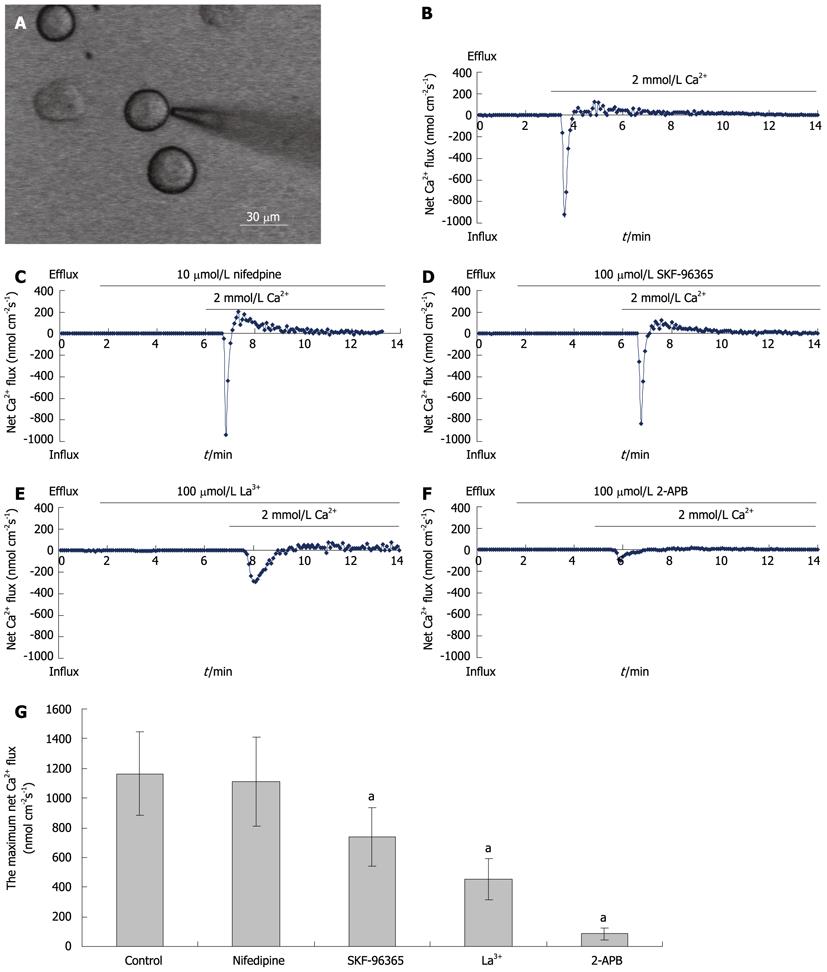
Measurements of net Ca2+ influx were performed using the non-invasive micro-test technique (NMT) system (BIO-IM, YoungerUnited States, Amherst, MA, United States) using our previously reported methods[24]. Briefly, isolated hepatocytes plated in a 35-mm dish (Figure 3A) were washed three times with a measuring solution containing (in mmol/L) NaCl, 136; KCl, 2.7; CaCl2, 0.2; KH2PO4, 1.5; Na2HPO4, 8; and glucose, 5.05 (pH 7.4). The electrode was controlled to move with an excursion of 10 μm at a programmable frequency in the range of 0.3-0.5 Hz, chosen to minimize mixing of the bathing saline. To construct the microelectrodes, borosilicate micropipettes (2-4 μm aperture, XYPG120-2, Xuyue Sci. and Tech. Co., Ltd., Beijing, 100080, China) were silanized with tributylchlorosilane, and the tips were filled with calcium ionophore I-cocktail A (Sigma-Aldrich, St Louis, MO, United States). An Ag/AgCl wire electrode holder (XYEH01-1) was inserted in the back of the electrode to make electrical contact with the electrolyte solution. Only electrodes with Nernstian slopes between 25 and 29 mV/decade were used. Ca2+ fluxes were calculated by Fick’s law of diffusion: J0 = -[D × (dC/dX)], where J0 represents the net Ca2+ flux (in μmol per cm per s), D is the self-diffusion coefficient for Ca2+ (in cm2/s), dC is the difference value of Ca2+ concentrations between the two positions, and dX is the 10 μm excursion over which the electrode moved in our experiments. Data and image acquisition, preliminary processing, control of the three-dimensional electrode positioner, and stepper-motor-controlled fine focus of the microscope stage were performed with imFlux® software.
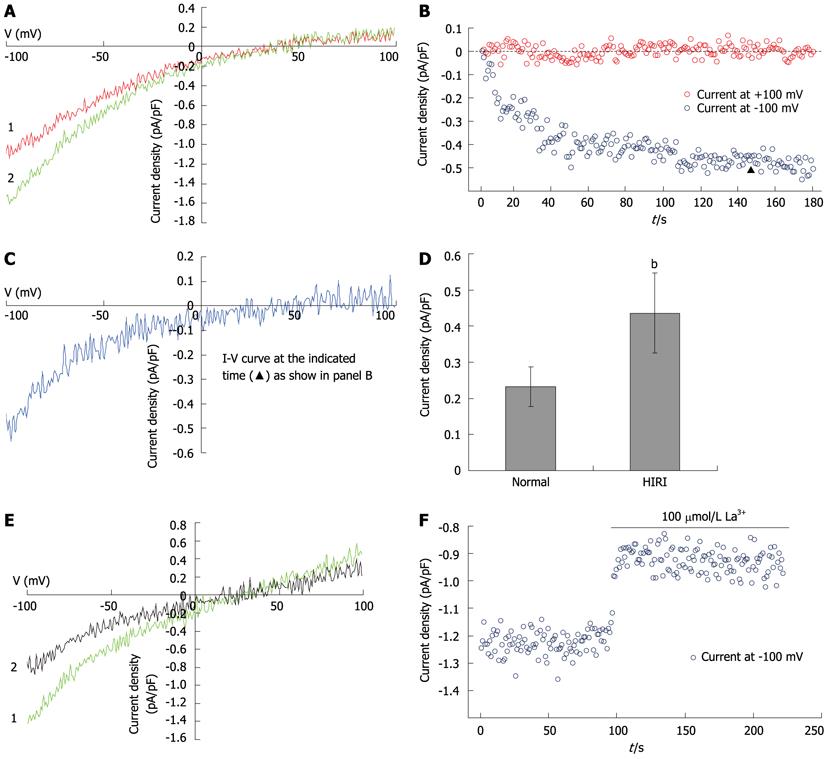
Whole-cell patch-clamp recording was performed at room temperature (22-25 °C) using a computer-based patch-clamp amplifier (EPC-10, HEKA Electronics, Lambrecht/Pfalz, Germany) and PatchMaster software (HEKA Electronics, Lambrecht/Pfalz, Germany). The isolated hepatocytes were plated in 35-mm dishes and washed with a standard external solution containing (in mmol/L) NaCl, 140; CsCl, 4; MgCl2, 2; CaCl2, 10; glucose, 10; and HEPES, 10 (pH 7.4; adjusted with NaOH). In experiments investigating Ba2+ current in rat hepatocytes, the NaCl, MgCl2 and CaCl2 in the external solution were replaced with 30 mmol/L of NaCl and 100 mmol/L of BaCl2. An automatic micropipette puller (Model P-97, Sutter Instruments, Novato, CA, United States) was used to pull the electrodes from the borosilicate glass. The pipette resistance was between 3 and 5 MΩ when filled with the pipette solution containing (in mmol/L) CsCl, 15; Cs glutamate, 135; EGTA, 10; and HEPES, 10 (pH 7.2; adjusted with CsOH). In all whole-cell patch-clamp recording experiments, recording started when the series resistance dropped to below 20 MΩ. After achieving the whole-cell configuration, voltage ramps of 50 ms duration, spanning a range from -100 mV to +100 mV, were immediately delivered from a holding potential of 0 mV every 2 s. Acquired currents were filtered at 2.9 kHz and sampled at 20 kHz. Capacitative currents were determined and compensated automatically by the EPC-10 amplifier. The voltages were corrected for a liquid-junction potential of 17 mV (estimated by JPCalc). The maximum SOC current (ISOC) at -100 mV was applied for statistical analysis.
High-performance liquid chromatography (HPLC) analysis was performed using LC-10A apparatus (Shimizu, Japan) with an Agilent Extend C18 column (416 × 150 mm, 5 μm). To create a fine chromatographic separation of taurocholate, a 60:20 (v/v) mixture of mobile phases A (methanol and acetonitrile 1:1) and B (5 mmol/L KH2PO4; pH 3.0) was employed. A constant flow rate of 0.8 mL/min was used for the quantitative determination of the standard solutions and test samples, with a column temperature of 30 °C, sample size of 10 μL, and detection wavelength of 210 nm. Freshly isolated hepatocytes were plated at 5 × 105/mL in 35-mm dishes at 37 °C in a 95% air-5% CO2 incubator and treated with 100 μmol/L 2-APB, 100 μmol/L La3+ or 10 μmol/L SKF-96365 for 12 h. The culture solution was collected and centrifuged at 500 rpm for 3 min. The supernatant was stored frozen at -20 °C. Supernatant (0.5 mL) was mixed intensively with 0.5 mL of absolute ethyl alcohol, incubated in a 60 °C water bath for 3 min, then centrifuged at 3000 rpm for 10 min. Supernatant (0.3 mL) was run through Sep-PAK columns, washed with 10 mL of pure water, and eluted with 3 mL of methanol. This eluent was placed in a water bath (70 °C), blown dry with nitrogen, and mixed with 3 mL of mobile phase.
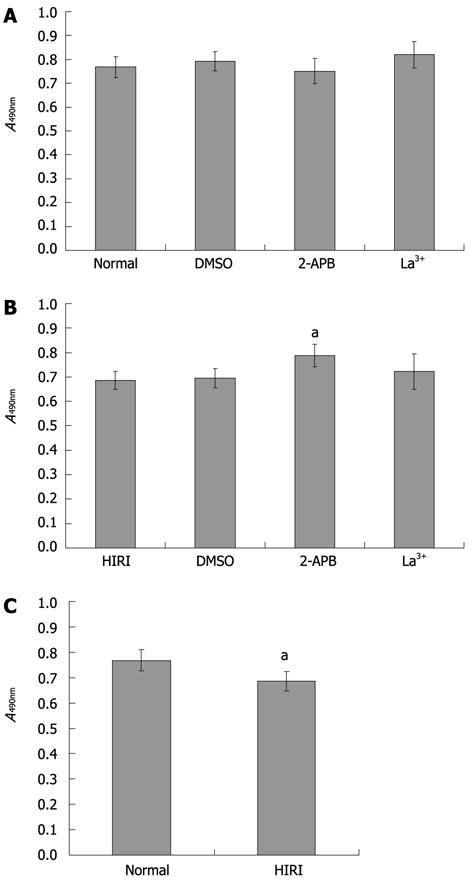
The method of Mosmann[25] for the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used with modifications for functional studies on cell survival/injury. Freshly isolated rat hepatocytes in 1640 medium were plated at 5 × 104 cells per well in 96-well microtiter plates. After 3 h, various SOC antagonists were added, or 0.025% (v/v) dimethylsulfoxide (DMSO) vehicle was used as control. Cells were cultured after drug exposure for 12 h at 37 °C in a 95% air-5% CO2 incubator, which is sufficient time for evidence of drug-induced cell death, increased cell survival or dampened cell injury to become apparent, as quantified by the generation of the formazan product from the MTT substrate. The number of hepatocytes per microtiter well was proportional to the absorbance of the solubilized formazan. After addition of MTT (5 mg/mL, 20 μL) and incubation for 4 h, the medium was discarded. MTT formazan crystals were then resolubilized with 150 μL DMSO per well and mixed on a microshaker for 10 min. The plate was then read immediately on a scanning multiwell spectrophotometer (Termo Multiscan MK3, Finland) at 490 nm.
IGOR Pro 5.01 software (Wavemetrics, Portland, OR, United States) was used to conduct an analysis of whole-cell patch-clamp recording data. All current traces were corrected for leak currents. Mageflux (http://www.xuyue.net/mageflux) was used to process the data of the NMT. All results are expressed as mean ± SD. Statistical significance of differences between test samples and controls was determined using the Student’s t-test. P values less than 0.05 were considered statistically significant differences.
Using calcium imaging, we investigated the relationship between Ca2+ entry and Ca2+ oscillations in hepatocytes under physiological conditions, with Ca2+ oscillations induced by noradrenaline in freshly isolated hepatocytes (Figure 1B). We found that Ca2+ oscillations were dependent on Ca2+ entry through the plasma membrane (Figure 1C). Ca2+ oscillations induced by ATP (activator of phospholipase C) were inhibited by 2-APB[26] (an inhibitor of SOCs, Figure 1D), which inhibited entry of extracellular Ca2+ but not release of Ca2+ from ER (Figure 1E).
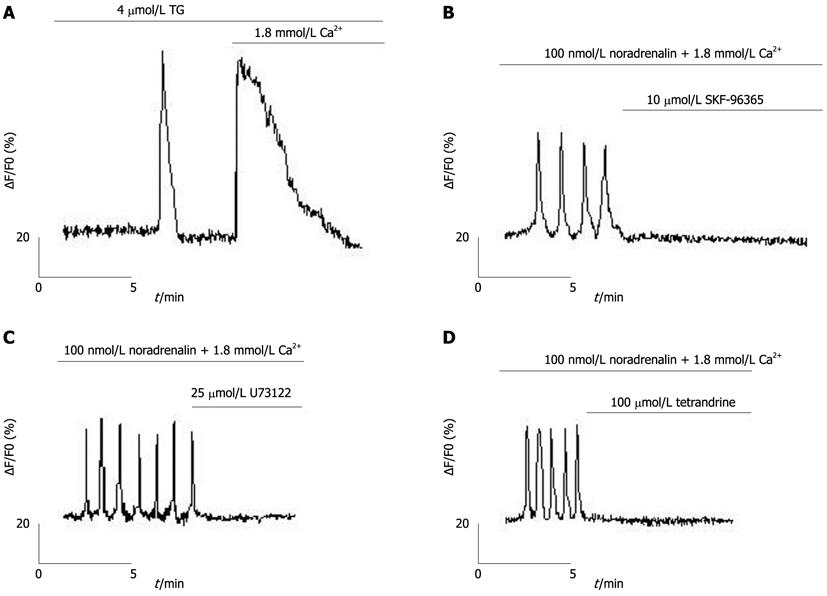
TG, an inhibitor of sarcoplasmic/endoplasmic reticulum Ca2+ pump (SERCA)[27], was used to passively eliminate hepatocyte ER calcium stores, evoking two peaks (one for the release of Ca2+ from the ER and the other for the subsequent Ca2+ entry through the plasma membrane; Figure 2A). Ca2+ oscillations in freshly isolated rat hepatocytes could be inhibited by 2-APB (Figures 1D and E). Moreover, Ca2+ oscillations could also be inhibited by the SOC inhibitor, SKF-96365[28,29] (Figure 2B), the phospholipase C (PLC) inhibitor, U73122[27], and the phospholipase A2 inhibitor, tetrandrine[30,31] (Figures 2C and D).
Using NMT, maximum net Ca2+ flux values of 1163 ± 279 nmol cm-2 s-1 were recorded in freshly isolated hepatocytes (n = 10; Figure 3B). Nifedipine (an inhibitor of VDCCs; 10 μmoL/L) had no effect on net Ca2+ fluxes (1108 ± 298 nmol cm-2 s-1) (n = 15; Figure 3C). The SOC blockers, SKF-96365, La3+ and 2-APB (all at 100 μmol/L), reduced net Ca2+ flux to 738 ± 195, 452 ± 136, and 84 ± 37 nmol cm-2 s-1, respectively (Figures 3D, E and F). These effects of SOC blockers on net Ca2+ fluxes were statistically significant (Figure 3G).
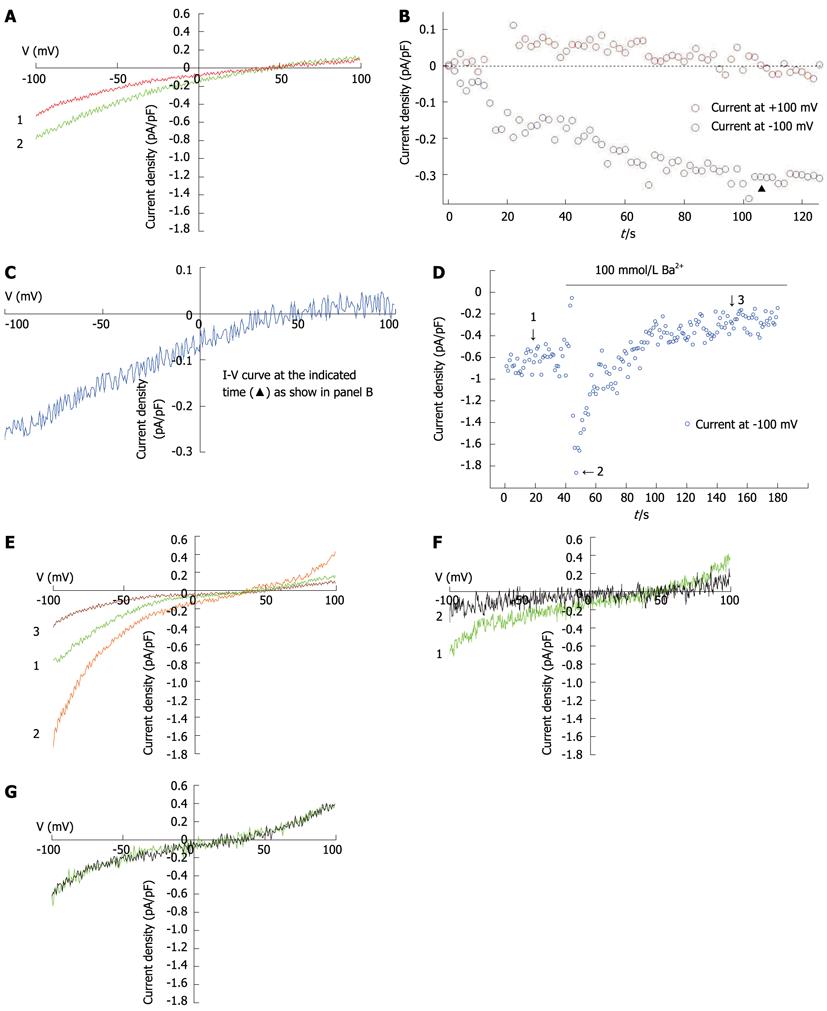
IP3 (10 μmol/L) and EGTA (10 mmol/L) induced currents in hepatocytes (Figures 4A and C), with a reverse potential of approximately +40 mV. The current-voltage relationship revealed that the currents were inwardly rectifying (Figures 4A, B and C). To further characterize the recorded currents, we substituted Ca2+ for Ba2+ in the external solution. The Ba2+ current (79 ± 7 pA) was significantly greater than the Ca2+ current (32 ± 1 pA, t = 5.683, P = 0.002), but much more transient, dropping to a lower level after less than 2 min (Figures 4D and E). The SOC inhibitor, 2-APB (100 mmol/L), inhibited the current induced by 10 mmol/L EGTA (Figure 4F). An inhibitor of non-selective cation channels, A9C (10 μmol/L), did not influence current magnitude (Figure 4G).
Hepatocellular Ca2+ overload is thought to play a crucial role in HIRI. To explore the effects of SOCs on the pathogenesis of HIRI, SOC currents induced by 10 mmol/L EGTA in hepatocytes were recorded in the HIRI and control groups (Figures 5A, B and C). The results showed that the SOC currents were significantly increased (from 31.6 ± 2.7 pA in controls to 57.0 ± 7.5 pA in HIRI hepatocytes; t = 2.682, P = 0.036; Figure 5D). In HIRI hepatocytes, the fully developed inward currents were rapidly reduced by 100 μmol/L La3+ (Figures 5E and F).
To explore the influence of SOC blockers on cell survival, we performed an MTT assay. No significant differences (P > 0.05) in cell survival rate (compared with normal hepatocytes) were observed after exposure to the DMSO vehicle or either of the two SOC inhibitors (Figure 6A). In addition, there was a similar survival rate in HIRI hepatocytes compared with hepatocytes pretreated with 0.025% (v/v) DMSO or 100 μmol/L La3+. However, the average optical absorbance value in hepatocytes pretreated with 100 μmol/L 2-APB (0.79 ± 0.05) was higher than in untreated HIRI hepatocytes (0.69 ± 0.04), P = 0.027, t = 4.047 (Figure 6B). Compared with normal hepatocytes, HIRI hepatocytes incurred cell damage (P = 0.029, t = 2.882; Figure 6C).
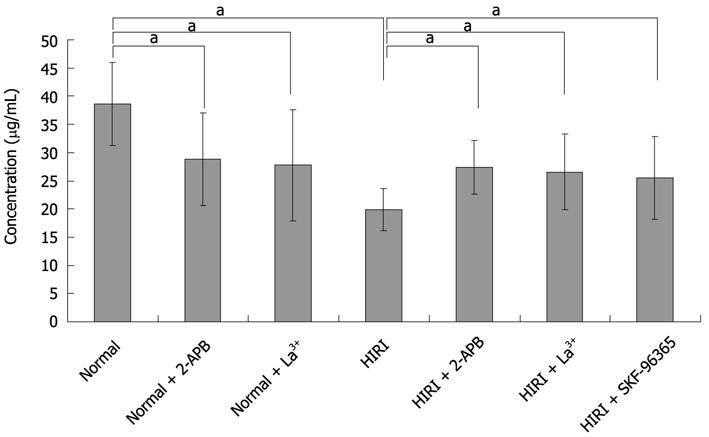
To explore the influence of SOCs on the secretory function of hepatocytes, taurocholate secretion by hepatocytes was investigated using HPLC analysis. Taurocholate concentration in the culture supernatant of normal hepatocytes was 38.58 ± 7.35 μg/mL (n = 6), notably higher than in hepatocytes treated with 100 μmol/L 2-APB (28.85 ± 8.18 μg/mL; P < 0.05, n = 6), 100 μmol/L La3+ (27.76 ± 9.86 μg/mL; P < 0.05, n = 6) or in HIRI hepatocytes (19.9 ± 3.8 μg/mL; P < 0.05, n = 6) (Figure 7). Interestingly, 100 μmol/L2-APB, 100 μmol/L La3+ and 10 μmol/L SKF-96365 could reverse the taurocholate level to 27.42 ± 4.74, 26.58 ± 6.67 and 25.52 ± 7.30 μg/mL, respectively, in HIRI hepatocytes, P < 0.05, n = 6.
Ca2+ is an important second messenger, and intracellular Ca2+ has been shown to play an important role in regulating a variety of physiological processes in both excitable and non-excitable cells. Intracellular Ca2+ homoeostasis and signaling are achieved by the complex interplay of Ca2+ fluxes among the cytosol, the intracellular stores and the extracellular environment[32]. SOCs are a family of Ca2+-permeable ion channels expressed by most cells. The signal for the activation of SOCs is a decrease in the Ca2+ concentration in the ER. Stimulation of a diverse range of plasma membrane receptors converges on and activates phosphatidylinositol 4,5-bisphosphate (PIP2) hydrolysis by PLC, which results in the generation of diacylglycerol and inositol 1,4,5-trisphosphate (IP3) and the subsequent activation of (1) Ca2+ release from the ER via IP3 receptors and (2) Ca2+ influx across the plasma membrane[33]; Stromal interaction molecule 1 (STIM1, identified as the ER Ca2+ sensor) and Orai1 (a pore-forming subunit of SOCs) have both been shown to be necessary for SOC function[33,34]. However, the role of SOCs during physiological activation of primary cells has not been extensively investigated, and there is little information on the roles of STIM and Orai proteins in primary cells. Our previous study found that functional interactions among STIM1, Orai1 and TRPC1 (transient receptor potential canonical 1) contribute to activating ISOC in human liver cells[35], but whether SOCs are the dominant Ca2+ channels in hepatocytes is still uncertain, and the nature of the Ca2+ influx mechanism following hormone activation of hepatocytes is controversial. For this purpose, we aimed to investigate the relationship between Ca2+ entry and Ca2+ oscillations in hepatocytes under physiological conditions and found that Ca2+ oscillations could be induced with noradrenaline, vasopressin or ATP, each of which stimulates PLC and activates both Ca2+ influx and intracellular Ca2+ release.
NMT is a novel non-invasive technology for obtaining dynamic information on specific ionic/molecular activities on material surfaces. This technique incorporates various temporal and spatial resolution domains from other traditional methods, and its three-dimensional measurement capability enables us to observe the physiological characteristics of biological phenomena that would be difficult or even impossible with other techniques[36]. To date, Ca2+, H+, K+, Cl-, NO-, Mg2+, Cd2+, Al3+, and O2 have been detected as sensors for ionic/molecular species. Our previous study found that NMT was a powerful tool for ion channel research, allowing us to demonstrate the existence of TRPC1-dependent Ca2+ channels in HL-7702 cells[24]. In the present study, we used NMT to investigate net Ca2+ fluxes in freshly isolated hepatocytes and showed that SOC inhibitors (2-APB, SKF-96365 and La3+), but not a VDCC inhibitor (nifedipine), blocked net Ca2+ fluxes, suggesting that these Ca2+ movements are mediated in rat hepatocytes by SOCs and not by VDCC.
To further explore the importance of SOCs in rat hepatocytes, we used calcium imaging and whole-cell patch-clamp recording techniques to separately measure the cytoplasmic-free Ca2+ concentrations and the SOC-mediated currents in rat hepatocytes. An increase in cytoplasmic free Ca2+ could be induced by TG-mediated passive depletion of ER calcium stores and subsequent Ca2+ entry through the plasma membrane. The Ca2+ oscillations could be antagonized by inhibitors of SOCs (2-APB, SKF-96365), PLC (U73122) and phospholipase A2 (tetrandrine). Tetrandrine, commonly used to inhibit activation of phospholipase A2 by receptors, is a potent blocker of ISOC in H4IIE cells. We recorded prominent inwardly rectifying currents that could be inhibited by 2-APB. A transient increase in inward current was observed when 100 mmol/L Ba2+ was applied to the bath (Figure 4D) which had similar amplitude and time-course properties to those of inward currents activated by IP3- or TG-mediated depletion of intracellular Ca2+ stores. Such changes in the presence of Ba2+ have previously been shown for ISOC in H4IIE liver cells[37]. These results are consistent with the properties of SOCs and, thus, strongly implicate the importance of SOCs in rat hepatocytes.
HIRI can occur during hemorrhagic shock or hepatic surgery, including trauma, tumor resection and transplantation. For the purpose of exploring the mechanism of HIRI in hepatocytes and searching for novel and clinically effective therapies, the investigation was based on validating the effects of SOCs on the pathogenesis of HIRI. A rat model of HIRI described previously by Yoshiyuki Yabe[38] that closely reflects the clinical condition was modified and established[10]. In these hepatocytes, SOC currents were significantly increased relative to controls, suggesting that SOCs could be involved in hepatocellular Ca2+ overload in HIRI.
The main function of hepatocytes is to synthesize and secrete bile acid; hence, we measured taurocholate secretion as an indicator of the ability of SOC inhibitors to protect and restore hepatocyte function. Taurocholate secretion in normal hepatocytes was significantly reduced in the presence of 100 μmol/L 2-APB and 100 μmol/L La3+, consistent with results from previous experiments[39]. Taurocholate secretion was also reduced in HIRI hepatocytes compared with normal cells. In these cells, non-toxic concentrations (as assessed by MTT assay) of 2-APB (100 μmol/L) or La3+ (100 μmol/L) actually reversed this suppression of taurocholate secretion, suggesting that inhibition of SOCs may have beneficial effects on HIRI hepatocyte health and function. Indeed, a concentration of 100 μmol/L 2-APB is in agreement with a previous report[40] that found that 2-APB is (1) effective in preventing HIRI in vivo when administered via the portal vein before ischemia and (2) able to attenuate HIRI when administered following an ischemic event. Thus, 2-APB could be a potential therapy for HIRI and offers a reduced side-effect profile compared with La3+, a nonspecific blocker of SOCs that also inhibits other types of Ca2+ channels on hepatocytes, which is consistent with previous findings by Nathanson et al[41]. Concerns about the safety of lanthanides have not yet been completely resolved, and drugs with greater specificity for SOCs are required if a blocker of SOCs is to be considered a viable therapeutic target for HIRI.
The physiological process of bile secretion has been studied extensively. It is believed that natural bile acids exist in the plasma which are taken up actively and concentrated, then secreted, at the biliary pole of the hepatocyte. The mechanisms whereby SOC inhibitors reversed the restraint of taurocholate secretion during HIRI in hepatocytes were determined by regulating the process of hepatic secretion, which is closely related to cytosolic Ca2+. Hepatocytes, as classic epithelial cells, are highly polarized, with transport directed from the sinusoidal or basolateral domain of the cell to the canalicular or apical domain in the physiological process of bile secretion. There is evidence that taurolithocholate and lithocholate increase the cytosolic Ca2+ concentration and, meanwhile, inhibit bile secretion[42].
HIRI that occurs in liver surgery, which can be caused by hepatic vascular clamping during partial hepatectomy or liver transplantation, frequently results in cellular damage and organ dysfunction. Although considerable investigation has provided insight into these processes of HIRI in the liver, the exact mechanisms remain only partially elucidated. Intracellular signaling pathways that have been identified as participating in the complex pathophysiological process correlating with cell necrosis and apoptosis involve: the release of multiple bioactive substances, such as cytokines, platelet activating factor (PAF), and free radicals; Ca2+-mediated intracellular Ca2+ overload; sinusoidal endothelial cells; Kupffer cells; and cholangiocytes. Among the significant factors is cytosolic Ca2+[43]. Considering that the increase of cytosolic Ca2+ appears in the earlier period of intracellular cascade of HIRI[44], the SOC blockers may be effective protective therapies for this clinical problem.
In our experimental results, we found that the sequence of taurocholate secretion seems to be: normal hepatocytes > normal hepatocytes + inhibitors of SOCs > HIRI hepatocytes + inhibitors of SOCs > HIRI hepatocytes. It is known that cellular secretion can not take place without the participation of Ca2+; accordingly, combined with the observation above, we classified the influential factors of cellular secretion roughly into two parts: SOCs and some unknown factors related to Ca2+ overload. Owing to blockage of Ca2+ influx induced by SOCs, taurocholate secretion was decreased in both normal and HIRI hepatocytes, which is consistent with earlier published papers by other researchers. However, there are unknown factors other than the inhibition of the Ca2+ channels that could induce Ca2+ overload; thus, it is reasonable that the secretion of HIRI hepatocytes pretreated with 2-APB was lower than normal hepatocytes. We concluded that HIRI hepatocytes recover part of their secretory function in the presence of SOC blockers.
Taken together, our results confirm the important role of SOCs in rat hepatocytes and point toward the effects of SOCs on HIRI. SOC inhibitors could protect against HIRI and are helpful in the recovery of secretory function in hepatocytes. We conclude that SOCs play a vital role in the pathogenesis of HIRI and that SOC blockers could represent a novel class of drugs for the prevention or therapy of HIRI.
Hepatic ischemia-reperfusion injury (HIRI) can occur in the liver in a wide variety of clinical and operative situations. The pathogenesis of HIRI is multifactorial, such as in hepatocellular Ca2+ overload. Variation of [Ca2+]i has been shown to play a significant role. [Ca2+]i may be increased by releasing Ca2+ from the endoplasmic reticulum (ER) or the sarcoplasmic reticulum (SR), or by stimulating Ca2+ entry from the extracellular space through calcium channels. The concept of a “store-operated calcium current” was proposed in 1986, and store-operated calcium channels (SOCs) may play an important role in HIRI.
SOCs have been verified as Ca2+ channels in that the amount of Ca2+ in the stores controls the extent of Ca2+ influx in non-excitable cells. However, the role of SOCs in rat hepatocytes has not been elucidated. In this study, the authors have further confirmed the important role of SOCs in rat hepatocytes and the pivotal role of SOCs in HIRI. SOC blockers assisted the recovery of secretory function in HIRI hepatocytes.
This is the first study that has used multiple experimental techniques to investigate the important role of SOCs in rat hepatocytes from various perspectives, particularly with regard to pathogenesis of HIRI on the cellular electrophysiological level and on the clinical research level.
Cytoplasmic Ca2+ overload could result in injury of liver cells. By understanding the role of SOCs in HIRI, this research will contribute to clarifying the exact mechanism of hepatocellular Ca2+ overload. This study might indicate a novel class of effective drugs targeted at Ca2+ channels in hepatocytes for the prevention or therapy of HIRI.
SOCs are a family of Ca2+-permeable ion channels expressed by most cells. The signal for the activation of SOCs is a decrease in the [Ca2+]i in the ER Ca2+ store, and this is believed to be an essential and ubiquitous component of Ca2+-signaling pathways. Stimulation of a diverse range of plasma membrane receptors converges on and activates phosphatidylinositol 4,5-bisphosphate hydrolysis by phospholipase C and results in the generation of diacylglycerol and IP3, which induces the subsequent activation of Ca2+ release from the ER via IP3 receptors and Ca2+ influx across the plasma membrane. STIM1, identified as the ER Ca2+ sensor, and Orai1, as a pore-forming subunit of SOCs, have both been shown to be necessary for SOC function.
This is a well conducted study with a clear objective and the data reflects the quality of the work by these investigators. Significance of the data from the study complies with the background objectives. All sections including Materials and Methods, Results, Discussion and References conform well to the style of the journal.
Peer reviewer: Parimal Chowdhury, PhD, Professor, Department of Physiology and Biophysics, College of Medicine, University of Arkansas for Medical Sciences, 4301 W Markham Street, Little Rock, AR 72205, United States
S- Editor Tian L L- Editor Logan S E- Editor Zhang DN
| 1. | Sato H, Takeo T, Liu Q, Nakano K, Osanai T, Suga S, Wakui M, Wu J. Hydrogen peroxide mobilizes Ca2+ through two distinct mechanisms in rat hepatocytes. Acta Pharmacol Sin. 2009;30:78-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 2. | Ye XT, Sha JH, Hui N. Hepatocytes injury and calcium ion distribution by cadmium induction. Dianzi Xianwei Xuebao. 2005;24:411. |
| 3. | Li XL, Liu YF, Wang FS, Liang J, Zhao N, He SG. Injurious effects of calcium overload on human hepatocytes during cold storage. Zhonghua Gandan Waike Zazhi. 2001;7:228-230. |
| 4. | Silomon M, Pizanis A, Rose S. Oxyradical-mediated hepatocellular Ca2+ alterations during hemorrhagic shock and resuscitation. Shock. 1999;11:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Silomon M, Rose S. Effect of sodium bicarbonate infusion on hepatocyte Ca2+ overload during resuscitation from hemorrhagic shock. Resuscitation. 1998;37:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 6. | Hahn O, Szijártó A, Lotz G, Schaff Z, Vígváry Z, Váli L, Kupcsulik PK. The effect of ischemic preconditioning prior to intraoperative radiotherapy on ischemic and on reperfused rat liver. J Surg Res. 2007;142:32-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cuzzocrea S, Riley DP, Caputi AP, Salvemini D. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol Rev. 2001;53:135-159. [PubMed] |
| 8. | Baía CE, Abdala E, Massarollo P, Beduschi T, Palma TM, Mies S. Inflammatory cytokines during liver transplantation: prospective randomized trial comparing conventional and piggyback techniques. Hepatogastroenterology. 2009;56:1445-1451. [PubMed] |
| 9. | Tomiyama K, Ikeda A, Ueki S, Nakao A, Stolz DB, Koike Y, Afrazi A, Gandhi C, Tokita D, Geller DA. Inhibition of Kupffer cell-mediated early proinflammatory response with carbon monoxide in transplant-induced hepatic ischemia/reperfusion injury in rats. Hepatology. 2008;48:1608-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Jiang N, Zhang ZM, Liu L, Zhang C, Zhang YL, Zhang ZC. Effects of Ca2+ channel blockers on store-operated Ca2+ channel currents of Kupffer cells after hepatic ischemia/reperfusion injury in rats. World J Gastroenterol. 2006;12:4694-4698. [PubMed] |
| 11. | Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 12. | Huber N, Sakai N, Eismann T, Shin T, Kuboki S, Blanchard J, Schuster R, Edwards MJ, Wong HR, Lentsch AB. Age-related decrease in proteasome expression contributes to defective nuclear factor-kappaB activation during hepatic ischemia/reperfusion. Hepatology. 2009;49:1718-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Barritt GJ. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem J. 1999;337:153-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 14. | Parekh AB, Putney JW. Store-operated calcium channels. Physiol Rev. 2005;85:757-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1661] [Article Influence: 83.1] [Reference Citation Analysis (0)] |
| 15. | Graf J, Häussinger D. Ion transport in hepatocytes: mechanisms and correlations to cell volume, hormone actions and metabolism. J Hepatol. 1996;24 Suppl 1:53-77. [PubMed] |
| 16. | Sawanobori T, Takanashi H, Hiraoka M, Iida Y, Kamisaka K, Maezawa H. Electrophysiological properties of isolated rat liver cells. J Cell Physiol. 1989;139:580-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Auld A, Chen J, Brereton HM, Wang YJ, Gregory RB, Barritt GJ. Store-operated Ca(2+) inflow in Reuber hepatoma cells is inhibited by voltage-operated Ca(2+) channel antagonists and, in contrast to freshly isolated hepatocytes, does not require a pertussis toxin-sensitive trimeric GTP-binding protein. Biochim Biophys Acta. 2000;1497:11-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Brereton HM, Harland ML, Froscio M, Petronijevic T, Barritt GJ. Novel variants of voltage-operated calcium channel alpha 1-subunit transcripts in a rat liver-derived cell line: deletion in the IVS4 voltage sensing region. Cell Calcium. 1997;22:39-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Gregory RB, Sykiotis D, Barritt GJ. Evidence that store-operated Ca2+ channels are more effective than intracellular messenger-activated non-selective cation channels in refilling rat hepatocyte intracellular Ca2+ stores. Cell Calcium. 2003;34:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Rychkov GY, Litjens T, Roberts ML, Barritt GJ. ATP and vasopressin activate a single type of store-operated Ca2+ channel, identified by patch-clamp recording, in rat hepatocytes. Cell Calcium. 2005;37:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Litjens T, Harland ML, Roberts ML, Barritt GJ, Rychkov GY. Fast Ca(2+)-dependent inactivation of the store-operated Ca2+ current (ISOC) in liver cells: a role for calmodulin. J Physiol. 2004;558:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2034] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 23. | Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3785] [Cited by in RCA: 3877] [Article Influence: 79.1] [Reference Citation Analysis (0)] |
| 24. | Zhang ZY, Wang WJ, Pan LJ, Xu Y, Zhang ZM. Measuring Ca2+ influxes of TRPC1-dependent Ca2+ channels in HL-7702 cells with non-invasive micro-test technique. World J Gastroenterol. 2009;15:4150-4155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38285] [Cited by in RCA: 39399] [Article Influence: 938.1] [Reference Citation Analysis (0)] |
| 26. | Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J. 2001;354:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 27. | Hu R, He ML, Hu H, Yuan BX, Zang WJ, Lau CP, Tse HF, Li GR. Characterization of calcium signaling pathways in human preadipocytes. J Cell Physiol. 2009;220:765-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Bouron A, Altafaj X, Boisseau S, De Waard M. A store-operated Ca2+ influx activated in response to the depletion of thapsigargin-sensitive Ca2+ stores is developmentally regulated in embryonic cortical neurons from mice. Brain Res Dev Brain Res. 2005;159:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Copanaki E, Schürmann T, Eckert A, Leuner K, Müller WE, Prehn JH, Kögel D. The amyloid precursor protein potentiates CHOP induction and cell death in response to ER Ca2+ depletion. Biochim Biophys Acta. 2007;1773:157-165. [PubMed] |
| 30. | Akiba S, Kato E, Sato T, Fujii T. Biscoclaurine alkaloids inhibit receptor-mediated phospholipase A2 activation probably through uncoupling of a GTP-binding protein from the enzyme in rat peritoneal mast cells. Biochem Pharmacol. 1992;44:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Rychkov GY, Litjens T, Roberts ML, Barritt GJ. Arachidonic acid inhibits the store-operated Ca2+ current in rat liver cells. Biochem J. 2005;385:551-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Green AK, Zolle O, Simpson AW. Regulation of [Ca2+]c oscillations by plasma membrane Ca2+ fluxes: a role for natriuretic peptides. Biochem Soc Trans. 2003;31:934-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Guo RW, Huang L. New insights into the activation mechanism of store-operated calcium channels: roles of STIM and Orai. J Zhejiang Univ Sci B. 2008;9:591-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 34. | Putney JW. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium. 2007;42:103-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 168] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 35. | Zhang ZY, Pan LJ, Zhang ZM. Functional interactions among STIM1, Orai1 and TRPC1 on the activation of SOCs in HL-7702 cells. Amino Acids. 2010;39:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Ding YN, Xu Y. Non-invasive micro-test technology and its applications in biology and medicine. Physics. 2007;36:548-558. |
| 37. | Rychkov G, Brereton HM, Harland ML, Barritt GJ. Plasma membrane Ca2+ release-activated Ca2+ channels with a high selectivity for Ca2+ identified by patch-clamp recording in rat liver cells. Hepatology. 2001;33:938-947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Yabe Y, Kobayashi N, Nishihashi T, Takahashi R, Nishikawa M, Takakura Y, Hashida M. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298:894-899. [PubMed] |
| 39. | Gregory RB, Hughes R, Barritt GJ. Induction of cholestasis in the perfused rat liver by 2-aminoethyl diphenylborate, an inhibitor of the hepatocyte plasma membrane Ca2+ channels. J Gastroenterol Hepatol. 2004;19:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Nicoud IB, Knox CD, Jones CM, Anderson CD, Pierce JM, Belous AE, Earl TM, Chari RS. 2-APB protects against liver ischemia-reperfusion injury by reducing cellular and mitochondrial calcium uptake. Am J Physiol Gastrointest Liver Physiol. 2007;293:G623-G630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Nathanson MH, Gautam A, Bruck R, Isales CM, Boyer JL. Effects of Ca2+ agonists on cytosolic Ca2+ in isolated hepatocytes and on bile secretion in the isolated perfused rat liver. Hepatology. 1992;15:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Combettes L, Dumont M, Berthon B, Erlinger S, Claret M. Release of calcium from the endoplasmic reticulum by bile acids in rat liver cells. J Biol Chem. 1988;263:2299-2303. [PubMed] |
| 43. | Sakon M, Ariyoshi H, Umeshita K, Monden M. Ischemia-reperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg Today. 2002;32:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 44. | Schanne FA, Kane AB, Young EE, Farber JL. Calcium dependence of toxic cell death: a final common pathway. Science. 1979;206:700-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1074] [Article Influence: 23.3] [Reference Citation Analysis (0)] |