Published online Oct 21, 2012. doi: 10.3748/wjg.v18.i39.5608
Revised: March 19, 2012
Accepted: May 5, 2012
Published online: October 21, 2012
AIM: To investigate the effects of emergent preoperative self-expandable metallic stent (SEMS) vs emergent surgery for acute left-sided malignant colonic obstruction.
METHODS: Two investigators independently searched the MEDLINE, EMBASE and Cochrane Central Register of Controlled Trials, as well as references of included studies to identify randomized controlled trials (RCTs) that compared two or more surgical approaches for acute colonic obstruction. Summary risk ratios (RR) and 95% CI for colonic stenting and emergent surgery were calculated.
RESULTS: Eight studies met the selection criteria, involving 444 patients, of whom 219 underwent SEMS and 225 underwent emergent surgery. Seven studies reported difference of the one-stage stoma rates between the two groups (RR, 0.60; 95% CI: 0.48-0.76; P < 0.0001). Only three RCTs described the follow-up stoma rates, which showed no significant difference between the two groups (RR, 0.80; 95% CI: 0.59-1.08; P = 0.14). Difference was not significant in the mortality between the two groups (RR, 0.91; 95% CI: 0.50-1.66; P = 0.77), but there was significant difference (RR, 0.57; 95% CI: 0.44-0.74; P < 0.0001) in the overall morbidity. There were no significant differences between the two groups in the anastomotic leak rate (RR, 0.60; 95% CI: 0.28-1.28; P = 0.19), occurrence of abscesses, including peristomal abscess, intraperitoneal abscess and parietal abscess (RR, 0.83; 95% CI: 0.36-1.95; P = 0.68), and other abdominal complications (RR: 0.67; 95% CI: 0.40-1.12; P = 0.13).
CONCLUSION: SEMS is not obviously more advantageous than emergent surgery for patients with acute left-sided malignant colonic obstruction.
-
Citation: Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting
vs emergent surgery for acute left-sided malignant colonic obstruction: A systematic review and meta-analysis. World J Gastroenterol 2012; 18(39): 5608-5615 - URL: https://www.wjgnet.com/1007-9327/full/v18/i39/5608.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i39.5608
Colorectal cancer is the fourth most common malignancy worldwide, with an estimated number of 1 023 000 new cases and 529 000 deaths each year[1]. The incidence of colorectal cancer has been increasing rapidly in Asia over the past few decades[2]. About 7%-29% of the patients with colorectal cancer present with bowel obstruction[3,4]. And benefit of surgical management of malignant large bowel obstruction remains controversial, especially for left-sided colonic obstruction. Emergent colorectal surgery for acute obstruction is associated with a mortality rate of 15%-20% and a morbidity rate of 15%-20%, both significantly higher than that in the elective situation[5-7]. Emergent surgery is an independent factor of mortality and morbidity, and about two-thirds of patients end up with a permanent stoma[3,6,8,9].
In 1991, colonic stenting was introduced to restore luminal patency in patients with malignant obstruction of the left side colon[10]. Tejero et al[11] used self-expandable metallic stent (SEMS) as a bridge to surgery in two patients with colonic obstruction in 1994. Stent placement before elective surgery, also known as a bridge to surgery, improved the clinical condition of the patient and seemed to decrease the mortality, morbidity, and number of colostomies in uncontrolled studies[12-15]. Although preoperative SEMS insertion has such advantages, it may result in the related complications such as perforation, stent migration, and reobstruction. As shown in the recent randomized controlled trials (RCTs), whether preoperative SEMS can reduce mortality, complication rate and stoma rate is still a big controversy[16-18]. Thus, this meta-analysis was performed to evaluate the effects of preoperative SEMS vs emergent surgery for acute left-sided malignant colonic obstruction.
SEMS was first used in 1991. We therefore, searched the databases, including the Cochrane Central Register of Controlled Trials (1991-April 2011), MEDLINE (1991-September 2011), EMBASE (1991-September 2008), Elsevier ScienceDirect (1998-September 2008), SpringLink (up to September 2011), Ovid LWW (1991-September 2011) and BMJ Journals Online (up to September 2008). The following keywords were used: “intestinal obstruction”, “colon”, “rectum”, “left-sided colon”, “surgery”, “resection“, “stents”, “randomized” and “controlled study”. The detailed search strategy is available from the authors. All included studies also had access to the PubMed “related articles” function and the Science Citation Index. In addition, the reference lists of included studies were scrutinized. No language restrictions were applied.
Data were independently abstracted from each study by two researchers, and disagreement was resolved by consensus. Data were extracted from each study using a predesigned review form. Data to be extracted were as follows: (1) treatment details: primary anastomosis rate, and the incidence of stoma creation; (2) short-term adverse events: mortality and morbidity such as anastomotic leak rate, abscess and extra abdominal complications; and (3) long-term outcomes: follow-up stoma rate.
Studies fulfilling the following criteria were included in the meta-analysis: (1) RCTs or other comparative studies comparing SEMS as a bridge to surgery and emergent surgery; (2) reports on at least one of the outcome measures mentioned below; and (3) studies reporting patients with malignant acute left-sided colonic obstruction.
The quality of nonrandomized studies was assessed using the Newcastle-Ottawa Scale with some modifications to meet the needs for this meta-analysis[19], and the quality of randomized studies was evaluated by means of the modified Jadad score[20]. The quality of the studies was evaluated based on three items: patient selection, comparability of study groups, and assessment of outcome. Studies achieving five or more stars were considered high quality. The quality of randomized studies was evaluated by means of the modified Jadad score including the following four areas: (1) randomization method; (2) hidden subgroups; (3) blinding; and (4) the description of the loss to follow-up and drop-out and the intention-to-treat. The total score of 1 to 3 points were ascribed to low-quality studies, whereas a total score of 4 to 7 points to high-quality researches.
Using the Cochrane Collaboration’s RevMan 5.1 software provided by meta-analysis, the results of included measurement of indicators were all count data, and 95% CI was used for the efficacy analysis. The heterogeneity between studies was tested. When there was homogeneity among studies (P > 0.1, I2 < 50%), a fixed effects model was used for meta-analysis; if there is significant heterogeneity among studies (P < 0.1, I2 > 50%), the random effects model was used. We also analyzed the different quality of the possible causes, and conducted subgroup analysis. If the heterogeneity among the studies was too large, descriptive analysis was performed.
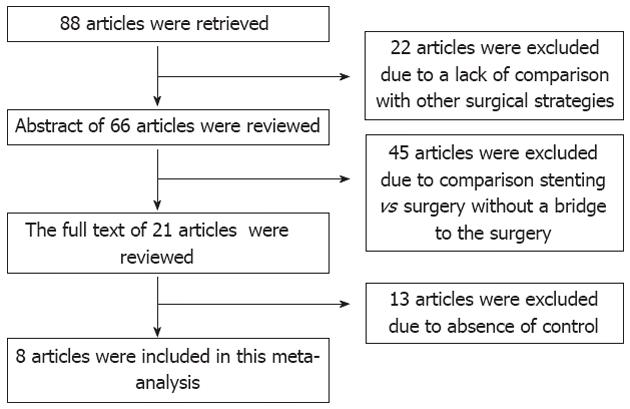
The initial search strategy retrieved 88 articles after screening all titles, abstracts and full texts. Twenty-two articles were excluded due to lack of comparison with other surgical strategies in most of the cohort studies, 45 articles were excluded because of comparison stenting vs surgery without a bridge to the surgery, and 13 studies were excluded because there was no control. Finally, 8 trials with 444 patients were included, of whom 219 (49.3%) successfully underwent stent insertion and 225 (50.7%) underwent emergent surgery. There were 17 (7.8%) deaths in the SEMS as a bridge to surgery group and 21 (9.3%) deaths in the emergent surgery group. There were only three RCTs[16-18] and five nonrandomized controlled studies (NRCTs)[12-14,21,22]. The flow chart of selection of studies and reasons for exclusion is presented in Figure 1. Characteristics of studies included in the meta-analysis are presented in Tables 1 and 2.
| Year | Author | Region | Total | SEMS | Surgery | Concealment of allocation | Jaded score | Quality |
| 2009 | Cheung | Hongkong | 60 | 30 | 30 | Appropriant | 5 | High |
| 2010 | Pirlet | France | 48 | 24 | 24 | Appropriant | 5 | High |
| 2011 | Van Hooft | Holland | 98 | 47 | 51 | Appropriant | 5 | High |
| Year | Author | Design | Total | SEMS | Surgery | Match | Study quality (rate, max 11) |
| 2008 | Dastur | R | 43 | 19 | 23 | 1,2,3,4,5,6 | 8 |
| 2002 | Martin | R | 52 | 26 | 26 | 1,2,3,4,5,8 | 7 |
| 2006 | Ng | R | 60 | 20 | 40 | 1,2,3,5,6,8 | 8 |
| 2007 | Pessione | R | 16 | 9 | 7 | 1,2,3,4,5,8 | 7 |
Three RCTs were of moderate to good methodological quality evaluated by the modified Jadad score[20]. Because of the special strategies under assessment, no blind method was used in all the RCTs. All five NRCTs contained groups matched for age, sex, and diagnosis; five articles contained information on tumor site, tumor stage, American Society of Anesthesiologists score, or body mass index, respectively. All studies were scored more than five stars using the modified Newcastle-Ottawa scale[19].
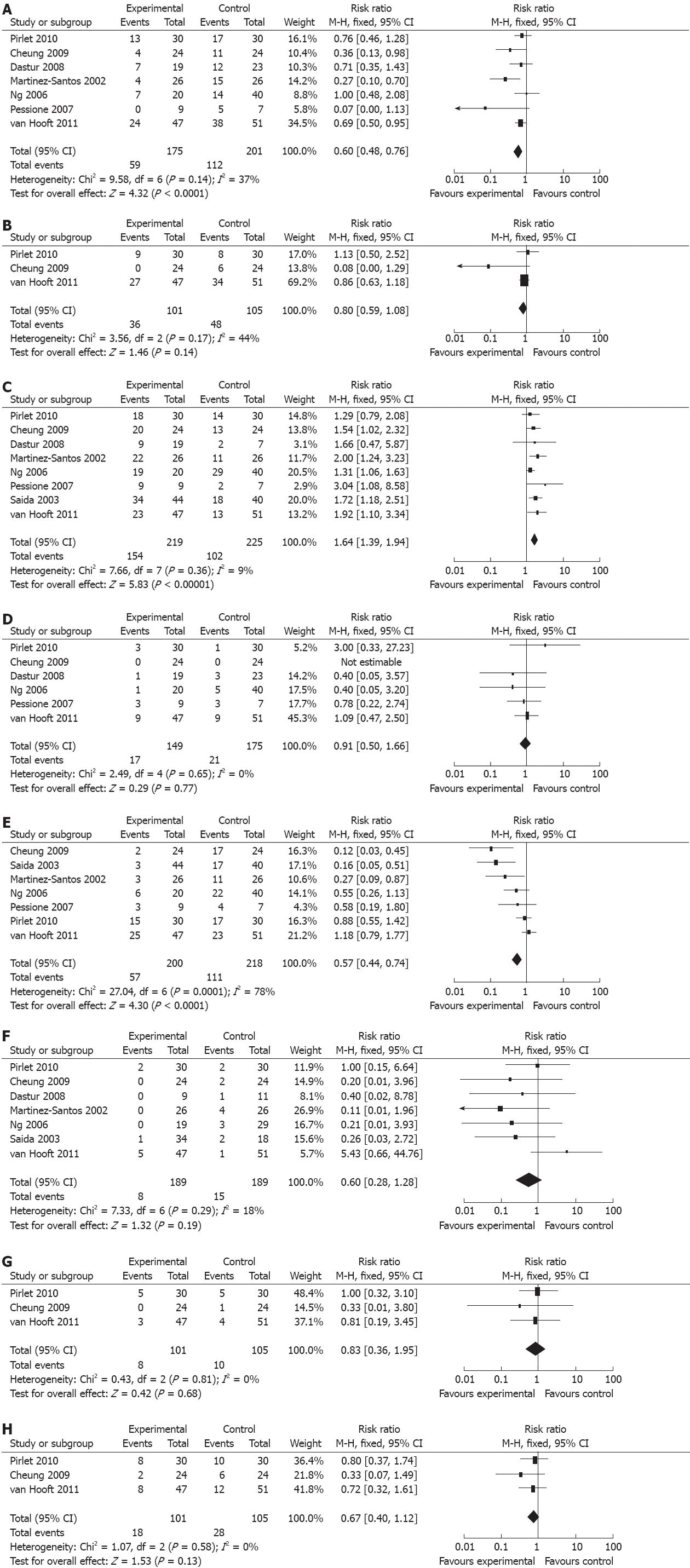
Our meta-analysis showed statistically significant difference between the SEMS group (175 patients) and the emergent surgery group (201 patients) in seven studies with regard to the one-stage stoma rate [risk ratios (RR): 0.60, 95% CI: 0.48-0.76; P < 0.0001]. There was no significant heterogeneity between the studies (P = 0.14, I2 = 37%) (Figure 2A). Only three RCTs compared the two groups (101 patients in the SEMS group and 105 in the emergent surgery group) and described the follow-up stoma rates. There was neither significant difference in follow-up stoma rates (RR: 0.80; 95% CI: 0.59-1.08; P = 0.14), nor heterogeneity (P = 0.17, I2 = 44%) between the two groups (Figure 2B).
Anastomosis rates were reported by all eight trials involving 219 patients in the SEMS group and 225 in the emergent surgery group. There was a significant difference between the two groups, with a pooled RR of 1.64 (95% CI: 1.39-1.94; P < 0.00001) (Figure 2C).
Six trials reported mortality in 149 patients in the SEMS group and 175 patients in the emergent surgery group. There was no significant difference (RR: 0.91; 95% CI: 0.50-1.66; P = 0.77) and heterogeneity (P = 0.65, I2 = 0%) between the two groups (Figure 2D).
Seven trials with 200 patients in the SEMS group and 218 patients in the emergent surgery group reported the overall morbidity. There was no significant difference between the two groups with a pooled RR of 0.57(95% CI; 0.44-0.74; P < 0.0001). However, significant heterogeneity was observed (P = 0.0001, I2 = 78%) (Figure 2E).
Further complication analysis was also performed, such as anastomotic leak, abscess and extra abdominal complications. The incidence of anastomotic leakage in the two groups was reported in seven studies. SEMS did not reduce the incidence of anastomotic leakage in patients treated with emergent surgery (RR: 0.60; 95% CI: 0.28-1.28; P = 0.19). No significant heterogeneity was observed between the two groups (P = 0.29, I2 = 18%) (Figure 2F).
Three studies compared the rates of abscesses, including peristomal abscess, intraperitoneal abscess and parietal abscess and extra abdominal complication. The analyses showed no significant difference in occurrence of abscess (RR: 0.83, 95% CI: 0.36-1.95; P = 0.68) and extra abdominal complications (RR: 0.67, 95% CI: 0.40-1.12; P = 0.13). No significant heterogeneity was found in abscess (P = 0.81, I2 = 0%) (Figure 2G) and extra abdominal complications (P = 0.58, I2 = 0%) (Figure 2H).
We also compared three RCTs with other NRCTs. There were significant differences in the morbidity (RCT group, RR 1.18; 95% CI: 0.79-1.77; P = 0.08 vs NRCTs group, RR 0.34; 95% CI: 0.21-0.56; P < 0.0001), anastomotic leak rate (RCT group, RR 5.43; 95% CI: 0.66-44.76; P = 0.53, vs NRCT group, RR 0.21; 95% CI: 0.05-0.82; P = 0.02), and anastomosis rates (RCT group, RR 1.57; 95% CI: 1.10-2.08; P = 0.002, vs NRCT group, RR 1.69; 95% CI: 1.38-2.08; P < 0.00001). There were no differences in the stoma rates (RCT group, RR 0.65; 95% CI: 0.50-0.85; P = 0.002, vs NRCT group, RR 0.52; 95% CI: 0.34-0.08; P = 0.003) and mortality (RCT group, RR 1.09; 95% CI: 0.47-2.50; P = 0.53, vs NRCT group, RR 0.54; 95% CI: 0.2-1.45; P = 0.22).
Meta-analysis can be used to evaluate the existing literature both qualitatively and quantitatively by comparing and integrating the results of different studies and taking into account the variations in characteristics that could influence the overall estimate of the outcome of interest[23]. Although meta-analysis is traditionally applied and best confined to RCTs, meta-analytical techniques using NRCTs might be a good method in some clinical settings in which either the number or the sample size of RCTs was insufficient[24].
The concept of colonic stenting as a bridge to elective surgery in patients with acute left-sided malignant colonic obstruction has been established to reduce the morbidity, mortality and number of colostomies. The nonrandomized or retrospective studies showed a significant reduction of morbidity and mortality, and need for stoma placement when SEMS was inserted before surgery with palliative intent. In contrast to these studies, two RCTs failed to confirm the findings.
Our meta-analysis illustrated that SEMS placement significantly decreased the one-stage stoma rates (RR: 0.60; 95% CI: 0.48-0.76; P < 0.0001) and increased anastomosis rates (RR: 1.64; 95% CI: 1.39-1.94; P < 0.00001), but no difference was found by the end of follow-up. The results of 206 patients in three RCTs which reported follow-up stoma rates demonstrated no difference. The difference of the stoma rate in the follow-up was partly caused by the high leakage rate of primary anastomosis in one stent group[16], probably because bowel decompression and improvement of the patients’ clinical condition were insignificant at the time of elective operation. Another reason might be that more patients with complete obstruction had been selected in a Holland study[16]. In a retrospective study from a renowned tertiary referral centre, complete obstruction has been identified as a risk factor for complications[25]. Additionally, the elective nature of the operation and the surgeons’ faith in the idea of bridge to surgery might have made the surgeons less conservative than the emergent surgery group.
The perioperative mortality is frequently used to evaluate the outcome of SEMS. Our study failed to reveal the difference between the two groups. Six studies including 324 patients came to a conclusion that there was no difference (RR: 0.91; 95% CI: 0.50-1.66; P = 0.77) between SEMS group and emergent surgery group. These outcomes might imply the potential benefits for preoperative SEMS as a bridge to surgery. It is hard to evaluate this outcome as the patients picked up did not match with the clinical stages. Clinical stage is considered as one of independent factors for prognosis. When SEMS is used as a bridge to surgery, there is concern about the oncologic outcome of those patients whose disease is potentially curable, because theoretically SEMS placement could induce tumor dissemination and worsen long-term survival[26]. More subgroup analyses should be performed to obtain a more accurate assessment.
In acute colonic obstruction, doctors and patients both want to remove the tumor with primary anastomosis with a shorter hospital stay. But the major concern is how to avoid complications. In right-sided bowel obstruction, a resection with primary anastomosis has been generally accepted by surgeons, but it is controversial in left-sided bowel obstruction. It is believed that the left colon obstruction has a high risk of radical resection and anastomosis with a high incidence of complications. Even with modern enema techniques and nutritional support, the rates of postoperative complications are still as high as 40%-50% (both significantly higher than in the elective situation with a < 14.0% anastomotic leakage rate and 10.0% operative mortality)[5,6,15,17,21-23,27,28] because of the long procedure time, peritoneal contamination, thin proximal wall of obstruction bowel, inflammatory edema, and poor blood supply. Although preoperative SEMS can potentially ameliorate bowel edema, there was no improvement in the overall situation. There was a significant difference between the two groups in the overall complications in seven studies with 418 patients, (RR: 0.57; 95% CI: 0.44-0.74; P < 0.0001). We found that SEMS, as a bridge to elective surgery, could decrease the incidence of anastomotic leakage. Leakage occurred in eight patients in the stenting group as compared with 15 patients in the control group. Anastomotic leakage could increase local recurrence and postoperative mortality. SEMS as a bridge to surgery can provide abundant bowel preparation to decrease tissue edema. But, there was no difference in the operation-related complications as shown in three studies involving 206 patients with abscess (RR: 0.83; 95% CI: 0.36-1.95; P = 0.68) and extra abdominal complications (RR: 0.67; 95% CI: 0.4-1.12; P = 0.13). Abscess was found as the main complication and pneumonia as the most common adverse event.
The major adverse events occurring in the SEMS group was bowel perforation during the stent placement procedure. Procedure- and stent-related complications were found in 5%-23.1% of patients, with an average rate of stent-related perforations of 5%[29,30]. The oncological consequences of potential tumor dissemination caused by perforations are unclear[15]. The data from NRCTs are inconsistent, ranging from no difference between colonic stenting and emergent surgery to a significantly reduced 5-year survival rate for patients treated with colonic stenting before elective surgery[27]. But the possibility of dissemination should be taken into account and the silent perforations should not be disregarded.
A cost-effectiveness analysis, including cost per quality-adjusted life-year as an outcome measure, was also performed in the meta-analysis. As a result, mortality, morbidity, quality-of-life dimensions, and stoma rates between treatment groups suggest that the probability of colonic stenting which could become more effective than emergent surgery is negligible. As only two authors have evaluated the cost-effectiveness, the results may have limitations, and a large sample would be gathered and assessed.
As this meta-analysis has a few limitations, there might be bias in the results. First, only three studies were RCTs, and confounding factors such as age and gender inevitably existed. Second, there was difference in the selection criteria, such as different protocols, method of procedures and so on. Finally, publication bias might exist when the meta-analysis was based on published studies, because positive results are more likely to be published than negative results.
In summary, the current meta-analysis demonstrated that SEMS as a bridge to surgery for obstructed left-sided colon cancer decreased the incidence of primary stoma rates and anastomotic leakage. But the consequence failed to show the effect on mortality and complications related to surgery. Therefore, preoperative SEMS can be used as an alternative approach for emergent surgery, but should be used with caution, mainly because of concerns of overt and silent perforations. Future studies are needed to further investigate the oncological outcomes and establish whether specific groups of patients could benefit more from either colonic stenting or emergent surgery.
Colorectal cancer is one of the most commonly diagnosed malignancies worldwide. And many patients with colorectal cancer present with an acute left-sided colonic obstruction. The benefit of surgical management of malignant large bowel obstruction remains controversial. Stent placement before elective surgery as a bridge to surgery is an alternative for emergent surgery in patients with acute left-sided malignant colonic obstruction. Its benefits are uncertain.
The authors performed a systematic review of the literature and meta-analysis of the one-stage stoma rates, follow-up stoma rates, anastomotic leakage rates, abscess, extra abdominal complications, morbidity and mortality of SEMS compared with emergent surgery.
The current meta-analysis demonstrated the advantage of SEMS as a bridge to surgery for obstructed left-sided colon cancer in term of decreasing the incidence of primary stoma rates and anastomotic leakage. But the consequence failed to show the effect on mortality and complications related to surgery.
The analysis has shown that preoperative SEMS can be used as an alternative approach for emergent surgery, but should be used with caution, mainly because of the concerns of overt and silent perforations. Future studies are needed to further investigate oncological outcomes and establish whether specific groups of patients could benefit more from either colonic stenting or emergent surgery.
SEMS: SEMS is a metallic tube or stent, used to hold a structure in the gastrointestinal tract in order to allow the passage of food, stool, or other secretions required for digestion; Systematic review: A literature review focused on a research question that tries to identify, appraise, select and synthesize all high quality research evidence relevant to that question; Meta-analysis: A combination of the results of several studies that address a set of related research hypotheses.
Overall, this is a nice review with good metrics and appropriate analysis.
Peer reviewer: Dr. Scott R Steele, MD, Department of Surgery, Madigan Army Medical Center, Fort Lewis, WA 98431, United States
S- Editor Wu X L- Editor A E- Editor Li JY
| 1. | Ferlay J, Autier P, Boniol M, Heanue M, Colombet M, Boyle P. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1761] [Cited by in RCA: 1707] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 2. | Hyodo I, Suzuki H, Takahashi K, Saito Y, Tanaka S, Chiu HM, Kim NK, Li J, Lim R, Villalon A. Present status and perspectives of colorectal cancer in Asia: Colorectal Cancer Working Group report in 30th Asia-Pacific Cancer Conference. Jpn J Clin Oncol. 2010;40 Suppl 1:i38-i43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg. 1994;81:1270-1276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 355] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 4. | Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T. Emergency surgery for colon carcinoma. Dis Colon Rectum. 2003;46:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Leitman IM, Sullivan JD, Brams D, DeCosse JJ. Multivariate analysis of morbidity and mortality from the initial surgical management of obstructing carcinoma of the colon. Surg Gynecol Obstet. 1992;174:513-518. [PubMed] |
| 6. | Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Runkel NS, Hinz U, Lehnert T, Buhr HJ, Herfarth Ch. Improved outcome after emergency surgery for cancer of the large intestine. Br J Surg. 1998;85:1260-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Alves A, Panis Y, Mathieu P, Mantion G, Kwiatkowski F, Slim K. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch Surg. 2005;140:278-283, discussion 284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 378] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 9. | Mealy K, O'Broin E, Donohue J, Tanner A, Keane FB. Reversible colostomy--what is the outcome? Dis Colon Rectum. 1996;39:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Dohmoto M. New method: endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507-1512. |
| 11. | Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA. New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum. 1994;37:1158-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 173] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballón P, Moreno-Azcoita M. Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum. 2002;45:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 183] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Saida Y, Sumiyama Y, Nagao J, Uramatsu M. Long-term prognosis of preoperative "bridge to surgery" expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum. 2003;46:S44-S49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 14. | Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JW. Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: a case-matched study. J Gastrointest Surg. 2006;10:798-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Park IJ, Choi GS, Kang BM, Lim KH, Lee IT, Jeon SW, Jun SH. Comparison of one-stage managements of obstructing left-sided colon and rectal cancer: stent-laparoscopic approach vs. intraoperative colonic lavage. J Gastrointest Surg. 2009;13:960-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 313] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 17. | Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 220] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 18. | Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg. 2009;144:1127-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Athanasiou T, Al-Ruzzeh S, Kumar P, Crossman MC, Amrani M, Pepper JR, Del Stanbridge R, Casula R, Glenville B. Off-pump myocardial revascularization is associated with less incidence of stroke in elderly patients. Ann Thorac Surg. 2004;77:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 189] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12862] [Article Influence: 443.5] [Reference Citation Analysis (1)] |
| 21. | Pessione S, Petruzzelli L, Gentilli S, Mioli P. [Treatment of neoplastic stenosis of the left colon: presurgical expandable metal stent vs emergency surgery. Comparison of results and survival rates]. Chir Ital. 2007;59:661-669. [PubMed] |
| 22. | Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol. 2008;12:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 287] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Mathurin P, Raynard B, Dharancy S, Kirzin S, Fallik D, Pruvot FR, Roumilhac D, Canva V, Paris JC, Chaput JC. Meta-analysis: evaluation of adjuvant therapy after curative liver resection for hepatocellular carcinoma. Aliment Pharmacol Ther. 2003;17:1247-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 26. | Maruthachalam K, Lash GE, Shenton BK, Horgan AF. Tumour cell dissemination following endoscopic stent insertion. Br J Surg. 2007;94:1151-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Fielding LP, Wells BW. Survival after primary and after staged resection for large bowel obstruction caused by cancer. Br J Surg. 1974;61:16-18. [PubMed] |
| 28. | Runkel NS, Schlag P, Schwarz V, Herfarth C. Outcome after emergency surgery for cancer of the large intestine. Br J Surg. 1991;78:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 138] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 408] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 30. | Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK. Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with nonobstructing elective surgery. World J Surg. 2009;33:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |