Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.323
Revised: May 28, 2011
Accepted: June 5, 2011
Published online: January 28, 2012
AIM: To investigate the relationship between donor liver cold preservation, lung surfactant (LS) changes and acute lung injury (ALI) after liver transplantation.
METHODS: Liver transplantation models were established using male Wistar rats. Donor livers were preserved in University of Wisconsin solution at 4 °C for different lengths of time. The effect of ammonium pyrrolidinedithiocarbamate (PDTC) on ALI was also detected. All samples were harvested after 3 h reperfusion. The severity of ALI was evaluated by lung weight/body weight ratio, lung histopathological score, serum nitric oxide (NO) and endothelin (ET)-1 levels, lung tumor necrosis factor (TNF)-α and interleukin (IL)-1β levels. Lung surfactants (LSs) were determined by micellar electrokinetic capillary chromatography.
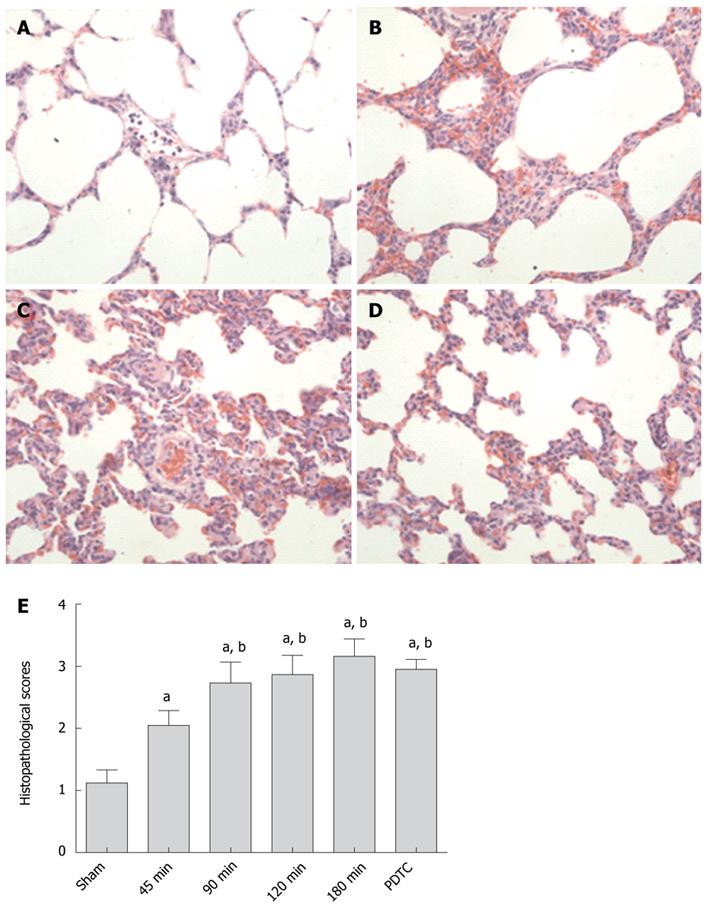
RESULTS: With extended donor liver cold preservation time (CPT), lung histopathological scores, serum ET-1 levels, lung weight/body weight ratio and the level of TNF-α and IL-1β in lung were increased significantly in the 180-min group compared with the sham group (3.16 ± 0.28 vs 1.12 ± 0.21, P < 0.001; 343.59 ± 53.97 vs 141.53 ± 48.48, P < 0.001; 0.00687 ± 0.00037 vs 0.00557 ± 0.00056, P < 0.001; 17.5 ± 3.0 vs 1.3 ± 0.3, P < 0.001; 10.8 ± 2.3 vs 1.8 ± 0.4, P < 0.001), but serum NO levels decreased remarkably (74.67 ± 10.01 vs 24.97 ± 3.18, P < 0.001). The expression of lung phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI) and phosphatidylserine (PS) increased when CPT was < 120 min, and decreased when CPT was > 180 min (PC: 1318.89 ± 54.79 vs 1011.18 ± 59.99, P < 0.001; PE: 1504.45 ± 119.96 vs 1340.80 ± 76.39, P = 0.0019; PI: 201.23 ± 34.82 vs 185.88 ± 17.04, P = 0.2265; PS: 300.43 ± 32.95 vs 286.55 ± 55.55, P = 0.5054). All these ALI-associated indexes could be partially reversed by PDTC treatment.
CONCLUSION: Prolonged CPT could induce or inhibit the expression of LSs at the compensation or decompensation stage, and some antioxidants (e.g., PDTC) may reverse the pathological process partially.
- Citation: Jiang A, Liu C, Liu F, Song YL, Li QY, Yu L, Lv Y. Liver cold preservation induce lung surfactant changes and acute lung injury in rat liver transplantation. World J Gastroenterol 2012; 18(4): 323-330
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/323.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.323
Acute lung injury (ALI) is a common complication of liver transplantation, which may develop into its severest form, acute respiratory distress syndrome (ARDS), and play a pivotal role in the death of patients post-transplantation[1]. It has been reported that the incidence of lung complications after liver transplantation is 60%-80%; the morbidity rate of ARDS is 4.5%-18%, and the case-fatality rate is 50%-70%[2,3]. Thus, prevention of the complications is important in reducing in-hospital mortality after liver transplantation, and investigation of the etiological factors of liver-transplantation-related ALI is therefore of great importance.
Lung surfactant (LS) is synthesized primarily by alveolar type II cells and is stored in lamellar bodies. In response to some stimuli, LS is secreted to supply the surface-active monolayer. LS is a complex mix of phospholipids, neutral lipids and proteins that lines the gas/liquid interface. LS is essential for normal breathing and the severity of ALI correlates with surfactant dysfunction and abnormalities in surfactant composition[4].
In our clinical practice, we have observed that ALI often occurs in the patients transplanted with long-persevered donor livers. Therefore, we raised a hypothesis that prolonged donor liver cold preservation time (CPT) might induce lung damage. Therefore, we conducted the present study to investigate the change of LS in liver-transplantation-induced ALI along with changes in donor liver CPT, aiming at uncovering the association between prolonged CPT and ALI.
Male Wistar rats (n = 110) were used as donors and recipients to establish orthotopic liver transplantation models. The rats were randomly divided into six groups (n = 10 in each group, n = 10 for donor in liver transplantation groups). Donor livers from each group were preserved in 4 °C University of Wisconsin solution respectively for 0 min (sham operation), 45 min, 90 min, 120 min, 180 min, and 180 min plus intravenous injection of ammonium pyrrolidinedithiocarbamate (PDTC) at a dosage of 100 mg/kg immediately after the onset of liver reperfusion. All recipients were sacrificed at 180 min after liver transplantation.
A total of 110 adult male Wistar rats (272 g ± 31 g; Tongji Medical Center, Central China University of Science and Technology) were used as donors and recipients. Prior to the experiment, the rats were fasted for 12 h and allowed free access to water. Liver harvesting and liver transplantation were performed under anesthesia with intraperitoneal injection of ketamine hydrochloride (80 mg/kg) and by using the method described by Kamada et al[5] and Hori et al[6]. Protocols for animal care and experimental management were approved by the ethics committee.
Blood samples were collected from the suprahepatic vena cava to determine the serum levels of nitric oxide (NO) and endothelin (ET)-1. Body weight and lung weight were measured and part of left lower lobe of lung was fixed with polyoxymethylene for histological examination. Lung tissue (200 mg) was harvested for the extraction of lung phospholipids.
Left lower lobe of lung was harvested, fixed in 10% formalin, and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin for light microscopy, and evaluated blindly by an independent consultant pathologist. Damage to the lung tissue was graded by the pathologist on a scale of 1 (no injury) to 4 (worst), as described previously[7].
Serum ET-1 was quantified with a radioimmunoassay kit (Radioimmunity Institute of PLA General Hospital, China) by Wizard gamma counter (PerkinElmer, United States) according to the manufacturer’s recommendations.
Serum NO was measured by an NO assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocol. The absorbance of the reaction mixture was read at 550 nm in a BioTek ELX 808 microplate reader (Bio-Tek Instruments, Winooski, Vermont, United States).
All recipients’ body weight and lung weight were measured by electronic balance.
Histological sections (4 μm) of lung were cut on a rotary microtome and stained to detect intragraft expression of TNF-α and IL-1β. Paraffin sections were spread on a slide, and rabbit anti-rat TNF-α, IL-1β polyclone antibodies (diluted 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, United States) were used to detect the intragraft expression of TNF-α, IL-1β respectively. Biotin-labeled goat anti-rabbit secondary antibody (Santa Cruz Biotechnology), horseradish-peroxidase-labeled anti-biotin, and 3,3-diaminobenzidine were used to visualize positive expression. We counted 10 randomly chosen fields per section using a light microscope at high power (400 × magnification; Leica Q550CW, Germany) and the results were expressed as absorbance units (A).
Fresh lung tissue (200 mg) was homogenized with 2 mL PBS on ice, and 1 mL tissue homogenate was dissolved in 2 mL chloroform/methanol (2:1, v/v). After thermal agitation and 30 min standing at room temperature, the solution was centrifuged at 2500 rpm at 4 °C for 5 min. The supernatant was transferred to a freezing tube. The above procedures were repeated, and then all the supernatants obtained were evaporated and frozen at -80 °C.
Chemicals and reagents used were of analytical reagent grade. Phospholipid standard solutions (Sigma, United States) included: phosphatidylcholine (PC), phosphatidylethanolaraine (PE), phosphatidylinositol (PI), and phosphatidylserine (PS). Micellar electrokinetic capillary chromatography (MECC) was performed in an Agilent G1600AX Capillary Electrophoresis (Agilent, Santa Clara, CA, United States). The electrophoresis buffer for MECC was composed of 35 mmol/L sodium deoxycholate, 6 mmol/L disodium tetraborate, 10 mmol/L disodium hydrogen phosphate, and 30% 1-propanol. The buffer pH value was adjusted to 8.5 with 1 mol/L HCl. MECC was conducted at a temperature of 40 °C and a voltage of 18 kV. The total length of capillary was 570 mm and the detection region was 500 mm away from the injection end. Vacuura injection was performed at 50 mbr for 5 s, and ultraviolet detection was performed at 200 nm[8].
All data were expressed as mean ± SEM, and analyzed with one-way ANOVA and Student’s t test. All the statistical analyses were carried out using SPSS version 13.0. P < 0.05 was considered to represent statistical significance.
The duration of donor operation was 45.6 ± 13.8 min, the time for donor liver preparation was 20.3 ± 6.7 min, and warm ischemia was avoided. The receptor operation took 26.7 ± 5.5 min, and anhepatic phase lasted for 16.1 ± 2.5 min. No significant difference was seen in portal clamping time among groups. No animal died before sample harvest.
The representative lung injuries in different groups are shown in Figure 1. Lung microscopic examination revealed alveolar, perivascular and interstitial edema, neutrophil infiltration, atelectasis, disruption of alveolar and bronchiolar epithelial cells, and local hemorrhage in severe cases with prolonged CPT. Consistent with these histopathological observations, the lung injury scores in the 180-min group were significantly higher than those in the sham group and 45-min group (3.16 ± 0.28 vs 1.12 ± 0.21, P < 0.001; 3.16 ± 0.28 vs 2.05 ± 0.24, P < 0.001), and were also higher than those in PDTC group although the difference was not significant (3.16 ± 0.28 vs 2.95 ± 0.16, P = 0.054).
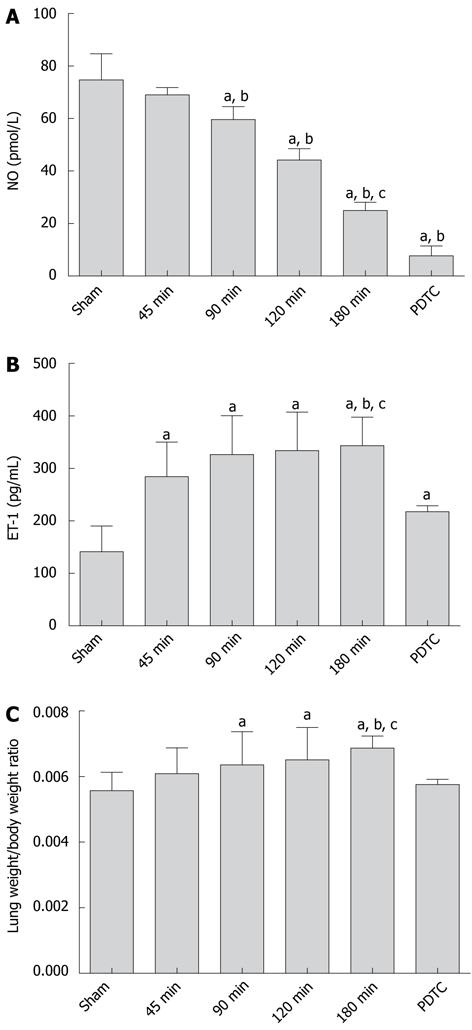
The experiment results showed that with donor liver CPT prolonged, the serum NO level in the 180-min group were significantly lower than those in the sham group and 45-min group (24.97 ± 3.18 vs 74.67 ± 10.01, P < 0.001; 24.97 ± 3.18 vs 69.05 ± 2.74, P < 0.001) and the decline could not be reversed by PDTC (24.97 ± 3.18 vs 7.67 ± 3.79, P < 0.001; Figure 2A).
With longer liver CPT, serum ET-1 levels increased significantly in the 180-min group compared with the sham group (343.59 ± 53.97 vs 141.53 ± 48.48, P < 0.001). There was a significant difference between each of the operation groups and the control group (P < 0.05). The expression of ET-1 was inhibited significantly by PDTC (343.59 ± 53.97 vs 217.80 ± 11.32, P < 0.001; Figure 2B).
Lung weight/body weight ratio increased with prolonged CPT. It peaked in the 180-min group compared with the sham group (0.00687 ± 0.00037 vs 0.00557 ± 0.00056, P < 0.001), but greatly decreased after PDTC was used 0.00687 ± 0.00037 vs 0.00576 ± 0.00016, P = 0.001; Figure 2C).
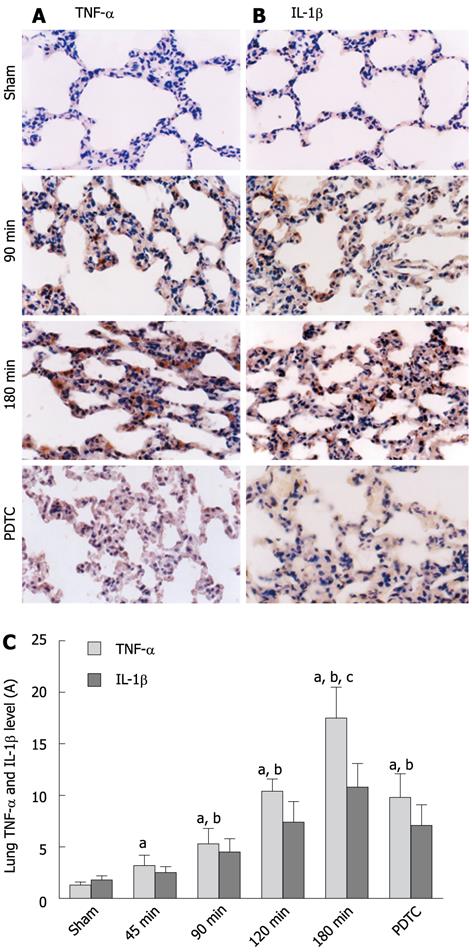
Figure 3 shows a significant increase in TNF-α and IL-1β in lung with prolonged CPT, which peaked in the 180-min group compared with the sham group (TNF-α: 17.5 ± 3.0 vs 1.3 ± 0.3, P < 0.001; IL-1β: 10.8 ± 2.3 vs 1.8 ± 0.4, P < 0.001). After administration of PDTC, TNF-α and IL-1β production was significantly attenuated (TNF-α: 17.5 ± 3.0 vs 9.8 ± 2.3, P < 0.001; IL-1β: 10.8 ± 2.3 vs 7.1 ± 2.0, P = 0.0012). We suggest that the prolonged CPT induced some inflammatory factors expressed in lung.
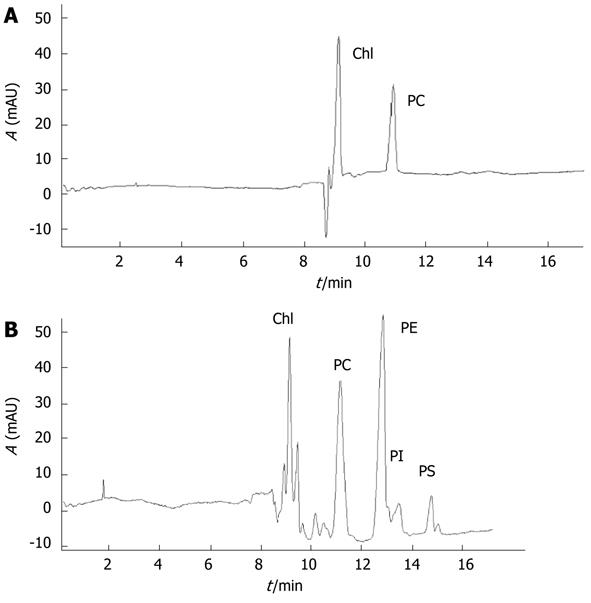
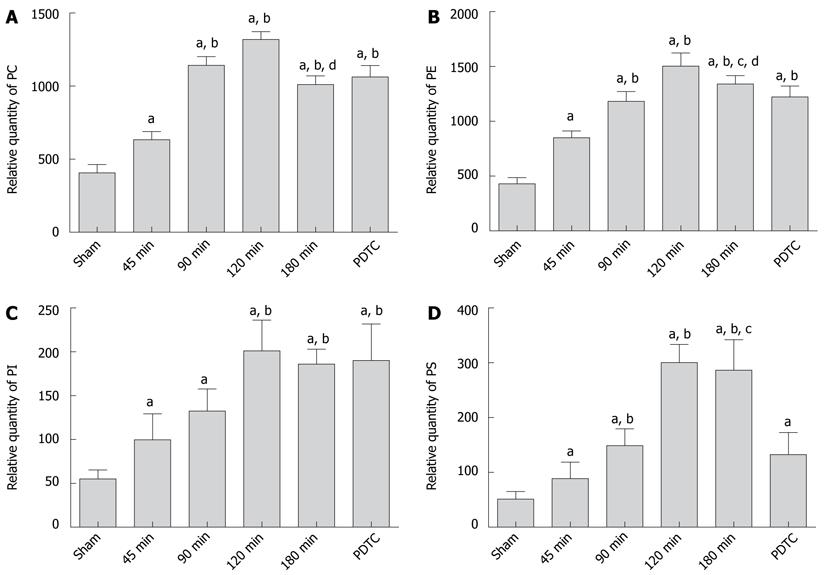
The area under curve (AUC) represents the composition of lung phospholipids (Figures 4 and 5). With the extension of CPT, PC levels increased significantly, reaching a maximum in the 120-min group compared with the sham group (1318.89 ± 54.79 vs 406.79 ± 56.49, P < 0.001), and then declined in notably the 180-min group (1318.89 ± 54.79 vs 1011.18 ± 59.99, P < 0.001). No significant difference was observed between the 180-min group and PDTC group (1011.18 ± 59.99 vs 1062.58 ± 78.49, P = 0.1173).
PE changed similarly with PC, reaching a maximum in the 120-min group compared with the sham group (1504.45 ± 119.96 vs 430.38 ± 57.91, P < 0.001), and decreased significantly in the 180-min group (1504.45 ± 119.96 vs 1340.80 ± 76.39, P = 0.0019). PE in the PDTC group decreased significantly compared with the 180-min group (1340.80 ± 76.39 vs 1222.18 ± 100.48, P = 0.0082).
PI kept rising gradually, and reached a peak in the 120-min group compared with the sham group (201.23 ± 34.82 vs 55.12 ± 10.14, P < 0.001), but no significant difference was found between the 120-min, 180-min and PDTC groups (201.23 ± 34.82 vs 185.88 ± 17.04, P = 0.2265; 185.88 ± 17.04 vs 190.10 ± 41.75, P = 0.7707).
PS reached a maximum in the 120-min group compared with the sham group (300.43 ± 32.95 vs 51.29 ± 13.89, P < 0.001), and decreased in the 180-min group (300.43 ± 32.95 vs 286.55 ± 55.55, P = 0.5054), but dropped significantly in the PDTC group (286.55 ± 55.55 vs 132.60 ± 40.27, P < 0.001).
The incidence of ALI post-liver transplantation has been reported to be 60%-80%[3]. Despite intense research and diverse therapeutic trials, there is no effective prevention or treatment for ALI at present[1]. Recent studies have shown that the pathogenesis of ALI involves disorders of oxidants/antioxidants, inflammation/anti-inflammation, and upregulation of inflammatory factors[9,10]. It has been suggested that the antioxidant PDTC can inhibit some oxidants and inflammatory damage[1].
In our study, levels of serum NO and ET-1 were used to evaluate the severity of ALI. NO and ET-1 are secreted by endothelial cells and are two important vasoactive substances that regulate mini-circulation. NO can expand blood vessels, prevent platelet aggregation, and therefore improve microcirculation. After liver transplantation, the implanted graft liberates high amounts of arginase and causes L-arginine deficiency. L-arginine is the substrate of NO synthesis reaction. Its depletion influences NO synthesis after liver transplantation[11]. This might be the reason why serum NO level drops after liver transplantation. An increase in NO level by L-arginine could attenuate LS depletion, and therefore, ameliorate postoperative pulmonary dysfunction[12]. PDTC could inhibits cytokine-induced NO production[13], as our study showed.
ET-1, as the most powerful vasoactive substance, can cause microcirculation disturbance and induce pulmonary injury[14]. The slowdown of liver blood flow can concentrate ET-1 in the serum and liver. We observed that the balance between NO and ET-1 was disrupted after liver transplantation.
Natural lung surfactants (LSs) are a mixture of phospholipids and specific proteins, and are produced by type II alveolar epithelial cells, stored in Golgi bodies and secreted into the alveolar space. They are important in maintaining alveolar expansion during breathing and physiological gas exchange. The pathological condition of ALI is often characterized with metabolic anomalies of LSs and disrupted lung function[15].
We found that with prolonged CPT of donor liver, that cold ischemia/warm reperfusion injury increased. The imbalanced levels of NO and ET-1 induced capillary endothelial dysfunction. Together with TNF-α and IL-1β produced in the lungs, the permeability of the air-blood barrier increases, just as shown by lung weight/body weight ratio and lung histopathological scores. Then, a variety of plasma compositions, such as serum, serum proteins, hemoglobin and proteases migrate into the alveolar space and inactivate LSs[16]. At the same time, ET-1 and some inflammatory factors, such as TNF-α and IL-1β, stimulate the production of cAMP, which can induce type II alveolar epithelial cells to secrete LSs[17]. Because of the anti-inflammatory properties of LSs[18], an increase in their concentration in the lungs indicates a strong compensatory ability in the defense against ALI[19]. Our study showed that, when CPT was < 2 h, the expression of LSs increased with longer CPT, however, when CPT was 3 h, more severe injury of type II alveolar epithelial cells inhibited secretion of LSs. This agrees with the results of Shu et al[20].
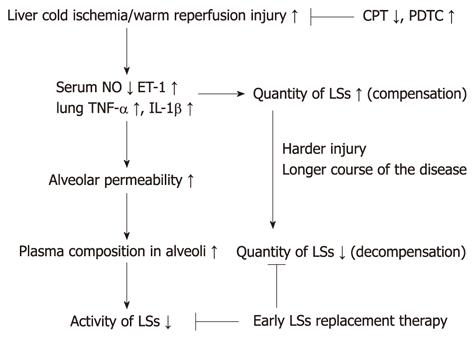
In contrast, the change in LSs after ALI is time-dependent, with early increases and late decreases. Therefore, surfactant replacement therapy should be administered at an early stage of ALI, the compensation stage, to alleviate injury[21]. If applied in the decompensation stage, the therapy may not provide satisfactory effects. This may be the reason why surfactant therapy has only limited success in ARDS[22,23]. Furthermore, as an antioxidant, PDTC can relieve cold ischemia/warm reperfusion injury of donor liver, and partly reverse the disturbance. Thus, shortening CPT or using PDTC may be useful for alleviation of ALI post-liver transplantation. A proposed scheme of the mechanism is given in Figure 6, summarizing how LS affects liver-transplantation-associated ALI.
The authors gratefully acknowledge Ke-Wei Meng and Jian Zhang for assistance with the experiments, and Qi Chen for polishing the manuscript.
Acute lung injury (ALI) and its severe subset acute respiratory distress syndrome (ARDS) have relatively high morbidity and mortality in post-liver transplantation patients. The pathogenesis and prevention of liver-transplantation-related ALI are not very clear. Lung surfactants (LSs) are a complicated mixture of approximately 90% lipids and 10% proteins. They are produced by type IIalveolar epithelial cells and secreted into the alveolar space to maintain normal respiratory mechanics by reducing alveolar surface tension to near-zero values.
Recent studies have shown that the pathogenesis of ALI involves disorders of oxidants/antioxidants, inflammation/anti-inflammation, and upregulation of inflammatory factors. Many antioxidants can inhibit some oxidants and inflammatory damage. The severity of ALI correlates with abnormalities in surfactant composition. Supplementing exogenous surfactant to newborns suffering from respiratory distress syndrome has a satisfactory therapeutic effect. Surfactant therapy has also been used in ALI/ARDS, but with only limited success.
We observed that ALI often occurred in patients transplanted with long-preserved donor livers. Therefore, we raised a hypothesis that cold preservation/warm reperfusion injury of donor liver may induce ALI after liver transplantation. To validate the hypothesis, we transplanted donor liver after different cold preservation times in rats. Prolonged cold preservation time had a positive correlation with the severity of ALI, and could induce or inhibit the expression of LSs in different time phases, and some antioxidants (e.g., ammonium pyrrolidinedithiocarbamate) may reverse the pathological process partially.
Shorter cold preservation time or some antioxidants may reduce liver-transplantation-related ALI, and surfactant replacement therapy should be useful in the early stage of ALI to achieve better results.
Cold ischemia/warm reperfusion injury, different from warm ischemia/reperfusion injury, is a characteristic injury in organ transplantation. It depends on the length of cold storage. Liver is one of the organs that are sensitive to ischemia/reperfusion injury. Emerging evidence suggests that the early stage of the injury can be regarded as part of the immune response to injury stress and sinusoidal endothelial cells are the targets of cold ischemia, whereas hepatocytes appear to be relatively unscathed.
This study suggests that liver transplantation in rats and the length of cold ischemia induce surfactant changes and ALI. The study was well designed and conducted.
Peer reviewers: Andrea De Gottardi, MD, PhD, Assistant Professor, Clinic of Visceral Surgery and Medicine-Hepatology, Freiburgstrasse, CH-3010 Berne-Inselspital, Switzerland; Christian Toso, MD, PhD, Abdominal and Transplant Surgery, Department of Surgery, Geneva University Hospitals, Rue Gabrielle-Perret-Gentil, 1211 Geneva 14, Switzerland
S- Editor Tian L L- Editor Kerr C E- Editor Zhang DN
| 1. | Yin H, Jin XB, Gong Q, Yang H, Hu LY, Gong FL, Zhu JY. Fructose-1,6-diphosphate attenuates acute lung injury induced by lipopolysaccharide in mice. Int Immunopharmacol. 2008;8:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170:22-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Yan LN. Modern liver transplantation. 1 ed. Beijing: People's Military Medical Press 2004; 295-296. |
| 4. | Bersten AD, Davidson K, Nicholas TE, Doyle IR. Respiratory mechanics and surfactant in the acute respiratory distress syndrome. Clin Exp Pharmacol Physiol. 1998;25:955-963. [PubMed] |
| 5. | Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979;28:47-50. [PubMed] |
| 6. | Hori T, Nguyen JH, Zhao X, Ogura Y, Hata T, Yagi S, Chen F, Baine AM, Ohashi N, Eckman CB. Comprehensive and innovative techniques for liver transplantation in rats: a surgical guide. World J Gastroenterol. 2010;16:3120-3132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Kostopanagiotou G, Avgerinos E, Costopanagiotou C, Arkadopoulos N, Andreadou I, Diamantopoulou K, Lekka M, Smyrniotis V, Nakos G. Acute lung injury in a rat model of intestinal ischemia-reperfusion: the potential time depended role of phospholipases A(2). J Surg Res. 2008;147:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Otieno AC, Mwongela SM. Capillary electrophoresis-based methods for the determination of lipids--a review. Anal Chim Acta. 2008;624:163-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Orfanos SE, Mavrommati I, Korovesi I, Roussos C. Pulmonary endothelium in acute lung injury: from basic science to the critically ill. Intensive Care Med. 2004;30:1702-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1038] [Cited by in RCA: 1111] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 11. | Roth E, Steininger R, Winkler S, Längle F, Grünberger T, Függer R, Mühlbacher F. L-Arginine deficiency after liver transplantation as an effect of arginase efflux from the graft. Influence on nitric oxide metabolism. Transplantation. 1994;57:665-669. [PubMed] |
| 12. | Yang Y, Su Z, Cai J, Wang S, Liu J, Xu Z, Ding W. Continuous pulmonary infusion of L-arginine during deep hypothermia and circulatory arrest improves pulmonary surfactant integrity in piglets. Ann Thorac Surg. 2008;86:429-435; discussion 435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Jang D, Murrell GA. Nitric oxide in arthritis. Free Radic Biol Med. 1998;24:1511-1519. [PubMed] |
| 14. | Hei ZQ, Luo CF, Li SR, Ma WH, Luo GJ. [Changes in hemodynamics and nitric oxide/endothelin-1 during liver transplantation in patients with cirrhosis]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005;17:592-594. [PubMed] |
| 15. | Pace PW, Yao LJ, Wilson JX, Possmayer F, Veldhuizen RA, Lewis JF. The effects of hyperoxia exposure on lung function and pulmonary surfactant in a rat model of acute lung injury. Exp Lung Res. 2009;35:380-398. [PubMed] |
| 16. | Taeusch HW, Keough KM. Inactivation of pulmonary surfactant and the treatment of acute lung injuries. Pediatr Pathol Mol Med. 2001;20:519-536. [PubMed] |
| 17. | Lazrak A, Thome U, Myles C, Ware J, Chen L, Venglarik CJ, Matalon S. cAMP regulation of Cl(-) and HCO(-)(3) secretion across rat fetal distal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L650-L658. [PubMed] |
| 18. | Kuronuma K, Mitsuzawa H, Takeda K, Nishitani C, Chan ED, Kuroki Y, Nakamura M, Voelker DR. Anionic pulmonary surfactant phospholipids inhibit inflammatory responses from alveolar macrophages and U937 cells by binding the lipopolysaccharide-interacting proteins CD14 and MD-2. J Biol Chem. 2009;284:25488-25500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Shu LH, Wei KL, Liu CF, Han XH, Shang YX, Cai XX, Li JJ, Wang LJ. [Changes of pulmonary surfactant protein A in young rats with acute lung injury induced by lipopolysaccharide]. Zhongguo Dang Dai Er Ke Zazhi. 2008;10:203-206. [PubMed] |
| 20. | Shu LH, Wei KL, Shang YX, Wu HM, Li J, Han XH, Cai XX, Liu CF, Li JJ, Wang LJ. [Relationship between alveolar epithelial type II cells and pulmonary surfactant protein A levels in young rats with acute lung injury]. Zhongguo Dang Dai Er Ke Zazhi. 2008;10:504-508. [PubMed] |
| 21. | Sun Y, Wang YQ, Zhong JG, Lu J. [Influence of porcine pulmonary surfactant administered at different time on therapeutic effects in rats with oleic acid induced acute lung injury]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2008;20:88-91. [PubMed] |
| 22. | Zuo YY, Veldhuizen RA, Neumann AW, Petersen NO, Possmayer F. Current perspectives in pulmonary surfactant--inhibition, enhancement and evaluation. Biochim Biophys Acta. 2008;1778:1947-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 381] [Article Influence: 22.4] [Reference Citation Analysis (0)] |