Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.349
Revised: June 16, 2011
Accepted: June 23, 2011
Published online: January 28, 2012
AIM: To investigate the inhibitory effect of hepatitis B virus (HBV) preS2 antibody (preS2Ab) against HBV infection and HBV-associated hepatic carcinogenesis.
METHODS: An adenoviral vector carrying the full-length light and heavy chains of the HBV preS2Ab gene, Ad315-preS2Ab, was constructed. Enzyme linked immunosorbent assay (ELISA) and Western blotting analyses were used to determine the preS2Ab expression levels in vitro. Immunofluorescent techniques were used to examine the binding affinity between the expressed HBV preS2Ab and HBV-positive liver cells. ELISAs were also used to determine hepatitis B surface antigen (HBsAg) levels to assess the inhibitory effect of the preS2Ab against HBV infection in L02 cells. The inhibitory effect of preS2Ab against hepatic carcinogenesis was studied with diethylnitrosamine (DEN)-induced hepatocellular carcinomas (HCCs) in HBV transgenic mice.
RESULTS: The expression of HBV preS2Ab increased with increases in the multiplicity of infection (MOI) of Ad315-preS2Ab in L02 cells, with 350.87 ± 17.37 μg/L of preS2Ab when the MOI was 100 plaque forming units (pfu)/cell. The expressed preS2Abs could recognize liver cells from HBV transgenic mice. ELISA results showed that L02 cells expressing preS2Ab produced less HBsAg after treatment with the serum of HBV patients than parental L02 cells expressing no preS2Ab. HBV transgenic mice treated with Ad315-preS2Ab had fewer and smaller cancerous nodes after induction with DEN than mice treated with a blank Ad315 vector or untreated mice. Additionally, the administration of Ad315-preS2Ab could alleviate hepatic cirrhosis and decrease the serum levels of alanine transaminase and aspartate transaminase.
CONCLUSION: Adenovirus-mediated HBV preS2Ab expression could inhibit HBV infection in L02 cells, and then inhibit DEN-induced hepatocellular carcinogenesis and protect hepatic function in HBV transgenic mice.
- Citation: Zhang Q, Li ZQ, Liu H, Yang JH. Adenovirus-expressed preS2 antibody inhibits hepatitis B virus infection and hepatic carcinogenesis. World J Gastroenterol 2012; 18(4): 349-355
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/349.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.349
Hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC) poses a severe threat to human health, and HBV is an important cause of the high HCC incidence in China. The available treatments for recurrent HBV infections in patients include immunization and antiviral therapies. For these therapies, hepatitis B immunoglobulin is administered, which has been proven effective for preventing maternal-infant vertical transmission and HBV recurrence after liver transplantation. However, the immunoglobulin is limited and expensive. Additionally, it has an unsatisfactory neutralization effect and may carry pathogens. Immunoprophylaxis of HBV infection with genetically engineered monoclonal antibodies that are specific to HBV envelope antigens is a promising approach in the clinic. HBV encodes an outer membrane protein, preS2, which binds to polyalbumin and helps the HBV gain entry into liver cells via albumin receptors. Blocking preS2 with its antibody may prevent or minimize HBV infection. However, full-length antibodies with large molecular weights have low permeability, and small-molecule antibodies, including Fab, scFv, and dsFv, have relatively low affinity and specificity for antigens.
To overcome these limitations, we have constructed an adenoviral vector carrying the full-length light and heavy chains of the human HBV preS2 antibody (preS2Ab) gene and tested its efficacy in the prevention and treatment of HBV-associated HCC. preS2Ab gene therapy has the advantages of lower production costs and longer expression durations. The adenoviral vector system can express the humanized HBV preS2Ab with high and stable efficiency, so this system may provide a novel approach for HBV gene therapy and may decrease the incidence of HCC.
Nucleic acid synthesis in this study was performed by Shanghai Shenergy Biocolor Bioscience and Technology Company (Shanghai, China). HEK293 and L02 cell lines were purchased from ATCC (Manassas, United States). HBV transgenic Imprinting Control Region (ICR) mice were provided by the Shanghai SLAC Laboratory Animal Center, Chinese Academy of Sciences, (Shanghai, China). Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Plasmids were purchased from Microbix Biosystems (Ontario, Canada). The Lipofectamine 2000 reagent is a product of Invitrogen (United States). Restriction endonucleases were from New England Biolabs (Ozyme, France). The enzyme linked immunosorbent assay (ELISA) kit was from R and D Systems (Minneapolis, MN, United States), and diethylnitrosamine (DEN) was from Sigma Chemical Co. (St. Louis, MO, United States).
The variable and constant regions of the humanized light and heavy chains of the HBV preS2Ab gene were synthesized. A BamHI site was introduced upstream of the heavy chain, and an Xbal site was introduced downstream. An EcoRI site was introduced upstream of the light chain, and a SalI site was introduced downstream. Other restriction sites in the encoding sequence were abolished by same-sense mutations. Next, the light and heavy chains of the preS2Ab gene were cloned into the corresponding restriction enzyme sites of pDC315 adenoviral shuttle plasmid. The light and heavy chains were bridged by an internal ribosome entry site (IRES) to yield pDC315-preS2Ab. The pDC315-preS2Ab plasmid was co-transfected with pBHGE3 into HEK293 cells using the Lipofectamine 2000 reagent. Twelve days after transfection, a recombinant adenoviral vector carrying the humanized HBV preS2Ab gene, Ad315-preS2Ab, was obtained. Ad315-preS2Ab was then amplified in HEK293 cells and purified by cesium chloride gradient centrifugation. The recombinant virus titer was determined by TCID50 analysis.
L02 cells were cultured in 6-well plates for 24 h and then subjected to serum-deprived medium. Ad315-preS2Ab was added for infection according to the multiplicity of infection (MOI) gradient. After 2 h, the cells were cultured with serum-containing medium for 72 h, and the supernatants and cells were collected. ELISA was used to determine the antibody levels in the supernatants; optical density (OD) values (A value) were read at 450 nm, and a standard concentration curve was plotted to calculate the antibody levels in the supernatants. The antibody content in the cell lysate solution was determined by Western blotting analysis.
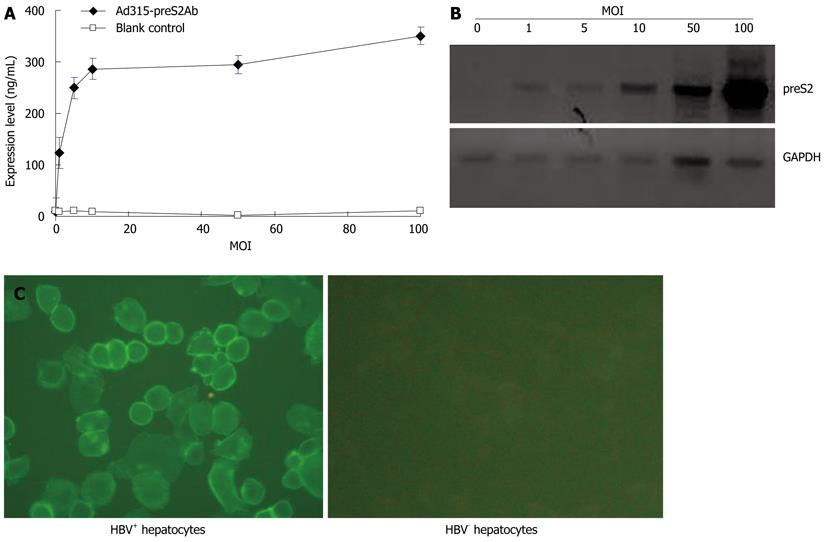
Liver samples from an HBV transgenic ICR mouse and a normal ICR mouse were obtained and made into single-cell suspensions. Cell smears were prepared for immunofluorescent examination using the supernatant of L02 cells infected with Ad315-preS2Ab [MOI = 100 plaque forming units (pfu)/cell] as the primary antibody and FITC-labeled goat anti-human IgG as the secondary antibody. Immunofluorescent labeling was observed under a fluorescence microscope, and photos were taken to assess the binding affinity of the expressed antibody.
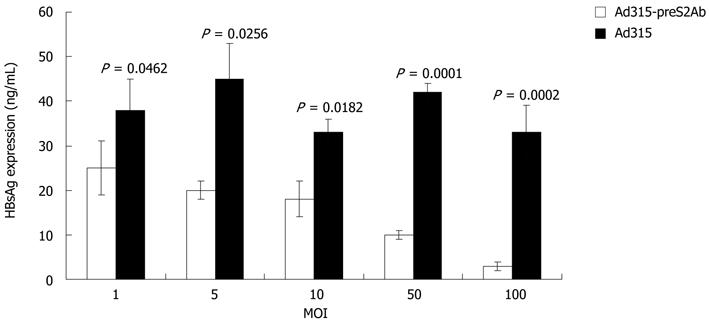
L02 cells were cultured in 6-well plates for 24 h and then subjected to serum-deprived medium. Ad315-preS2Ab was added for infection at an MOI of 50 pfu/cell, and after 2 h, the cells were cultured with serum-containing medium for 72 h. Sera from HBV patients with HBsAg (+), HBeAg (+) and anti-HBc (+) were collected and added to L02 cells infected with either Ad315-preS2Ab or the Ad315 blank vector for 7 d. ELISAs were used to determine the HBsAg levels in the supernatants.
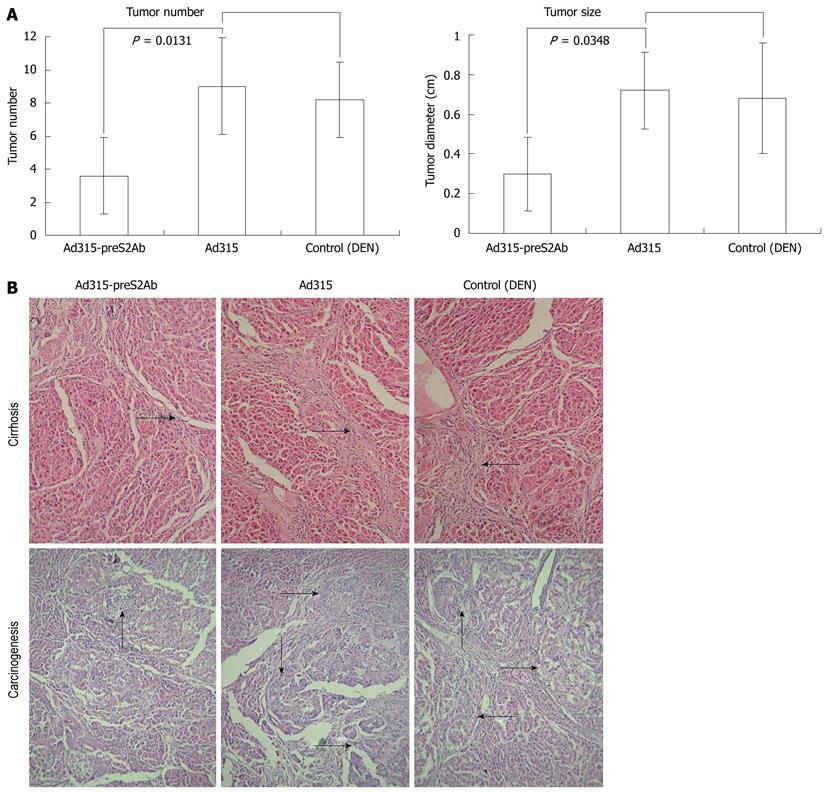
A total of 24 HBV transgenic ICR mice, aged 4 to 6 wk, were evenly divided into 4 groups. Animals in the Ad315-preS2Ab group and Ad315 blank vector group were given the corresponding adenovirus particles via tail vein injections; each mouse was injected with 2 × 108 pfu adenoviruses every other day for a total of 5 injections. The total amount for each animal was 1 × 109 pfu. Mice in the non-virus control group and the blank control group were given the same volume of normal saline. DEN was intraperitoneally injected (1 mg/kg, once a week for 4 wk) into animals in the Ad315-preS2Ab, Ad315 vector and non-virus groups one week after the initial injection of adenovirus or normal saline. Mice in the blank control group were not treated with DEN. The animals were sacrificed after 8 mo, and the liver tissues were sectioned at 0.5 cm intervals to observe the diameters of the cancerous nodes. Serial pathological sections were also subjected to hematoxylin/eosin (HE) staining, and all cancerous nodes under a microscope within 5 medium power fields were counted and represented as the mean ± SD.
Sera samples were collected when animals were sacrificed. Hepatic enzyme levels in the sera, including alanine transaminase (ALT) and aspartate transaminase (AST), were measured using an automated biochemical analyzer (SIEMENS ADVIA 2400, Siemens Healthcare Diagnostics, IL, United States).
All data are presented as the mean ± SD. Statistical significance was calculated using unpaired Student’s t-tests. A P < 0.05 was considered significant. All analyses were performed using SPSS version 13.0 (SPSS Inc., United States).
TCID50 analysis showed that the titer of Ad315-preS2Ab was 2.1 × 1010 pfu/mL after amplification in HEK293 cells. L02 cells were infected with Ad315-preS2Ab with MOI = 1, 5, 10, 50, and 100 pfu/cell. Seventy-two hours after infection, ELISA and Western blotting results showed that the expression of preS2Ab in cell supernatants or lysates increased with increases in MOI (Figures 1A and B), with a final antibody concentration of 350.87 ± 17.37 ng/mL at an MOI of 100 pfu/cell. The preS2Ab from the supernatant of L02 cells infected with Ad315-preS2Ab (MOI = 100 pfu/cell) recognized liver cells from HBV transgenic ICR mice, as shown by strong immunofluorescent reactions. Liver cells from normal ICR mice demonstrated a lack of immunofluorescent labeling (Figure 1C).
ELISA results showed that, compared with the Ad315 blank vector, Ad315-preS2Ab-mediated preS2Ab expression effectively decreased HBsAg levels in the supernatants of L02 cells treated with the serum of HBV patients. This finding indicated that the preS2Ab could inhibit HBV infection and replication (Figure 2).
The Ad315 blank vector group and the non-virus group each had one mouse death during the experiment, so the data from 5 mice in each group were analyzed. In the blank control group, there were no cancerous nodes. Slight fibroblastic proliferation and inflammatory cell infiltration were noticed; however, severe hepatic cirrhosis with fibrous septa and pseudolobule formation and cancerous nodes of different numbers and various sizes were found in the non-virus control group after DEN induction. In contrast, only slight-to-moderate hepatic cirrhosis was found in the Ad315-preS2Ab group, and the cancerous nodes were both fewer in number and smaller than those in the non-virus control group (Figure 3).
To investigate whether the adenovirus Ad315-preS2Ab could protect hepatic function in HBV transgenic mice, the ALT and AST levels in sera were measured to assess hepatic function. Quantitative results for the liver enzyme assays showed that the DEN-treated HBV transgenic mice had higher levels of ALT and AST in sera than the ICR mice that were not treated with DEN. The Ad315 blank vector did not decrease enzyme levels, but 1 × 109 pfu of Ad315-preS2Ab resulted in an obvious decrease of ALT (P = 0.0141) and AST (P = 0.0243) compared with the DEN-treated control group (Table 1).
Globally, there are approximately 300 million HBV carriers. Therefore, it is of great importance to prevent and treat both HBV infection and the subsequent hepatic carcinogenesis. HBV infection is closely associated with the viral proteins. The HBV envelope proteins consist mainly of preS1, preS2 and HBsAg antigens, which can induce production of the corresponding antibodies[1-3]. The HBV preS2 protein is found mainly on tubulose particles and Dane particles, and it is a component of the HBV outer capsid antigen. The preS2 protein possesses stronger antigenicity than the HBsAg; moreover, it also possesses strong antigenic determinants for T and B cells and plays important roles in virus infection, assembly, replication and stimulation of the immune reaction[4,5]. preS2 protein has binding sites for polymerized human serum albumin (pHSA), which possesses determinants for binding with liver cell receptors. Therefore, HBV can enter liver cells by binding to pHSA; this ability is an important reason for the hepatotropism of HBV[6]. It has been found that the strong antigenicity of preS2 can induce immune responses during HBV infection and cause production of the preS2Ab. This antibody can help eliminate HBV and prevent the virus from entering normal liver cells[7]. Therefore, using an antibody against the HBV protein to block HBV infection and replication is an important strategy for preventing and treating HBV infection and hepatocellular carcinogenesis.
Hepatitis B immunoglobulin has been shown to be effective in preventing maternal-infant vertical transmission and HBV recurrence after liver transplantation; however, the immunoglobulin has limited sources and is expensive and nonspecific. Additionally, it has an unsatisfactory neutralization effect and may carry pathogens. Artificially-expressed HBsAg antibodies and gene therapy can overcome these shortcomings, indicating a bright future for HBV prevention and treatment. Presently, the best-studied genetically-engineered antibodies are the small molecular antibodies, which mainly include Fab, ScFv, dsFv and single-domain antibodies. These small antibodies have the advantages of low molecular weight, high permeability, and easy construction and expression[8,9]. However, their low molecular weights also result in a short half-life period in vivo, making it difficult for them to reach effective concentrations in the blood. This limitation greatly restricts their clinical applications. Moreover, small molecular antibodies have no Fc segment, which is known to play important roles in the therapeutic effects of antibodies. Currently, there are a dozen monoclonal antibodies which have been used in clinical settings, and even more have been tested in clinical trials[10-12].
To overcome these barriers, we constructed an adenoviral vector carrying the full-length humanized HBV preS2Ab gene and examined its expression and inhibitory effects on HBV infection. We also examined the effects of the antibody on the carcinogenesis of HBV-positive liver cells. There are many advantages to gene therapy with adenoviral vectors. The adenoviral particles are stable, and the virus genome rarely undergoes rearrangement, so the inserted genes are kept unchanged after continuous passage of the virus. In addition, the virus genome is easily manageable, and adenovirus can be produced on a large scale. Moreover, adenovirus vectors can infect both dividing and non-dividing cells, and they can be used to transfect pulmonary cells, liver cells, bone cells, blood vessels, muscle cells, and central nervous system cells. Finally, these vectors can be used to achieve high expression of exogenous genes[13-16]. For these reasons, adenoviral vectors have been increasingly used for gene therapy. Additionally, related studies have yielded impressive achievements in China and in other parts of the world. The adenoviral vector constructed by Kim et al[17] permanently improved hyperlipidemia in mice, with the induced protein expression lasting for 2.5 years. In a rat hemophilia model, the adenoviral vector constructed by Reddy et al[18] continuously expressed factor VIII for more than 9 mo. These findings demonstrate the advantages of adenoviral vectors and indicate a bright future for adenoviral vectors in gene therapy.
In the present study, we successfully constructed an Ad315-preS2Ab vector carrying the full-length preS2Ab genes and used this vector to infect L02 liver cells. The production of the preS2Ab increased with increases in the MOI of the Ad315-preS2Ab. The expressed preS2Ab recognized liver cells from HBV transgenic ICR mice, as shown by strong immunofluorescent reactions, demonstrating that the Ad315-preS2Ab-mediated preS2Ab possesses a satisfactory binding affinity for the corresponding antigen. We also found that the Ad315-preS2Ab-mediated preS2Ab could efficiently decrease the level of HBsAg in L02 cells infected with the sera of HBV patients, indicating that the expressed preS2Ab can inhibit HBV infection and replication. Our in vivo study showed that the administration of Ad315-preS2Ab could alleviate hepatic cirrhosis and decrease the number and size of cancerous nodes induced by DEN in HBV transgenic ICR mice, suggesting that Ad315-preS2Ab has an inhibitory effect against hepatocellular carcinogenesis. Our in vivo experiment also demonstrated that Ad315-preS2Ab can decrease ALT and AST levels in mouse sera and protect hepatic function in HBV transgenic mice.
In this study, we attempted to establish a complete antibody gene therapy expression system for the prevention and treatment of HBV infection and HBV-associated hepatocellular carcinogenesis. This study will pave the way for gene therapies for HBV infection and HCC.
Hepatitis B virus (HBV)-associated hepatocellular carcinoma (HCC) remains a severe threat to human health. Genetically engineered monoclonal antibodies that are specific to HBV envelope antigens are a promising approach for the treatment of recurrent HBV infection. However, full-length antibodies with large molecular weights have low permeability, and small-molecule antibodies have relatively low affinity and specificity for antigens, which affects the efficacy of these antibodies in the clinic.
An adenoviral vector carrying the HBV preS2 antibody genes can express the humanized HBV preS2 antibody with high and stable efficiency to overcome the limitations of antibody permeability, affinity and specificity.
By encoding the full-length preS2 antibody genes, the Ad315-preS2Ab adenovirus efficiently expressed the HBV preS2 antibody in L02 liver cells, inhibiting HBV infection and replication. Administration of Ad315-preS2Ab could protect hepatic function, alleviate hepatic cirrhosis and suppress hepatocellular carcinogenesis in diethylnitrosamine-treated HBV transgenic mice.
The preS2 antibody gene therapy may provide a novel approach for inhibiting HBV infection and decreasing the incidence of HCC.
HBV encodes an outer membrane protein, preS2, which binds to polyalbumin and helps HBV gain entry into liver cells via albumin receptors. This study demonstrates that the adenovirus-expressed preS2 antibody can block preS2, which then prevents or minimizes HBV infection to inhibit HBV-associated hepatocellular carcinogenesis.
This is an interesting paper describing the inhibition of HBV infection and subsequent carcinogenesis using a vector carrying the anti-HBV Ab gene.
Peer reviewer: Zenichi Morise, MD, PhD, Professor and Chairman, Department of Surgery, Banbuntane Houtokukai Hospital, Fujita Health University School of Medicine, 3-6-10 Otobashi Nakagawa-ku, Nagoya, AICHI 454-8509, Japan
S- Editor Zhang SJ L- Editor Logan S E- Editor Zhang DN
| 1. | Huang X, Qin Y, Zhang P, Tang G, Shi Q, Xu J, Qi F, Shen Q. PreS deletion mutations of hepatitis B virus in chronically infected patients with simultaneous seropositivity for hepatitis-B surface antigen and anti-HBS antibodies. J Med Virol. 2010;82:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Gous N, Bhimma R, Kew M, Kramvis A. Retrospective characterization of the S open reading frame of HBV isolated from children with membranous nephropathy treated with interferon-alpha2b. Antivir Ther. 2010;15:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Tadokoro K, Kobayashi M, Yamaguchi T, Suzuki F, Miyauchi S, Egashira T, Kumada H. Classification of hepatitis B virus genotypes by the PCR-Invader method with genotype-specific probes. J Virol Methods. 2006;138:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Ochoa-Callejero L, Otano I, Vales A, Olagüe C, Sarobe P, Lasarte JJ, Prieto J, Menne S, González-Aseguinolaza G. Identification of CD4+ and CD8+ T cell epitopes of woodchuck hepatitis virus core and surface antigens in BALB/c mice. Vaccine. 2010;28:5323-5331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Bayard F, Malmassari S, Deng Q, Lone YC, Michel ML. Hepatitis B virus (HBV)-derived DRB1*0101-restricted CD4 T-cell epitopes help in the development of HBV-specific CD8+ T cells in vivo. Vaccine. 2010;28:3818-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Park JH, Lee MK, Kim HS, Kim KL, Cho EW. Targeted destruction of the polymerized human serum albumin binding site within the preS2 region of the HBV surface antigen while retaining full immunogenicity for this epitope. J Viral Hepat. 2003;10:70-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Guo M, Kang B, Zheng Q, Qian Q, Su C, Wu M, Yang J. An anti-preS2 antibody protects human hepatocytes from hepatitis B virus infection. Acta Gastroenterol Belg. 2009;72:306-311. [PubMed] |
| 8. | Chen DJ, Tan Z, Chen F, Du T. Construction of humanized carcinoembryonic antigen specific single chain variable fragment and mitomycin conjugate. World J Gastroenterol. 2007;13:5765-5770. [PubMed] |
| 9. | Menezes MA, Aires KA, Ozaki CY, Ruiz RM, Pereira MC, Abreu PA, Elias WP, Ramos OH, Piazza RM. Cloning approach and functional analysis of anti-intimin single-chain variable fragment (scFv). BMC Res Notes. 2011;4:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Ocvirk J, Brodowicz T, Wrba F, Ciuleanu TE, Kurteva G, Beslija S, Koza I, Pápai Z, Messinger D, Yilmaz U. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J Gastroenterol. 2010;16:3133-3143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 79] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Hayes DF. Bevacizumab treatment for solid tumors: boon or bust? JAMA. 2011;305:506-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Kurzeja M, Rudnicka L, Olszewska M. New interleukin-23 pathway inhibitors in dermatology: ustekinumab, briakinumab, and secukinumab. Am J Clin Dermatol. 2011;12:113-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Mason MR, Tannemaat MR, Malessy MJ, Verhaagen J. Gene therapy for the peripheral nervous system: a strategy to repair the injured nerve? Curr Gene Ther. 2011;11:75-89. [PubMed] |
| 14. | Roy S, Medina-Jaszek A, Wilson MJ, Sandhu A, Calcedo R, Lin J, Wilson JM. Creation of a panel of vectors based on ape adenovirus isolates. J Gene Med. 2011;13:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Rebetz J, Na M, Su C, Holmqvist B, Edqvist A, Nyberg C, Widegren B, Salford LG, Sjögren HO, Arnberg N. Fiber mediated receptor masking in non-infected bystander cells restricts adenovirus cell killing effect but promotes adenovirus host co-existence. PLoS One. 2009;4:e8484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Fang L, Pu YY, Hu XC, Sun LJ, Luo HM, Pan SK, Gu JZ, Cao XR, Su CQ. Antiangiogenesis gene armed tumor-targeting adenovirus yields multiple antitumor activities in human HCC xenografts in nude mice. Hepatol Res. 2010;40:216-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Kim IH, Józkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci USA. 2001;98:13282-13287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Reddy PS, Sakhuja K, Ganesh S, Yang L, Kayda D, Brann T, Pattison S, Golightly D, Idamakanti N, Pinkstaff A. Sustained human factor VIII expression in hemophilia A mice following systemic delivery of a gutless adenoviral vector. Mol Ther. 2002;5:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 83] [Article Influence: 3.6] [Reference Citation Analysis (0)] |