Published online Jan 28, 2012. doi: 10.3748/wjg.v18.i4.295
Revised: June 27, 2011
Accepted: July 4, 2011
Published online: January 28, 2012
The latest avenue of research is revealing the existence of and role for the colonic stem cells in the physiological renewal of the mucosa and in pathological circumstances where they have both positive and negative effects. In the case of human colon, different levels of stem cell compartments exist. First, the crypt epithelial stem cells, which have a role in the normal crypt epithelial cell dynamics and in colorectal carcinogenesis. Close to the crypts, the second layer of stem cells can be found; the local subepithelial stem cell niche, including the pericryptic subepithelial myofibroblasts that regulate the epithelial cell differentiation and have a crucial role in cancer progression and chronic inflammation-related fibrosis. The third level of stem cell compartment is the immigrating bone-marrow-derived stem cells, which have an important role in wound healing after severe mucosal inflammation, but are also involved in cancer invasion. This paper focuses on stem cell biology in the context of physiological and pathological processes in the human colon.
- Citation: Sipos F, Valcz G, Molnár B. Physiological and pathological role of local and immigrating colonic stem cells. World J Gastroenterol 2012; 18(4): 295-301
- URL: https://www.wjgnet.com/1007-9327/full/v18/i4/295.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i4.295
Beside water and electrolyte absorption, barrier function is a major physiological function of the colonic mucosa, which is the basis of the defense against the luminal pathogens and toxins. Keeping the integrity of the epithelial layer therefore is of great importance, in which stem cells as the key elements of the mucosal regeneration have an important role.
The epithelial layer of the colon consists of a single sheet of columnar epithelial cells folded into finger-like invaginations that are surrounded by the lamina propria to form a functional unit, called Lieberkühn’s crypt. It has been estimated that there are about 20 million of these crypts in the human colon[1]. There are four epithelial cell lineages within the crypt. Enterocytes, goblet cells and endocrine cells are terminally differentiated cells, which are found in the upper third of the crypt, and are derived from multipotent stem cells located at the bottom of the crypt. During asymmetric division, these epithelial stem cells undergo self-renewal and generate a population of transit amplifying cells that, upon migration upward through the crypt, proliferate and differentiate into one of the epithelial cell types of the crypt wall. The fourth type is the Paneth cell, which differentiates during a downward migration to the base of the crypt, where they reside below the crypt epithelial stem cell population[2].
Extensive experimental evidence has demonstrated that Wnt/β-catenin signaling plays a central role in maintaining the intestinal stem-cell niche and regulating normal crypt dynamics[3-7].
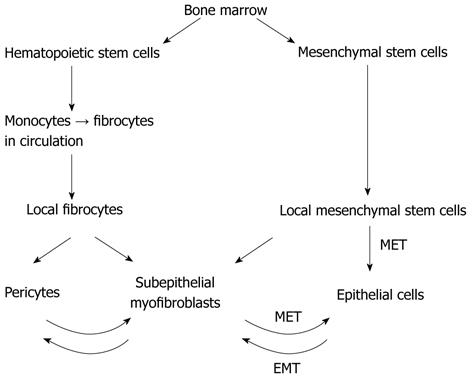
The origin of crypt epithelial stem cells is probably dual. Regarding their origin, there are two major kinds of stem cells in the subepithelial layer of the colonic mucosa: the local mesenchymal stem cells (MSCs) and the immigrating bone-marrow-derived stem cells[8,9] (Figures 1 and 2). In the case of mild to moderate mucosal injury, the local subepithelial stem cell niche is enough for differentiating to the epithelial lineage[8]. In the adult gut, both the number and differentiation capacity of the local stem cells are low. In the case of serious tissue injury [i.e., graft-versus-host disease, inflammatory bowel disease (IBD)] the regenerative capacity of local stem cells is not enough to complete tissue healing. In this case, bone-marrow-derived MSCs (BM-MSCs) migrate into the gastrointestinal wall, where they may contribute to the repair progress[9-11] as differentiated mesenchymal cells (e.g., myofibroblasts)[12,13]. Intestinal subepithelial myofibroblasts have a key role in the support of the epithelial stem cell compartment. They can originate from local subepithelial fibroblasts[14], and from immigrating bone marrow derived cells[15]. The bone marrow origin of these cells may be supposed by such observations in which co-expression of epithelial and hematopoietic lineage markers on them were found in inflamed mucosa adjacent to subepithelial lymphoid aggregates[16-20]. Both processes are regulated by transforming growth β-1 (TGF-β1), which plays an important role in intestinal mucosal healing[21].
Although our knowledge is expanding about the origin and behavior of subepithelial colonic stem cells, data about their effect in pathological circumstances like inflammation, fibrosis or cancer development are scattered throughout the literature. A better understanding of the regulation of their differentiation may help to establish new therapeutic strategies.
The local MSCs, namely the pericryptal myofibroblasts, which form the epithelial stem cell niche and also regulate epithelial cell differentiation are important cells orchestrating many diverse functions in the intestine, and are involved in growth and repair, tumorigenesis, inflammation, and fibrosis[22]. Adult myofibroblasts are derived or replenished after injury or in response to neoplastic transformation from several sources: differentiation or activation of resident fibroblasts, dedifferentiation from perivascular smooth muscle cells and adipocytes, epithelial-to-mesenchymal transition of epithelial and endothelial cells, and bone-marrow-derived stem cells. MSCs or hematopoietic stem cells, via CD14+ monocytes, transdifferentiate into circulating CD34+ fibrocytes, which become resident CD34+ fibrocytes[7,12].
Migration of colonic fibroblasts into and through the extracellular matrix during the initial phase of mucosal healing appears to be a fundamental component of wound contraction. In recent studies, it has been shown that colonic lamina propria fibroblast-conditioned media induce migration of primary human colonic fibroblasts in the modified Boyden chamber, and fibronectin is mainly responsible for this autocrine migration induction[23]. The differentiation of fibroblasts into myofibroblasts is an important step in tissue repair. It has been described that TGF-β1 potently stimulates the production of smooth muscle actin and stress fiber formation in fibroblasts and therefore their differentiation into myofibroblasts[24]; moreover, it also regulates their migration[14]. Only fibroblasts expressing Thy-1 (CD90), a cell-surface glycoprotein of T-cells, can differentiate into myofibroblasts after treatment with TGF-β, whereas only Thy-1- fibroblasts differentiate into lipofibroblasts upon exposure to 15-deoxy-δ-prostaglandin J2[25]. After differentiation, subepithelial myofibroblasts form pericryptal fibroblast sheet adjacent to the basal lamina of colonic crypts[10,26]. Intestinal subepithelial myofibroblasts contribute to the coordination of tissue regeneration by producing TGF-β, epidermal growth factor, basic fibroblast growth factor, proinflammatory cytokines, and the formation of new basement membrane[27].
Brown et al[28] have reported a cyclooxygenase-2-expressing stromal cell that moves in response to Toll-like receptor (TLR) signals from a position in the upper aspect of the lamina propria to a position adjacent to the pericryptal myofibroblasts in the base of the crypts, where the crypt epithelial stem cells reside. This relocation and the prostaglandin secretion appear critical to colonic epithelial repair in a dextran sodium sulfate colitis model. Their study has not ruled out a role for prostaglandin production or TLR responses by the conventional myofibroblasts or pericytes that are also present.
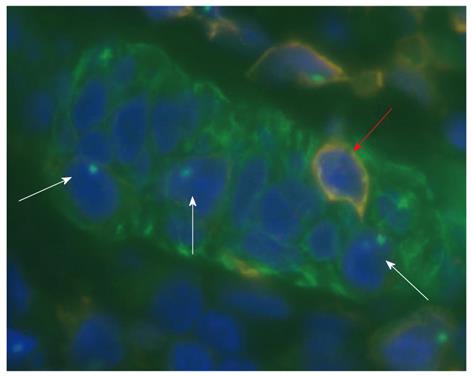
The number of myofibroblasts originating from the bone marrow significantly increased in the lamina propria of severe colitis compared to the healthy colon[9,13]. This homing process is driven by chemokines and adhesion molecules[29] (Table 1). Based on the former results[30-32], emerging evidence suggests that bone-marrow-derived stem cells contribute to tissue regeneration in the colon partly by promoting neovascularization or arteriogenesis. After human hematopoietic cell transplantation, epithelial tissue chimerism appears[33-35]. The bone marrow origin of subepithelial stem cells may be supposed by observations in which epithelial cell markers and leukocyte markers show that double positive cells are found in inflamed mucosa adjacent to lymphoid aggregates[18,19,36]. The presence of cytokeratin, epithelial growth factor receptor, hepatocyte-derived growth factor receptor or CDX2 co-expression in CD45+ cells of subepithelial lymphoid aggregates may support the mesenchymal origin of epithelial stem cells.
| Crypt epithelial stem cells | Subepithelial (pericryptal) myofibroblasts-local stem cell niche | BM-MSCs | |
| Origin | Pericryptal myofibroblasts | Subepithelial CD34+ fibroblasts, perivascular smooth muscle cells, adipocytes, endothelial- and epithelial cells (via EMT), BM-MSCs | Bone marrow |
| Daughter cells | Enterocytes, goblet cells, endocrine cells, paneth cells | Follicular dendritic cells, crypt epithelial stem cells, pericytes | Subepithelial myofibroblasts, fibrocytes, pericytes, adipocytes, crypt epithelial stem cells |
| Main regulator pathways of differentiation and/or homing | Wnt/β-catenin, Lgr-5, integrins, growth factor receptors (EGFR, HGFR, I1GFR) | TGF-β1, cytokines (IL-1, -6, -10, TNFα), growth factors (TGFα, GM-CSF, PDGF-AA, -BB, bFGF, KGF, HGF), chemokines (IL-8, MCP1, MIP-1α,-2), inflammatory mediators (PGE2, PAF, PGI2) | Chemokine receptors (CCR-1,-2,-7,-8,-9; CXCR-1,-2,-4,-5,-6), cell adhesion molecules involved in extravasation (VCAM, ICAM, selectins), proinflammatory cytokines (TNFα, IL-8), von Willebrand factor |
| Physiological function | Epithelial renewal | Growth, repair | Growth, repair, wound healing |
| Pathological function | Tumorigenesis, ulcer development | Tumorigenesis, cancer progression, inflammation, fibrosis | Tumorigenesis, inflammation, fibrosis |
| Cell type specific markers | Positive markers: Lgr-5, Musashi-1, CDX-2 | Negative markers: smoothelin, caldesmon, desmin | Negative markers: CD13, -14, -45, c-Kit, MHC class I and II |
| Positive markers: α-SMA, vimentin, SMM, prolyl 4-hydroxylase, CD90 | Positive markers: CD54,-90,-133,-146,-166, Flk-1, Sca-1, stage-specific antigen I, musashi-1, HLA class I | ||
| References | 1-6, 35, 50 | 12, 15, 25, 37, 48, 49 | 8, 9, 11, 20, 26, 29, 35, 37, 41, 58 |
Presumably, intestinal subepithelial myofibroblasts originated from BM-MSCs and may create a local microenvironment for the immigrated BM-MSCs that are committed to the epithelial lineage. The high percentages of intraepithelial cells of bone marrow origin are immune cells, such as CD45+ leukocytes. In bone-marrow-transplanted patients, the numbers of CD45+ and Y-FISH+ (male donor origin) double-positive, intraepithelial lymphocytes were significantly higher number in regenerating colonic epithelium than in the normal samples[35].
Previous studies using animal models of IBD have shown that transplanted bone marrow cells contribute to tissue repair by forming epithelial cells, activated myofibroblasts, and can also contribute to neovasculogenesis in the inflamed colon via the formation of entire new blood vessels[37]. It has also been shown that myofibroblasts of bone marrow origin are functional in their production of pro-collagen α1 mRNA[38]. These studies showing bone marrow contribution to tissue regeneration in IBD are now supported by the results of Khalil et al[39], who have further shown that regeneration can occur from a defined subpopulation of CD34- stem cells, present in both the bone marrow and peripheral blood, and moreover, that these stem cells can significantly enhance tissue regeneration in IBD without the need for prior ablation of the recipient’s immune system by irradiation.
The homing of BM-MSCs to colonic mucosa has been poorly revealed to date. BM-MSCs migrate via the bloodstream to the sites of colonic mucosal damage, which have been certified in several in vivo experiments[11,35,40]. Regulation of BM-MSC migration may happen as an effect of chemical signals, which are upregulated during injury.
Systemically delivered or natively circulating MSCs accumulate in injured tissues. During homing, MSCs adhere to endothelial cells and infiltrate underlying tissue. Previously, it has been shown that adhesiveness of endothelial cells for MSCs correlates with the inhibition of mitochondrial function of endothelial cells and secretion of von Willebrand factor (vWF)[41]. Potapova et al[42] have demonstrated that the treatment of endothelial cells with vWF stimulates MSC adhesion in a time- and concentration-dependent manner. MSCs do not adhere to immobilized vWF and do not express receptors for vWF, suggesting that the stimulation of MSC adhesion is a result of endothelial cell activation with vWF. In cell culture experiments, it has also been shown that normal colonic endothelial cells highly express vWF[43]. Based on these results, vWF seems to be an auto/paracrine regulator of colonic endothelial cells. Activation of p38 mitogen-activate protein kinase (MAPK) in endothelial cells by vWF may be responsible for the regulation of endothelial cell adhesiveness for MSCs in the colon.
CXC chemokine receptor (CXCR)4 has also a pivotal role in stem cell homing. It has recently been shown that CXC chemokine ligand 12 and CXCR4 are constitutively expressed on intestinal epithelial cells, lamina propria T cells, and the expression is increased in those of ulcerative colitis patients[44,45]. Induction of CXCR4 is associated with upregulation of two genes encoding transcription factors previously shown to control CXCR4 expression (hypoxia-inducible factor-2α and achaete scute complex like protein 2) and maintenance of crypt epithelial stem cells[46,47].
Subepithelial isolated lymphoid follicles and lymphoid aggregates of the colon are supposed to be the central organizer elements of stem cell homing and the mesenchymal-to-epithelial transition by producing an ideal cytokine, chemokine and cellular milieu[48,49].
Cancer stem cells are defined as cells that are endowed with both self-renewal and multilineage differentiation potential and, as such, are believed to expand clonally and repopulate the various types of differentiation lineages present within the tumor[50,51].
Crypt epithelial stem cells appear to be the cell of origin of colorectal cancer, based on their existence throughout the lifetime of an individual, and thus, their capacity to acquire multiple genetic mutations leads to carcinogenesis. Direct evidence for crypt epithelial stem cells as the source of intestinal tumors has come from a study of tissue-specific expression of Cre recombinases to inactivate a conditional Apc allele[52]. Although deletion of Apc in epithelial cells results in adenomas at very low frequency and with long latency, inactivation of Apc within epithelial stem cells leads to formation of macroscopic adenomas within 36 d. Moreover, these adenomas retain a small percentage of cells expressing Lgr5, an intestinal stem cell marker. These data support the view that crypt epithelial stem cells are the target of the origin of colorectal cancer. Alteration of crypt epithelial stem cell number or proliferation state may increase the probability of intestinal tumorigenesis.
Múnera et al[53] have tested the epithelial cell autonomous function of Ets2, a member of the Ets family of transcription factors, which is located on human chromosome 21, and has been identified as a Wnt target in colorectal cancer cells and crypt epithelial stem cells[54], during chemical carcinogenesis of the colon by using a conditional Ets2 allele and a transgene expressing Cre recombinase only in intestinal epithelial cells. Their results indicate that, although Ets2 is a Wnt pathway target gene within crypt epithelial stem cells, its loss provides a competitive advantage for crypt epithelial stem cells to colonize crypts, increase basal crypt cell proliferation, and increase crypt fission. Ets2 loss may increase the number or sensitivity of colon stem cells for tumor initiation.
The results of Deka et al[55] have revealed the essential role for Bcl9/Bcl9l in regulating a subset of Wnt target genes involved in controlling epithelial-to-mesenchymal transition and stem-cell-related features and suggest that targeting the Bcl9/Bcl9l arm of Wnt signaling in Wnt-activated cancers might attenuate these traits, which are associated with tumor invasion, metastasis, and resistance to therapy.
The interaction between cancer cells and non-transformed cells in the tumor microenvironment is essential for tumor growth and progression[56]. The tumor stroma includes several non-transformed cell types, such as endothelial cells, immune cells, and fibroblastic stromal cells (cancer-associated fibroblasts). This latter cell type plays an important role in cancer progression by promoting angiogenesis, epithelial-to-mesenchymal transition, and genetic instability[57-59]. Although tumor stromal fibroblasts are mainly recruited from local tissue fibroblasts, it has been proposed that BM-MSCs are recruited into the stroma of developing tumors[57,60]. Several studies have demonstrated that BM-MSCs can selectively migrate to sites of mucosal damage and wound healing including colorectal cancers, where a number of tumor-related inflammatory reactions and abnormal tissue regeneration phenomena take place actively. It also has been shown that cancer cells release specific factors that induce BM-MSC mobilization and recruitment to the tumor stroma where they eventually contribute to the formation of a tumor-supportive microenvironment[57].
The cause of metastasis remains elusive despite a vast amount of information on cancer cells. According to recent research, cancer cell fusion with macrophages or immigrating BM-MSCs provides an explanation[49,61,62]. BM-MSCs fused with tumor cells are present not just in animal tumor xenografts where they are associated with metastases, but in human carcinomas, including colon cancer. BM-MSC-tumor cell fusion explains the epithelial-to-mesenchymal transition in cancer since BM-MSCs express mesodermal traits and epithelial-to-mesenchymal transition regulators like Twist and SPARC (secreted protein acidic and rich in cysteine). If bone-marrow-derived-tumor cell fusion underlies invasion and metastasis in human cancer, new therapeutic strategies would be mandated.
A new association between parathyroid hormone (PTH) and cancer development has been revealed recently. Based on recent results, PTH can stimulate the phosphoinositide 3-kinase/MAPK-mediated proliferation of rat enterocytes, and primary hyperparathyroidism in humans is associated with an increased incidence of colon cancer[63]. It has been shown in a large cohort[64] that high serum PTH levels may be associated with incident, sporadic colorectal cancer in Western European populations, and in particular among men. PTH has its effect on MSCs/progenitors as they express PTH receptor[65]. Taking together these data, further investigations on PTH and colorectal carcinogenesis would be of great clinical importance.
Intestinal fibrosis is among the most common complications of IBD, especially Crohn’s disease (CD), resulting in stricture formation in the small intestine and colon. About 75% of CD patients will undergo surgery at least once over the course of their disease, and fibrotic strictures represent the main indication for surgery and the first cause of hospitalization and costs for CD patients[66]. Fibrosis in CD is the result of transmural chronic inflammation with repeated episodes of immune-mediated damage and repair[67]. Key factors for intestinal fibrosis are excessive deposition of extracellular matrix, proliferation of profibrogenic mesenchymal cells in the colon, thickening of all layers of the bowel wall, overgrowth of muscular layers of the intestine, enhanced local Th-1 type immune response, and overexpression of profibrogenic cytokines and growth factors[68]. As mentioned above, stromal cells derived from MSCs, fibrocytes, or BM-MSCs may also home to sites of inflammation and, in the presence of ongoing inflammation, become activated myofibroblasts/fibroblasts and contribute to tissue fibrosis[22]. Specific inhibition of the TGF-β signaling pathway, the key regulator of this pathologic process may be a promising therapeutic strategy to reduce the number of profibrogenic mesenchymal cells in chronic intestinal fibrosis[69].
The local and immigrating stem cells of the human colon are of major clinical importance because they are all involved in the regeneration of the damaged mucosa (Table 1). The results of the first attempts of MSC therapy in IBD are promising[70-72]. The regulation of the homing and differentiation of stem cells also provides new, individual and disease-specific therapeutic targets in the case of colorectal cancer. Inhibition of the TGF-β signaling pathway may be a promising therapeutic strategy in chronic inflammation-related colon fibrosis. The expected results of the ongoing and forthcoming studies hopefully will open the door to the development of new cures for old diseases.
Peer reviewers: Hang Nguyen, PhD, University of Auvergne, Pathogénie Bactérienne Intestinale, Centre Biomédical de Recherche et Valorisation, 28 Place Henri-Dunant, 63000 Clermont-Ferrand, France; Scott Steele, MD, FACS, FASCRS, Chief, Colon and Rectal Surgery, Department of Surgery, Madigan Army Medical Center, Fort Lewis, WA 98431, United States; Benjamin Perakath, Professor, Dr., Department of Surgery Unit 5, Christian Medical College, Vellore 632004, Tamil Nadu, India
S- Editor Sun H L- Editor Kerr C E- Editor Zhang DN
| 1. | Potten CS, Booth C, Hargreaves D. The small intestine as a model for evaluating adult tissue stem cell drug targets. Cell Prolif. 2003;36:115-129. [PubMed] |
| 2. | Abdul Khalek FJ, Gallicano GI, Mishra L. Colon cancer stem cells. Gastrointest Cancer Res. 2010;S16-S23. [PubMed] |
| 3. | Ilyas M. Wnt signalling and the mechanistic basis of tumour development. J Pathol. 2005;205:130-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 4. | Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, Batlle E, Simon-Assmann P, Clevers H, Nathke IS. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 632] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 5. | van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241-250. [PubMed] |
| 6. | van Leeuwen IM, Mirams GR, Walter A, Fletcher A, Murray P, Osborne J, Varma S, Young SJ, Cooper J, Doyle B. An integrative computational model for intestinal tissue renewal. Cell Prolif. 2009;42:617-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC. Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol. 2011;73:213-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 8. | Valcz G, Krenács T, Sipos F, Leiszter K, Tóth K, Balogh Z, Csizmadia A, Muzes G, Molnár B, Tulassay Z. The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. Pathol Oncol Res. 2011;17:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Brittan M, Wright NA. Stem cell in gastrointestinal structure and neoplastic development. Gut. 2004;53:899-910. [PubMed] |
| 10. | Tanaka F, Tominaga K, Ochi M, Tanigawa T, Watanabe T, Fujiwara Y, Ohta K, Oshitani N, Higuchi K, Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Wei Y, Nie Y, Lai J, Wan YJ, Li Y. Comparison of the population capacity of hematopoietic and mesenchymal stem cells in experimental colitis rat model. Transplantation. 2009;88:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123:2229-2238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 531] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 13. | Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes SJ, Hodivala-Dilke K, Goldman J, Alison MR, Wright NA. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752-757. [PubMed] |
| 14. | Brenmoehl J, Miller SN, Hofmann C, Vogl D, Falk W, Schölmerich J, Rogler G. Transforming growth factor-beta 1 induces intestinal myofibroblast differentiation and modulates their migration. World J Gastroenterol. 2009;15:1431-1442. [PubMed] |
| 15. | Emura M, Ochiai A, Horino M, Arndt W, Kamino K, Hirohashi S. Development of myofibroblasts from human bone marrow mesenchymal stem cells cocultured with human colon carcinoma cells and TGF beta 1. In Vitro Cell Dev Biol Anim. 2000;36:77-80. [PubMed] |
| 16. | Sipos F, Muzes G, Valcz G, Galamb O, Tóth K, Leiszter K, Krenács T, Tulassay Z, Molnár B. Regeneration associated growth factor receptor and epithelial marker expression in lymphoid aggregates of ulcerative colitis. Scand J Gastroenterol. 2010;45:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Gould VE, Bloom KJ, Franke WW, Warren WH, Moll R. Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression? Virchows Arch. 1995;425:617-629. [PubMed] |
| 18. | Sipos F, Molnár B, Zágoni T, Berczi L, Tulassay Z. Growth in epithelial cell proliferation and apoptosis correlates specifically to the inflammation activity of inflammatory bowel diseases: ulcerative colitis shows specific p53- and EGFR expression alterations. Dis Colon Rectum. 2005;48:775-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Sipos F, Galamb O, Herszényi L, Molnár B, Solymosi N, Zágoni T, Berczi L, Tulassay Z. Elevated insulin-like growth factor 1 receptor, hepatocyte growth factor receptor and telomerase protein expression in mild ulcerative colitis. Scand J Gastroenterol. 2008;43:289-298. [PubMed] |
| 20. | Valcz G, Krenács T, Sipos F, Patai AV, Wichmann B, Leiszter K, Tóth K, Balogh Z, Csizmadia A, Hagymási K. Lymphoid aggregates may contribute to the migration and epithelial commitment of bone marrow-derived cells in colonic mucosa. J Clin Pathol. 2011;64:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597-608. [PubMed] |
| 22. | Mifflin RC, Pinchuk IV, Saada JI, Powell DW. Intestinal myofibroblasts: targets for stem cell therapy. Am J Physiol Gastrointest Liver Physiol. 2011;300:G684-G696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Leeb SN, Vogl D, Grossmann J, Falk W, Schölmerich J, Rogler G, Gelbmann CM. Autocrine fibronectin-induced migration of human colonic fibroblasts. Am J Gastroenterol. 2004;99:335-340. [PubMed] |
| 24. | Simmons JG, Pucilowska JB, Keku TO, Lund PK. IGF-I and TGF-beta1 have distinct effects on phenotype and proliferation of intestinal fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2002;283:G809-G818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol. 2003;163:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 199] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Andoh A, Yoshida T, Yagi Y, Bamba S, Hata K, Tsujikawa T, Kitoh K, Sasaki M, Fujiyama Y. Increased aggregation response of platelets in patients with inflammatory bowel disease. J Gastroenterol. 2006;41:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, Stappenbeck TS. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 29. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1672] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 30. | Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1184] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 31. | Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1255] [Cited by in RCA: 1375] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 32. | MacDermott RP, Sanderson IR, Reinecker HC. The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 1998;4:54-67. [PubMed] |
| 33. | Spyridonidis A, Schmitt-Gräff A, Tomann T, Dwenger A, Follo M, Behringer D, Finke J. Epithelial tissue chimerism after human hematopoietic cell transplantation is a real phenomenon. Am J Pathol. 2004;164:1147-1155. [PubMed] |
| 34. | Körbling M, Katz RL, Khanna A, Ruifrok AC, Rondon G, Albitar M, Champlin RE, Estrov Z. Hepatocytes and epithelial cells of donor origin in recipients of peripheral-blood stem cells. N Engl J Med. 2002;346:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 545] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 35. | Okamoto R, Yajima T, Yamazaki M, Kanai T, Mukai M, Okamoto S, Ikeda Y, Hibi T, Inazawa J, Watanabe M. Damaged epithelia regenerated by bone marrow-derived cells in the human gastrointestinal tract. Nat Med. 2002;8:1011-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 36. | Gould VE, Bloom KJ, Franke WW, Warren WH, Moll R. Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression? Virchows Arch. 1995;425:617-629. [PubMed] |
| 37. | Brittan M, Chance V, Elia G, Poulsom R, Alison MR, MacDonald TT, Wright NA. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984-1995. [PubMed] |
| 38. | Direkze NC, Jeffery R, Hodivala-Dilke K, Hunt T, Playford RJ, Elia G, Poulsom R, Wright NA, Alison MR. Bone marrow-derived stromal cells express lineage-related messenger RNA species. Cancer Res. 2006;66:1265-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Khalil PN, Weiler V, Nelson PJ, Khalil MN, Moosmann S, Mutschler WE, Siebeck M, Huss R. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Matsumoto T, Okamoto R, Yajima T, Mori T, Okamoto S, Ikeda Y, Mukai M, Yamazaki M, Oshima S, Tsuchiya K. Increase of bone marrow-derived secretory lineage epithelial cells during regeneration in the human intestine. Gastroenterology. 2005;128:1851-1867. [PubMed] |
| 41. | Potapova IA, Cohen IS, Doronin SV. Apoptotic endothelial cells demonstrate increased adhesiveness for human mesenchymal stem cells. J Cell Physiol. 2009;219:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Potapova IA, Cohen IS, Doronin SV. Von willebrand factor increases endothelial cell adhesiveness for human mesenchymal stem cells by activating p38 mitogen-activated protein kinase. Stem Cell Res Ther. 2010;1:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Schellerer VS, Croner RS, Weinländer K, Hohenberger W, Stürzl M, Naschberger E. Endothelial cells of human colorectal cancer and healthy colon reveal phenotypic differences in culture. Lab Invest. 2007;87:1159-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Nakase H, Mikami S, Chiba T. Alteration of CXCR4 expression and Th1/Th2 balance of peripheral CD4-positive T cells can be a biomarker for leukocytapheresis therapy for patients with refractory ulcerative colitis. Inflamm Bowel Dis. 2009;15:963-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Dotan I, Werner L, Vigodman S, Weiss S, Brazowski E, Maharshak N, Chen O, Tulchinsky H, Halpern Z, Guzner-Gur H. CXCL12 is a constitutive and inflammatory chemokine in the intestinal immune system. Inflamm Bowel Dis. 2010;16:583-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 565] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 47. | Ahmadbeigi N, Seyedjafari E, Gheisari Y, Atashi A, Omidkhoda A, Soleimani M. Surface expression of CXCR4 in unrestricted somatic stem cells and its regulation by growth factors. Cell Biol Int. 2010;34:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Sipos F, Muzes G, Galamb O, Spisák S, Krenács T, Tóth K, Tulassay Z, Molnár B. The possible role of isolated lymphoid follicles in colonic mucosal repair. Pathol Oncol Res. 2010;16:11-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Sipos F, Muzes G. Isolated lymphoid follicles in colon: switch points between inflammation and colorectal cancer? World J Gastroenterol. 2011;17:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ. 2008;15:947-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 51. | Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339-9344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2176] [Cited by in RCA: 2199] [Article Influence: 115.7] [Reference Citation Analysis (0)] |
| 52. | Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1797] [Cited by in RCA: 1668] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 53. | Múnera J, Ceceña G, Jedlicka P, Wankell M, Oshima RG. Ets2 regulates colonic stem cells and sensitivity to tumorigenesis. Stem Cells. 2011;29:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 54. | van de Wetering M, Sancho E, Verweij C, de Lau W, Oving I, Hurlstone A, van der Horn K, Batlle E, Coudreuse D, Haramis AP. The beta-catenin/TCF-4 complex imposes a crypt progenitor phenotype on colorectal cancer cells. Cell. 2002;111:241-250. [PubMed] |
| 55. | Deka J, Wiedemann N, Anderle P, Murphy-Seiler F, Bultinck J, Eyckerman S, Stehle JC, André S, Vilain N, Zilian O. Bcl9/Bcl9l are critical for Wnt-mediated regulation of stem cell traits in colon epithelium and adenocarcinomas. Cancer Res. 2010;70:6619-6628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46-54. [PubMed] |
| 57. | Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007;263-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 58. | Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, Ganesan S, Glod JW, Banerjee D. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331-4339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 725] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 59. | Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 857] [Cited by in RCA: 869] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 60. | Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2949] [Cited by in RCA: 2767] [Article Influence: 172.9] [Reference Citation Analysis (0)] |
| 61. | Pawelek JM, Chakraborty AK. The cancer cell--leukocyte fusion theory of metastasis. Adv Cancer Res. 2008;101:397-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 62. | Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nat Rev Cancer. 2008;8:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 63. | Whitfield J, Bird RP, Morley P, Willick GE, Barbier JR, MacLean S, Ross V. The effects of parathyroid hormone fragments on bone formation and their lack of effects on the initiation of colon carcinogenesis in rats as indicated by preneoplastic aberrant crypt formation. Cancer Lett. 2003;200:107-113. [PubMed] |
| 64. | Fedirko V, Riboli E, Bueno-de-Mesquita HB, Rinaldi S, Pischon T, Norat T, Jansen EH, van Duijnhoven FJ, Tjønneland A, Olsen A. Prediagnostic circulating parathyroid hormone concentration and colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2011;20:767-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Ohishi M, Schipani E. PTH and stem cells. J Endocrinol Invest. 2011;34:552-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 66. | Spinelli A, Correale C, Szabo H, Montorsi M. Intestinal fibrosis in Crohn's disease: medical treatment or surgery? Curr Drug Targets. 2010;11:242-248. [PubMed] |
| 67. | Van Assche G, Geboes K, Rutgeerts P. Medical therapy for Crohn's disease strictures. Inflamm Bowel Dis. 2004;10:55-60. [PubMed] |
| 68. | Suzuki K, Sun X, Nagata M, Kawase T, Yamaguchi H, Sukumaran V, Kawauchi Y, Kawachi H, Nishino T, Watanabe K. Analysis of intestinal fibrosis in chronic colitis in mice induced by dextran sulfate sodium. Pathol Int. 2011;61:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 69. | Fiocchi C, Lund PK. Themes in fibrosis and gastrointestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2011;300:G677-G683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Singh UP, Singh NP, Singh B, Mishra MK, Nagarkatti M, Nagarkatti PS, Singh SR. Stem cells as potential therapeutic targets for inflammatory bowel disease. Front Biosci (Schol Ed). 2010;2:993-1008. [PubMed] |
| 71. | Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 72. | Wood NJ. IBD: Stem cell therapy feasible, safe and beneficial for fistulizing Crohn's disease. Nat Rev Gastroenterol Hepatol. 2011;8:181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |