Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.302
Revised: August 26, 2011
Accepted: September 2, 2011
Published online: January 28, 2012
Reactive oxygen species (ROS) attack guanine bases in DNA easily and form 8-hydroxydeoxyguanosine (8-OHdG), which can bind to thymidine rather than cytosine, based on which, the level of 8-OHdG is generally regarded as a biomarker of mutagenesis consequent to oxidative stress. For example, higher levels of 8-OHdG are noted in Helicobacter pylori-associated chronic atrophic gastritis as well as gastric cancer. However, we have found that exogenous 8-OHdG can paradoxically reduce ROS production, attenuate the nuclear factor-κB signaling pathway, and ameliorate the expression of proinflammatory mediators such as interleukin (IL)-1, IL-6, cyclo-oxygenase-2, and inducible nitric oxide synthase in addition to expression of nicotinamide adenine dinucleotide phosphate oxidase (NOX)-1, NOX organizer-1 and NOX activator-1 in various conditions of inflammation-based gastrointestinal (GI) diseases including gastritis, inflammatory bowel disease, pancreatitis, and even colitis-associated carcinogenesis. Our recent finding that exogenous 8-OHdG was very effective in either inflammation-based or oxidative-stress-associated diseases of stress-related mucosal damage has inspired the hope that synthetic 8-OHdG can be a potential candidate for the treatment of inflammation-based GI diseases, as well as the prevention of inflammation-associated GI cancer. In this editorial review, the novel fact that exogenous 8-OHdG can be a functional molecule regulating oxidative-stress-induced gastritis through either antagonizing Rac-guanosine triphosphate binding or blocking the signals responsible for gastric inflammatory cascade is introduced.
- Citation: Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-Hydroxydeoxyguanosine: Not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol 2012; 18(4): 302-308
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/302.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.302
When we consume a soft drink or eat a meal, the gastrointestinal (GI) mucosa is continuously stressed with various antigens that we have ingested because the GI lumen is actually outside the body[1]. As far as the interaction between materials from outside and the physiological barriers of the GI tract is concerned, everything existing outside the GI lumen can cause stressful reactions at the cellular level through mechanisms including antigen challenge, concurring oxidative stress, and some inflammatory assaults. The GI lumen can come under attack by many factors, including solid food, Helicobacter pylori (H. pylori) and other commensal bacteria, non-steroidal anti-inflammatory drugs, and gastric acid[2-4]. In particular, one important means of attack is mediated by oxygen, which is also the most important metabolic substance in the body. In spite of being essential for life, oxygen can cause various cellular stresses called oxidative stress by generating reactive oxygen species (ROS)[5]. The definition of oxidative stress is a disturbance of oxidant-antioxidant homeostasis, leading to potential cellular damage. Even though the existence of ROS and their pathological implications were discovered < 50 years ago, it is surprising that many diseases can be explained by oxidative stress and its subsequent dysregulation. Although ROS are a crucial regulator of cellular signal transduction and energy transmission, disturbance of the balance between generating and scavenging capability of ROS might lead to cell damage. ROS can react with cellular proteins or lipids, transforming them into oxidized forms, or bind with nucleic acids, turning them into mutated forms. It is particularly interesting that oxidative stress is closely associated with carcinogenesis.
Fortunately, to cope with these harmful effects of oxidative stress, cells may endeavor to enhance defensive factors of various types. The common examples of defense factors are reduced glutathione/oxidized glutathione, superoxide dismutase, catalase, heme oxygenase 1, G protein gamma-like, and nicotinamide adenine dinucleotide phosphate (NADPH) dehydrogenase, quinine 1[6-8]. However, disease can occur if there are insufficient defense factors or overwhelming offensive factors. Therefore, if we set up a sensitive marker that predicts the degree of oxidative stress, we can prevent the initiation or progression of disease by measuring this marker[9]. Moreover, if the level of this marker reflects the severity of disease, appropriate levels of scavenging agents can prevent complications of the disease, because the extreme is organ dysfunction or cancer. Several biomarkers to estimate oxidative stress have been suggested, but most of them have failed to reach clinical significance. One successful discovery of the late 1980s was the level of 8-hydroxydeoxyguanosine (8-oxo-7, 8-dihydroguanosine, 8-OHdG), because it has been proven to be increased in serum or urine of patients who have oxidative-stress-associated disease[10].
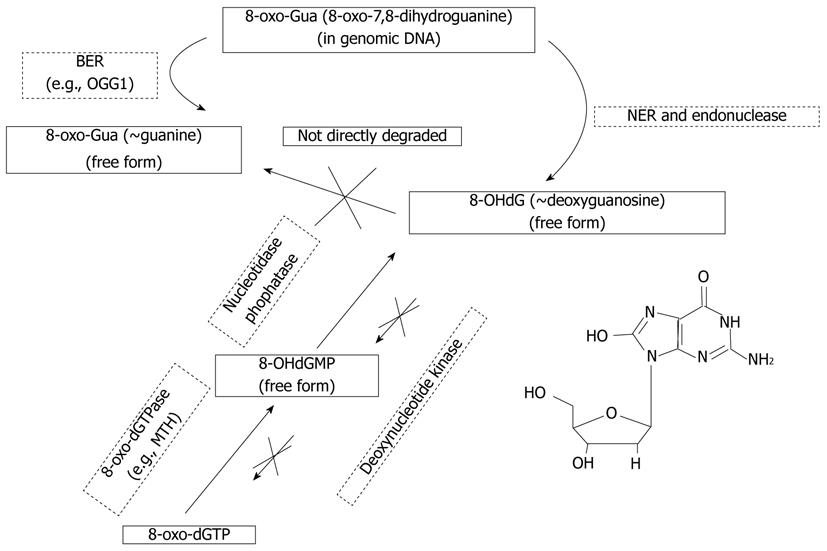
When DNA is attacked by oxidative stress such as ROS, ultraviolet light, or genotoxic agents, guanine is easily oxidized into 8-oxo-7,8-dihydroguanine (8-oxo-Gua)[11]. The existence of this oxidized guanine in genomic DNA can cause transversion mutation such as G-T or G-A binding, accumulation of which can lead to detrimental consequences[12-14]. Fortunately, mammalian cells have multiple repair systems such as base excision repair enzymes or nucleotide excision repair (NER) enzymes, which counteract the hazardous effects of 8-oxo-Gua. Consequently, 8-OHdG, a nucleoside form of 8-oxo-Gua, is generated from either damaged oligomer which contains 8-oxo-Gua by NER or from cytoplasmic oxidized nucleotides like 8-hydroxy-deoxyguanosine triphosphate (8-hydroxy-dGTP). Fortunately, exogenously administered 8-OHdG cannot reincorporate into genomic DNA because the activity of deoxynucleotide kinase which converts 8-OHdG into 8-hydroxy-dGTP is very low, although wild deoxyguanosine can be actively converted to deoxyguanosine triphosphate which can be used as a substrate of DNA polymerase[15-22].
8-OHdG can cross the cell membrane unlike any other species that contains oxidized guanine, thus, it is usually detected in the urine or serum of patients who have diseases associated with oxidative stress[23]. Examples of application of 8-OHdG as a disease-associated clinical marker are summarized in Table 1.
| Diseases | References |
| Helicobacter pylori infection | Hahm et al[58], Baik et al[59] |
| Colorectal tumor | Sato et al[60], Gushima et al[61] |
| Breast cancer | Matsui et al[62], Djuric et al[63], Musarrat et al[64] |
| Bladder/prostate cancer | Chiou et al[65] |
| Lung cancer | Erhola et al[66] |
| Atherosclerosis | Martinet et al[67] |
| Diabetes | Kanauchi et al[68] |
| Smoking | Asami et al[69], Kiyosawa et al[70] |
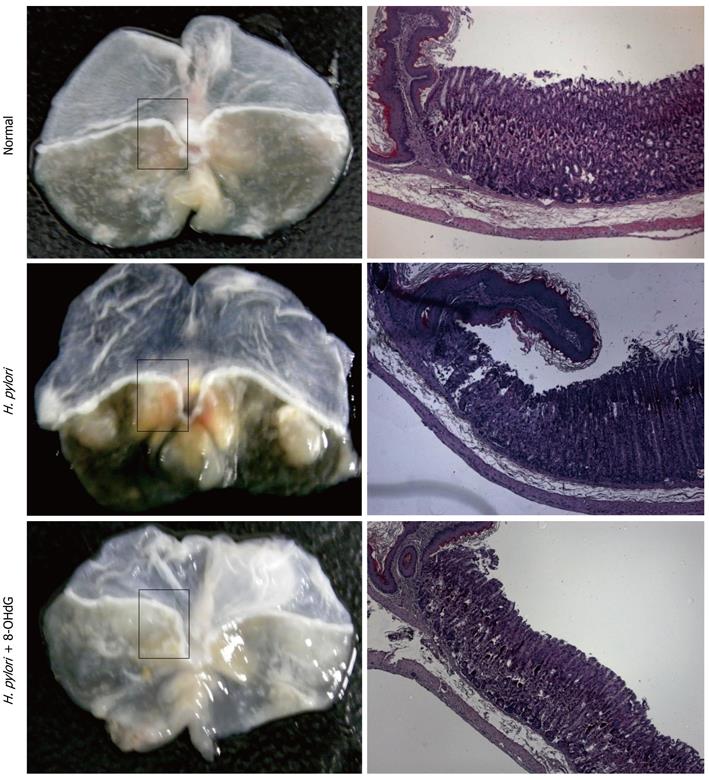
Although many studies have shown increased levels of 8-OHdG in oxidative-stress-associated diseases, the exact biological role of 8-OHdG has not been investigated. Oxidized deoxyguanosine is notorious for inducing mutagenesis, therefore, most researchers have felt that 8-OHdG might have mutagenic or at least harmful effects in cells, and that is why mammalian physiology tries hard to excrete this oxidized guanosine. However, under the innovative hypothesis that the generation of this molecule can be one of the defense mechanisms of cells against oxidative-stress-induced inflammation, we have tried to obtain evidence that oxidized guanosine can interact with the GTPase family, which is broadly involved in cytoskeleton modification, triggering inflammation, regulating apoptosis, and carcinogenesis[24-27]. Interestingly, genetically modified oxidized GTP, 8-oxo-GTPγS, seems to interact with the small GTPase family such as Ras, Rho, Rac and cdc42[28]. Among these, we have focused on the role of Rac1 in inflammatory cascades[29,30] because Rac1 activation is crucial for aggregating NADPH oxidase (NOX) complex and subsequent ROS production[31]. As a result, we have concluded that inhibition of Rac1 by exogenous 8-OHdG, which is a transmittable form of oxidized guanosine, can significantly block ROS-mediated inflammation. Compared with other nucleoside products, 8-OHdG has a potent anti-inflammatory effect by inhibiting the activity of Rac1 on lipopolysaccharide (LPS)-stimulated microglial cells, chemokine-activated neutrophils, and inflammatory mediator-stimulated macrophages[21,32-34]. Moreover, since endogenously produced 8-OHdG is much lower than exogenously treated concentrations of 8-OHdG, we propose the biological role of the antioxidative and anti-inflammatory actions of 8-OHdG, implying that 8-OHdG formation can be a defense mechanism against oxidative stress, and enrichment with exogenous 8-OHdG can be a strategy to prevent the initiation or progression of inflammatory disease, backed up with additional fact that only pretreatment or earlier administration of 8-OHdH is effective. To clarify and compare the cellular effect of 8-OHdG with existing anti-inflammatory agents, we have also investigated several animal models of acute inflammation. Intraperitoneal LPS injection to mice causes severe inflammation in lung tissues by inducing tumor necrosis factor (TNF)-α, interleukins, and myeloperoxidase activity and recruiting neutrophils. Simultaneous treatment with 8-OHdG significantly decreases the level of the above markers, and the efficacy of 8-OHdG is even more potent than that of aspirin; a conventional anti-inflammatory agent[35]. We also have investigated the antiallergic effects of 8-OHdG in ovalbumin-sensitized mice[36,37]. One of the major phenomena of oxidative stress is stress-related mucosal disease (SRMD), the mechanism of which is mucosal damage induced by ROS involved in reperfusion injury after local ischemia. We established a water-immersion restraint stress model, which mimics SRMD, causing severe ulceration and hemorrhagic lesions in gastric mucosa. Treatment with 8-OHdG reduces the pathological lesions, as well as other angiogenesis mediators such as TNF-α and vascular endothelial growth factor[21]. Recently, we have established an animal model of H. pylori-infected gastric inflammation by infecting four times with bacteria, followed by ingestion of a high-salt diet for > 16 wk. The degree of gastric inflammation induced by H. pylori is significantly decreased by continuous ingestion of 8-OHdG-dissolved water ad libitum. Moreover, dysplastic and precancerous lesions are observed in this animal model with chronic H. pylori infection, but curiously, the carcinogenesis that results from chronic H. pylori infection is apparently ameliorated with exogenous administration of 8-OHdG (Figure 1).
Although the possible mechanism to explain how 8-OHdG inhibits the activity of Rac1 has not been clearly documented yet, we hypothesize that 8-OHdG can interfere with the GTP-binding pocket of Rac1, because GTP and 8-OHdG share a similar conformation with a guanine base. 8-OHdG does not affect the activity of phosphoinositide 3-kinase/AKT or that of Rac1-guanosine exchange factor, which is an upstream pathway of Rac1 activation by exchanging Rac1-bound-GDP into GTP, turning inactive Rac1 into the active form[38-40]. However, treatment with 8-OHdG dramatically decreases the portion of Rac1-GTP, implying the specific molecular target of 8-OHdG might be Rac1 inactivation[21]. Rac1 is a crucial mediator of activating NOX complex[41,42], therefore, inactivating Rac1 could decrease the generation of ROS, and block the redox-sensitive nuclear factor (NF)-κB pathway. Rac1 also directly binds to signal transducer and activator of transcription (STAT)3 and regulates its activity[43], therefore, the ratio of phosphorylated to total STAT3 would be decreased by treatment with 8-OHdG[32]. The regulation of Rac1-mediated ROS, NF-κB and STAT3, which are the important mediators of inflammatory cascades, is the latest possibility of how 8-OHdG exerts an anti-inflammatory action.
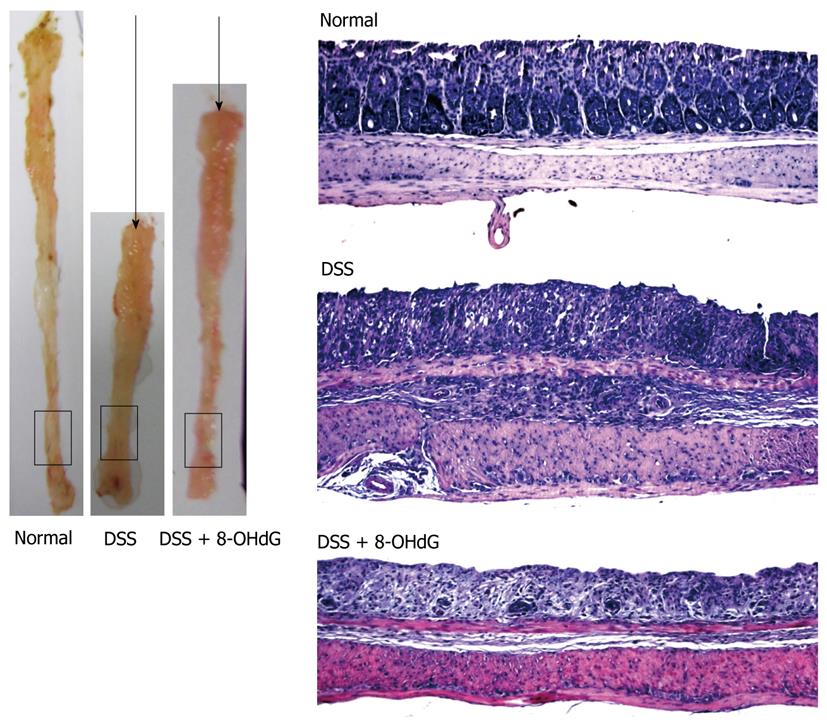
8-oxoguanine DNA glycosylase 1 (OGG1) is an endogenous DNA repair enzyme that repairs 8-OH-Gua in genomic DNA, cutting it into 8-OHdG (Figure 2). Although 8-OHdG does not reincorporate into genomic DNA as explained above, exogenously administered 8-OHdG can increase the ratio of 8-OH-Gua in genomic DNA by increasing error-prone DNA polymerase in the specific types of cells that have mutated forms of OGG1[19,44]. Although wild-type OGG1 can correct and repair 8-OH-Gua, OGG1 mutation cannot remove this harmful oxidized guanine, causing mutation and apoptosis. Treatment with 8-OHdG induces G1 arrest and apoptosis in KG-1 leukemia cells, which have an OGG1 mutation, but not in U937 leukemia cells, which have wild-type OGG1[45-48]. The wide profile of OGG1 mutations in human cancer is now actively under investigation[49-54], therefore, treatment of cancer that has OGG1 mutations with 8-OHdG might be developed as a new model of targeted chemotherapy. For example, we have investigated models of colitis induced by dextran sodium sulfate (DSS) and colitis-associated cancer induced by DSS combined with azoxymethane (AOM + DSS)[55-57], and determined whether exogenous 8-OHdG has a preventive effect on colitis and colitis-associated cancer[57]. Daily injection of 8-OHdG significantly inhibits recruitment of inflammatory cells in DSS-induced colitis, and chronic ingestion of diet containing 8-OHdG exerts a chemopreventive role in AOM + DSS-induced colitis-associated cancer (Figure 3)[47].
There is extensive experimental evidence that oxidative damage permanently occurs to lipids of cellular membranes, proteins and DNA. In nuclear and mitochondrial DNA, 8-OHdG or 8-oxodG is one of the predominant agents of free-radical-induced oxidative lesions. This is why 8-OHdG has been used widely in many studies as a biomarker for the measurement of endogenous oxidative DNA damage, and as a risk factor for many diseases including cancer, because urinary 8-OHdG is a good biomarker for risk assessment of various cancers and other degenerative diseases. Several lines of evidence[7,29-32,47] show that exogenous 8-OHdG can: inhibit allergy-induced inflammation; remodel airway and lung tissues through Rac inactivation; regulate oxidative-stress-induced gastritis through antagonizing Rac-GTP binding or blocking the signals responsible for gastric inflammatory cascade; play a role in anti-inflammatory actions via suppression of intercellular adhesion molecule-1 gene expression by blockade of the Toll-like receptor 4/STAT3 signal cascade in inflammation-enhanced brain microglia; and prevent colitis-associated carcinogenesis based on efficient TNF-α inhibition or H. pylori-associated gastric carcinogenesis based on inhibition of cytokine generation. All this clearly suggests that 8-OHdG could be used as a potential tool to modulate GI tract inflammation as well as allergy-related bronchial disease, and is especially applicable to diverse inflammation-based diseases including gastritis, colitis, and esophagitis, as well as GI cancer, including esophageal, gastric and colon cancer, for which more extensive and well-designed clinical trials are required.
Peer reviewers: Hartmut Jaeschke, Professor, Department of Pharmacology, Toxicology and Therapeutics, University of Kansas Medical Center, 3901 Rainbow Blvd, MS 1018, Kansas City, KS 66160, United States; Dae-Yeul Yu, PhD, Professor, Aging Research Center, Korea Research Institute of Bioscience and Biotechnology, 111 Gwahangno, Yuseong-gu, Daejeon 305-806, Korea; SV Rana, PhD, Professor, Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh 160012, India
S- Editor Lv S L- Editor Kerr C E- Editor Li JY
| 1. | Kim JH, Kim EH, Ock C, Hong H, Kim YJ, Kwon KA, Park DK, Hahm KB. Mitigating endoplasmic reticulum stress with revaprazan ameliorates stress-related mucosal disease. J Gastroenterol Hepatol. 2012;27:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Won I, Kim YJ, Kim SJ, Kim EH, Hahm KB. Nutrigenomic approach to tackle the unpleasant journey to Helicobacter pylori-associated gastric carcinogenesis. J Dig Dis. 2011;12:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Handa O, Naito Y, Yoshikawa T. Helicobacter pylori: a ROS-inducing bacterial species in the stomach. Inflamm Res. 2010;59:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 4. | Farinati F, Cardin R, Cassaro M, Bortolami M, Nitti D, Tieppo C, Zaninotto G, Rugge M. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3513] [Cited by in RCA: 4190] [Article Influence: 261.9] [Reference Citation Analysis (0)] |
| 6. | Kundu JK, Surh YJ. Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis. Pharm Res. 2010;27:999-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 7. | Kim J, Cha YN, Surh YJ. A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res. 2010;690:12-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 8. | Surh YJ, Kundu JK, Li MH, Na HK, Cha YN. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch Pharm Res. 2009;32:1163-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Dong MH, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2006;22:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Lunec J, Holloway KA, Cooke MS, Faux S, Griffiths HR, Evans MD. Urinary 8-oxo-2'-deoxyguanosine: redox regulation of DNA repair in vivo? Free Radic Biol Med. 2002;33:875-885. [PubMed] |
| 11. | Cheng TJ, Kao HP, Chan CC, Chang WP. Effects of ozone on DNA single-strand breaks and 8-oxoguanine formation in A549 cells. Environ Res. 2003;93:279-284. [PubMed] |
| 12. | Lieberman HB. DNA damage repair and response proteins as targets for cancer therapy. Curr Med Chem. 2008;15:360-367. [PubMed] |
| 13. | Hakem R. DNA-damage repair; the good, the bad, and the ugly. EMBO J. 2008;27:589-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 347] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 14. | Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1412] [Cited by in RCA: 1530] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 15. | Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1634] [Cited by in RCA: 1682] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 16. | Hayakawa H, Hofer A, Thelander L, Kitajima S, Cai Y, Oshiro S, Yakushiji H, Nakabeppu Y, Kuwano M, Sekiguchi M. Metabolic fate of oxidized guanine ribonucleotides in mammalian cells. Biochemistry. 1999;38:3610-3614. [PubMed] |
| 17. | Moriya M. Single-stranded shuttle phagemid for mutagenesis studies in mammalian cells: 8-oxoguanine in DNA induces targeted G.C--& gt; T.A transversions in simian kidney cells. Proc Natl Acad Sci USA. 1993;90:1122-1126. [PubMed] |
| 18. | Reardon JT, Bessho T, Kung HC, Bolton PH, Sancar A. In vitro repair of oxidative DNA damage by human nucleotide excision repair system: possible explanation for neurodegeneration in xeroderma pigmentosum patients. Proc Natl Acad Sci USA. 1997;94:9463-9468. [PubMed] |
| 19. | Kim JE, Chung MH. 8-Oxo-7,8-dihydro-2'-deoxyguanosine is not salvaged for DNA synthesis in human leukemic U937 cells. Free Radic Res. 2006;40:461-466. [PubMed] |
| 20. | Kim JE, Hyun JW, Hayakawa H, Choi S, Choi J, Chung MH. Exogenous 8-oxo-dG is not utilized for nucleotide synthesis but enhances the accumulation of 8-oxo-Gua in DNA through error-prone DNA synthesis. Mutat Res. 2006;596:128-136. [PubMed] |
| 21. | Ock CY, Hong KS, Choi KS, Chung MH, Kim Y, Kim JH, Hahm KB. A novel approach for stress-induced gastritis based on paradoxical anti-oxidative and anti-inflammatory action of exogenous 8-hydroxydeoxyguanosine. Biochem Pharmacol. 2011;81:111-122. [PubMed] |
| 22. | Won MH, Kang TC, Jeon GS, Lee JC, Kim DY, Choi EM, Lee KH, Choi CD, Chung MH, Cho SS. Immunohistochemical detection of oxidative DNA damage induced by ischemia-reperfusion insults in gerbil hippocampus in vivo. Brain Res. 1999;836:70-78. [PubMed] |
| 23. | Svoboda P, Ko SH, Cho B, Yoo SH, Choi SW, Ye SK, Kasai H, Chung MH. Neopterin, a marker of immune response, and 8-hydroxy-2'-deoxyguanosine, a marker of oxidative stress, correlate at high age as determined by automated simultaneous high-performance liquid chromatography analysis of human urine. Anal Biochem. 2008;383:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 25. | Embade N, Valerón PF, Aznar S, López-Collazo E, Lacal JC. Apoptosis induced by Rac GTPase correlates with induction of FasL and ceramides production. Mol Biol Cell. 2000;11:4347-4358. [PubMed] |
| 26. | Murga C, Zohar M, Teramoto H, Gutkind JS. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene. 2002;21:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Park KS, Kim JH, Kim MS, Kim JM, Kim SK, Choi JY, Chung MH, Han B, Kim SY, Lee HK. Effects of insulin and antioxidant on plasma 8-hydroxyguanine and tissue 8-hydroxydeoxyguanosine in streptozotocin-induced diabetic rats. Diabetes. 2001;50:2837-2841. [PubMed] |
| 28. | Yoon SH, Hyun JW, Choi J, Choi EY, Kim HJ, Lee SJ, Chung MH. In vitro evidence for the recognition of 8-oxoGTP by Ras, a small GTP-binding protein. Biochem Biophys Res Commun. 2005;327:342-348. [PubMed] |
| 29. | Sulciner DJ, Irani K, Yu ZX, Ferrans VJ, Goldschmidt-Clermont P, Finkel T. rac1 regulates a cytokine-stimulated, redox-dependent pathway necessary for NF-kappaB activation. Mol Cell Biol. 1996;16:7115-7121. [PubMed] |
| 30. | Park EJ, Ji KA, Jeon SB, Choi WH, Han IO, You HJ, Kim JH, Jou I, Joe EH. Rac1 contributes to maximal activation of STAT1 and STAT3 in IFN-gamma-stimulated rat astrocytes. J Immunol. 2004;173:5697-5703. [PubMed] |
| 31. | Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245-313. [PubMed] |
| 32. | Kim HS, Ye SK, Cho IH, Jung JE, Kim DH, Choi S, Kim YS, Park CG, Kim TY, Lee JW. 8-hydroxydeoxyguanosine suppresses NO production and COX-2 activity via Rac1/STATs signaling in LPS-induced brain microglia. Free Radic Biol Med. 2006;41:1392-1403. [PubMed] |
| 33. | Kim HJ, Yoon SH, Ryu HO, Yoon BH, Choi S, Ye SK, Chung MH. 8-oxo-7,8-dihydroguanosine triphosphate(8-oxoGTP) down-regulates respiratory burst of neutrophils by antagonizing GTP toward Rac, a small GTP binding protein. Free Radic Res. 2007;41:655-662. [PubMed] |
| 34. | Kim DH, Cho IH, Kim HS, Jung JE, Kim JE, Lee KH, Park T, Yang YM, Seong SY, Ye SK. Anti-inflammatory effects of 8-hydroxydeoxyguanosine in LPS-induced microglia activation: suppression of STAT3-mediated intercellular adhesion molecule-1 expression. Exp Mol Med. 2006;38:417-427. [PubMed] |
| 35. | Choi S, Choi HH, Lee SH, Ko SH, You HJ, Ye SK, Chung MH. Anti-inflammatory effects of 8-hydroxy-2'-deoxyguanosine on lipopolysaccharide-induced inflammation via Rac suppression in Balb/c mice. Free Radic Biol Med. 2007;43:1594-1603. [PubMed] |
| 36. | Ro JY, Kim DY, Lee SH, Park JW, Chung MH. Effects of 7,8-dihydro-8-oxo-deoxyguanosine on antigen challenge in ovalbumin-sensitized mice may be mediated by suppression of Rac. Br J Pharmacol. 2009;158:1743-1752. [PubMed] |
| 37. | Kim JS, Kim DY, Lee JK, Ro JY, Chung MH. 8-oxo-2'-deoxyguanosine suppresses allergy-induced lung tissue remodeling in mice. Eur J Pharmacol. 2011;651:218-226. [PubMed] |
| 38. | Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6:167-180. [PubMed] |
| 39. | Karnoub AE, Worthylake DK, Rossman KL, Pruitt WM, Campbell SL, Sondek J, Der CJ. Molecular basis for Rac1 recognition by guanine nucleotide exchange factors. Nat Struct Biol. 2001;8:1037-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Miyano K, Sumimoto H. Role of the small GTPase Rac in p22phox-dependent NADPH oxidases. Biochimie. 2007;89:1133-1144. [PubMed] |
| 41. | Takemura Y, Goodson P, Bao HF, Jain L, Helms MN. Rac1-mediated NADPH oxidase release of O2- regulates epithelial sodium channel activity in the alveolar epithelium. Am J Physiol Lung Cell Mol Physiol. 2010;298:L509-L520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res. 2006;98:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 431] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 43. | Simon AR, Vikis HG, Stewart S, Fanburg BL, Cochran BH, Guan KL. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290:144-147. [PubMed] |
| 44. | Choi J, Kim DY, Hyun JW, Yoon SH, Choi EM, Hahm KB, Rhee KH, Chung MH. Measurement of oxidative damage at individual gene levels by quantitative PCR using 8-hydroxyguanine glycosylase (OGG1). Mutat Res. 2003;523-524:225-235. [PubMed] |
| 45. | Choi S, Choi HH, Choi JH, Yoon BH, You HJ, Hyun JW, Kim JE, Ye SK, Chung MH. Inhibitory effect of 8-oxo-7,8-dihydro-2'-deoxyguanosine on the growth of KG-1 myelosarcoma in Balb/c nude mice. Leuk Res. 2006;30:1425-1436. [PubMed] |
| 46. | Hyun JW, Jung YC, Kim HS, Choi EY, Kim JE, Yoon BH, Yoon SH, Lee YS, Choi J, You HJ. 8-hydroxydeoxyguanosine causes death of human leukemia cells deficient in 8-oxoguanine glycosylase 1 activity by inducing apoptosis. Mol Cancer Res. 2003;1:290-299. [PubMed] |
| 47. | Hyun JW, Yoon SH, Yu Y, Han CS, Park JS, Kim HS, Lee SJ, Lee YS, You HJ, Chung MH. Oh8dG induces G1 arrest in a human acute leukemia cell line by upregulating P21 and blocking the RAS to ERK signaling pathway. Int J Cancer. 2006;118:302-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Hyun JW, Choi JY, Zeng HH, Lee YS, Kim HS, Yoon SH, Chung MH. Leukemic cell line, KG-1 has a functional loss of hOGG1 enzyme due to a point mutation and 8-hydroxydeoxyguanosine can kill KG-1. Oncogene. 2000;19:4476-4479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 49. | Dallosso AR, Dolwani S, Jones N, Jones S, Colley J, Maynard J, Idziaszczyk S, Humphreys V, Arnold J, Donaldson A. Inherited predisposition to colorectal adenomas caused by multiple rare alleles of MUTYH but not OGG1, NUDT1, NTH1 or NEIL 1, 2 or 3. Gut. 2008;57:1252-1255. [PubMed] |
| 50. | Kim JC, Ka IH, Lee YM, Koo KH, Kim HC, Yu CS, Jang SJ, Kim YS, Lee HI, Lee KH. MYH, OGG1, MTH1, and APC alterations involved in the colorectal tumorigenesis of Korean patients with multiple adenomas. Virchows Arch. 2007;450:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Görgens H, Müller A, Krüger S, Kuhlisch E, König IR, Ziegler A, Schackert HK, Eckelt U. Analysis of the base excision repair genes MTH1, OGG1 and MUTYH in patients with squamous oral carcinomas. Oral Oncol. 2007;43:791-795. [PubMed] |
| 52. | Arcand SL, Provencher D, Mes-Masson AM, Tonin PN. OGG1 Cys326 variant, allelic imbalance of chromosome band 3p25.3 and TP53 mutations in ovarian cancer. Int J Oncol. 2005;27:1315-1320. [PubMed] |
| 53. | Hagiwara A, Kitajima Y, Sato S, Miyazaki K. Allelic loss of the DNA repair gene OGG1 against oxidative damage in esophageal squamous cell carcinoma. Oncol Rep. 2005;13:1009-1016. [PubMed] |
| 54. | Kim IJ, Ku JL, Kang HC, Park JH, Yoon KA, Shin Y, Park HW, Jang SG, Lim SK, Han SY. Mutational analysis of OGG1, MYH, MTH1 in FAP, HNPCC and sporadic colorectal cancer patients: R154H OGG1 polymorphism is associated with sporadic colorectal cancer patients. Hum Genet. 2004;115:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 2010;3:1314-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Kim YJ, Lee JS, Hong KS, Chung JW, Kim JH, Hahm KB. Novel application of proton pump inhibitor for the prevention of colitis-induced colorectal carcinogenesis beyond acid suppression. Cancer Prev Res (Phila). 2010;3:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Ock CY, Kim EH, Hong H, Hong KS, Han YM, Choi KS, Hahm KB, Chung MH. Prevention of colitis-associated colorectal cancer with 8-hydroxydeoxyguanosine. Cancer Prev Res (Phila). 2011;4:1507-1521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Hahm KB, Lee KJ, Choi SY, Kim JH, Cho SW, Yim H, Park SJ, Chung MH. Possibility of chemoprevention by the eradication of Helicobacter pylori: oxidative DNA damage and apoptosis in H. pylori infection. Am J Gastroenterol. 1997;92:1853-1857. [PubMed] |
| 59. | Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, Park CK, Kasai H, Rhee KH. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996;56:1279-1282. [PubMed] |
| 60. | Sato T, Takeda H, Otake S, Yokozawa J, Nishise S, Fujishima S, Orii T, Fukui T, Takano J, Sasaki Y. Increased plasma levels of 8-hydroxydeoxyguanosine are associated with development of colorectal tumors. J Clin Biochem Nutr. 2010;47:59-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Gushima M, Hirahashi M, Matsumoto T, Fujita K, Fujisawa R, Mizumoto K, Nakabeppu Y, Iida M, Yao T, Tsuneyoshi M. Altered expression of MUTYH and an increase in 8-hydroxydeoxyguanosine are early events in ulcerative colitis-associated carcinogenesis. J Pathol. 2009;219:77-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Matsui A, Ikeda T, Enomoto K, Hosoda K, Nakashima H, Omae K, Watanabe M, Hibi T, Kitajima M. Increased formation of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, in human breast cancer tissue and its relationship to GSTP1 and COMT genotypes. Cancer Lett. 2000;151:87-95. [PubMed] |
| 63. | Djuric Z, Heilbrun LK, Simon MS, Smith D, Luongo DA, LoRusso PM, Martino S. Levels of 5-hydroxymethyl-2'-deoxyuridine in DNA from blood as a marker of breast cancer. Cancer. 1996;77:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 64. | Musarrat J, Arezina-Wilson J, Wani AA. Prognostic and aetiological relevance of 8-hydroxyguanosine in human breast carcinogenesis. Eur J Cancer. 1996;32A:1209-1214. [PubMed] |
| 65. | Chiou CC, Chang PY, Chan EC, Wu TL, Tsao KC, Wu JT. Urinary 8-hydroxydeoxyguanosine and its analogs as DNA marker of oxidative stress: development of an ELISA and measurement in both bladder and prostate cancers. Clin Chim Acta. 2003;334:87-94. [PubMed] |
| 66. | Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, Uchida K, Osawa T, Nieminen MM, Alho H. Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2'-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett. 1997;409:287-291. [PubMed] |
| 67. | Martinet W, Knaapen MW, De Meyer GR, Herman AG, Kockx MM. Elevated levels of oxidative DNA damage and DNA repair enzymes in human atherosclerotic plaques. Circulation. 2002;106:927-932. [PubMed] |
| 68. | Kanauchi M, Nishioka H, Hashimoto T. Oxidative DNA damage and tubulointerstitial injury in diabetic nephropathy. Nephron. 2002;91:327-329. [PubMed] |
| 69. | Asami S, Manabe H, Miyake J, Tsurudome Y, Hirano T, Yamaguchi R, Itoh H, Kasai H. Cigarette smoking induces an increase in oxidative DNA damage, 8-hydroxydeoxyguanosine, in a central site of the human lung. Carcinogenesis. 1997;18:1763-1766. [PubMed] |
| 70. | Kiyosawa H, Suko M, Okudaira H, Murata K, Miyamoto T, Chung MH, Kasai H, Nishimura S. Cigarette smoking induces formation of 8-hydroxydeoxyguanosine, one of the oxidative DNA damages in human peripheral leukocytes. Free Radic Res Commun. 1990;11:23-27. [PubMed] |