Published online Oct 14, 2012. doi: 10.3748/wjg.v18.i38.5462
Revised: May 8, 2012
Accepted: May 13, 2012
Published online: October 14, 2012
AIM: To investigate the effect and the possible mechanism of ginsenoside Rb1 on small intestinal smooth muscle motility in mice.
METHODS: Intestinal smooth muscle strips were isolated from male ICR mice (5 wk old), and the effect of ginsenoside Rb1 on spontaneous contraction was recorded with an electrophysiolograph. The effect of ginsenoside Rb1 on ion channel currents, including the voltage-gated K+ channel current (IKV), calcium-activated potassium channel currents (IKCa), spontaneous transient outward currents and ATP-sensitive potassium channel current (IKATP), was recorded on freshly isolated single cells using the whole-cell patch clamp technique.
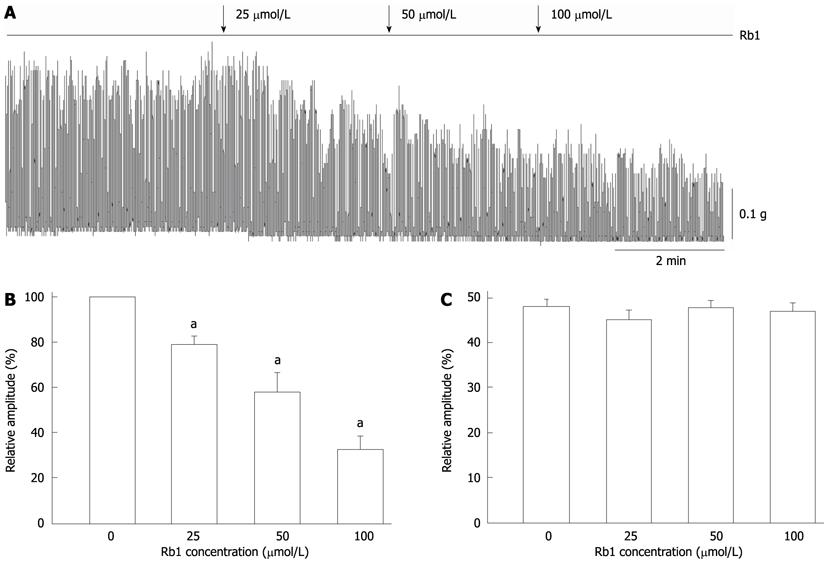
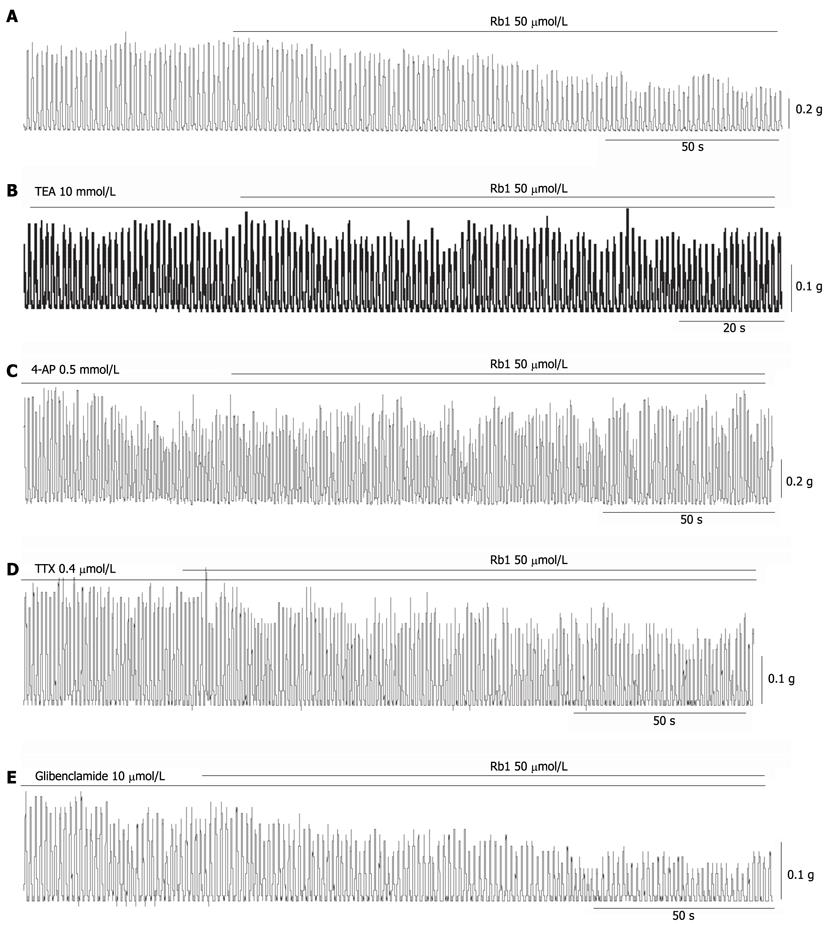
RESULTS: Ginsenoside Rb1 dose-dependently inhibited the spontaneous contraction of intestinal smooth muscle by 21.15% ± 3.31%, 42.03% ± 8.23% and 67.23% ± 5.63% at concentrations of 25 μmol/L, 50 μmol/L and 100 μmol/L, respectively (n = 5, P < 0.05). The inhibitory effect of ginsenoside Rb1 on spontaneous contraction was significantly but incompletely blocked by 10 mmol/L tetraethylammonium or 0.5 mmol/L 4-aminopyridine, respectively (n = 5, P < 0.05). However, the inhibitory effect of ginsenoside Rb1 on spontaneous contraction was not affected by 10 μmol/L glibenclamide or 0.4 μmol/L tetrodotoxin. At the cell level, ginsenoside Rb1 increased outward potassium currents, and IKV was enhanced from 1137.71 ± 171.62 pA to 1449.73 ± 162.39 pA by 50 μmol/L Rb1 at +60 mV (n = 6, P < 0.05). Ginsenoside Rb1 increased IKCa and enhanced the amplitudes of spontaneous transient outward currents from 582.77 ± 179.09 mV to 788.12 ± 278.34 mV (n = 5, P < 0.05). However, ginsenoside Rb1 (50 μmol/L) had no significant effect on IKATP (n = 3, P < 0.05).
CONCLUSION: These results suggest that ginsenoside Rb1 has an inhibitory effect on the spontaneous contraction of mouse intestinal smooth muscle mediated by the activation of IKV and IKCa, but the KATP channel was not involved in this effect.
- Citation: Xu L, Huang SP. Effect of the ginsenoside Rb1 on the spontaneous contraction of intestinal smooth muscle in mice. World J Gastroenterol 2012; 18(38): 5462-5469
- URL: https://www.wjgnet.com/1007-9327/full/v18/i38/5462.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i38.5462
Sijunzi decoction (SJZD) is one of the most famous and widely used traditional prescriptions. This prescription contains four common herbs, including Panax ginseng, Poria cocos, Atractylodes macrocephala and Glycyrrhiza uralensis, and it has been used either alone to replenish or invigorate intestinal and stomach function or as a complement to other herbs during treatment of other diseases, such as poor health and cancer[1,2]. Ginseng, the root of Panax ginseng C. A. Meyer (Araliaceae), is a principal component of SJZD. Ginsenoside, a component of ginseng, has a four-ring steroid-like structure with attached sugar moieties. Recently, ginseng’s chemical and pharmacological properties have been reported by many investigators[3,4]. Approximately 30 ginsenosides have been isolated and identified from the Panax ginseng root. These ginsenosides appear to be responsible for most of the pharmacological effects of ginseng. Many reports made it evident that ginseng saponins, or ginsenosides, have various effects on gastrointestinal motility. Ginsenosides modulate the pacemaker activities of the interstitial cells of Cajal (ICCs), making the ICCs targets for ginsenosides, and their interaction can affect intestinal motility[5]. The aqueous extract of Ginseng Radix possesses ameliorative properties and improves carbachol-induced accelerated small intestinal transit, and Rb1 contributed to the suppressive effects of Ginseng Radix on intestinal motility. Rb1 is one representative of the compounds contained in Ginseng Radix that is capable of ameliorating the accelerated transit of the small intestine[6]. However, the mechanism of Rb1 modulation of gastrointestinal motility has not been clearly demonstrated. Based on the studies cited above, it could be deduced that ICCs and gastrointestinal smooth muscle cells might be targets for Rb1. In this study, we attempted to determine the effect of ginsenoside Rb1 on the motility of intestinal smooth muscle and determine its mechanism.
Five-week-old male ICR mice (provided by the Experimental Animal Centre of the Chinese Academy of Sciences, Shanghai) weighing approximately 30 g were sacrificed by cervical dislocation. The small intestines were removed and kept in Krebs solution. After removing the mucosal and submucosal layers, single circular muscle bundles with the attached longitudinal muscle layer were prepared. Approximately 2 mm × 6 mm muscle strips were needed and were fixed in a vertical chamber (5-mL capacity containing 5 mL CO2/bicarbonate-buffered Krebs solution bubbled with 5% CO2/95% O2). The chamber was maintained at 37 °C using a water jacket. One end of the chamber was attached to an isometric force transducer (RM6240C, Chengdu Instrument Factory, China) to record the contraction. The muscle strip was incubated at the appropriate tension[7].
Intestinal smooth muscle cells were freshly isolated from mice. The intestine was rapidly cut, and the mucosal layer was separated from the muscle layers in a Ca2+-free physiological salt solution (Ca2+-free PSS). The circular muscle layer was dissected from the longitudinal layer using fine scissors and was cut into small segments (2 mm × 3 mm). These segments were incubated in a medium modified from Kraft-Bruhe (K-B) solution for 30 min at 4 °C. The segments were subsequently incubated for 10-12 min at 36 °C in Ca2+-free PSS digestion medium containing collagenase (0.5 mg/mL, Worthington), DTT [0.5 mg/mL, Sigma Aldrich (St. Louis, MO, United States)], papain [1.5 mg/mL, Sigma Aldrich (St. Louis, MO, United States)] and bovine serum albumin (4 mg/mL, Biotech Grade)[7,8]. After digestion, the supernatant was discarded, and the softened muscle segments were transferred into the modified K-B solution. The single cells were dispersed by gentle trituration using a wide-bore fire-polished glass pipette. The isolated intestinal smooth muscle cells were incubated in a modified K-B solution at 4 °C until use on the same day. Several drops of the cell suspension were dropped into a perfusion bath, which was fixed on the stage of an inverted phase-contrast microscope for 15-20 min before the experiments. Next, the cells were perfused with PSS at a rate of 1-1.5 mL/min. A single 4-channel perfusion system (BPS-4, ALA, United States) was used to exchange the solution.
A conventional whole-cell patch clamp configuration was used to record the KATP channel current (IKATP), the spontaneous transient outward currents (STOC) and the voltage-gated K+ channel current (IKV). To record IKATP, the membrane potential was clamped at -60 mV. The pipette solution consisted of the following (mmol/L): KCl 107, KOH 33, Hepes 10, MgCl2 1, Na2ATP 0.1, NaADP 0.1, and GTP 0.3, adjusted to a pH of 7.2 with NaOH. To observe the effect of Rb1 on IKv, we applied a depolarising step pulse to the cells, and the membrane potential was clamped at -60 mV. The pipettes were filled with solution containing the following (mmol/L): KCl 20, potassium-aspartic acid 110, di-tris-creatine phosphate 2.5, disodium creatine phosphate 2.5, MgATP 5, Hepes 5, MgCl2 1.0, and EGTA 10, adjusted to a pH of 7.3 with KOH. To record STOC, the holding potential was clamped at -20 mV. The pipettes were filled with a solution containing the following (mmol/L): KCl 140, MgCl2 5, K2ATP 2.7, Na2GTP 0.1, disodium salt 2.5, Hepes 5, and EGTA 0.1, adjusted to a pH of 7.2 with Tris. The patch pipettes were pulled from borosilicate glass capillaries using a pipette puller (PC-10, Narishige Group, Japan). The current was amplified with an EPC-10 patch clamp amplifier (HEKA Instruments, Germany) and digitised with a PCI-16 A/D converter (HEKA Instrument). All pipettes had a resistance of 3-5 MΩ[7,9,10].
All experimental protocols included in this manuscript were approved by the local animal care committee and conformed to the Guide for the Care and Use of Laboratory Animals published by the Science and Technology Commission of the PRC (STCC Publication No. 2, revised 1988).
Chemicals used included Ginsenoside Rb1 (purchased from Sichuan Weiqi Biological Technology CO., Ltd.), Glibenclamide [a KATP channel blocker, purchased from Tocris (Ellisville, Misso6yuri, United States)], tetraethylammonium (TEA, a non-selective potassium channel blocker), 4-aminopyridine (4-AP, a voltage-gated K+ channel blocker), and tetrodotoxin (TTX, a blocker of voltage-dependent Na+ channels) purchased from Sigma Aldrich (St. Louis, MO, United States). Ginsenoside Rb1 was dissolved first in dimethyl sulphoxide (DMSO) at a concentration of 200 mmol. For the intestinal smooth muscle isometric measurements, all chemicals were further diluted with Krebs solution to prepare the desired concentrations before use. In the electrophysiological recording experiment, Ginsenoside Rb1 was diluted with PSS to the final concentration immediately before use.
The ionic composition of the Krebs solution was as follows (in mmol/L): Na+ 137.4, K+ 5.9, Ca2+ 2.5, Mg2+ 1.2, HCO3- 15.5, H2PO4- 1.2, Cl- 134, and glucose 11.5. The solution was aerated with O2 containing 5% CO2, and the pH was maintained at 7.2-7.3. The composition of Kraft-Bruhe (K-B) solution was as follows (in mmol/L): EGTA 0.5, Hepes 10, MgCl2 3, KCl 50, glucose 10, KH2PO4 20, Taurine 20, and L-Glutamic acid 50, adjusted to a pH of 7.4 with KOH. The composition of Ca2+-free PSS was as follows (in mmol/L): NaCl 134.8, KCl 4.5, Hepes 10, MgCl2 1, and glucose 10, adjusted to a pH of 7.4 with Tris. The composition of PSS was as follows (in mmol/L): NaCl 134.8, KCl 4.5, Hepes 10, MgCl2 1, glucose 10, and CaCl2 2, adjusted to pH 7.4 with Tris. The pipette solution for recording the KATP channel current contained the following (mmol/L): KCl 107, KOH 33, Hepes 10, MgCl2 1, Na2ATP 0.1, NaADP 0.1, and GTP 0.3, adjusted to a pH of 7.2 with NaOH. The pipettes were filled with a solution for IKCa containing the following (in mmol/L): KCl 140, MgCl2 5, K2ATP 2.7, Na2GTP 0.1, disodium salt 2.5, Hepes 5, and EGTA 0.1, adjusted to a pH of 7.2 with Tris. The pipettes were filled with solution for IKV containing the following (in mmol/L): KCl 20, potassium-aspartic acid 110, di-tris-creatine phosphate 2.5, disodium-creatine phosphate 2.5, MgATP 5, Hepes 5, MgCl2 1.0, and EGTA 10, adjusted to a pH of 7.3 with KOH.
Experimental values were expressed as the mean ± SD. Statistical significance was tested using Student’s t-test, and probabilities of less than 5% (P < 0.05) were considered to be significant.
In this study, Rb1 exhibited an inhibitory effect on the spontaneous contraction of intestinal smooth muscle strips in a dose-dependent manner (Figure 1). Rb1 suppressed spontaneous contraction by 21.15% ± 3.31%, 42.03% ± 8.23% and 67.23% ± 5.63% (Figure 1B, n = 5, P < 0.05) at concentrations of 25 μmol/L, 50 μmol/L and 100 μmol/L, respectively. Rb1-induced inhibition of spontaneous contraction appeared to decrease the amplitude of spontaneous contractions (Figure 1B), but the frequency was not changed (Figure 1C).
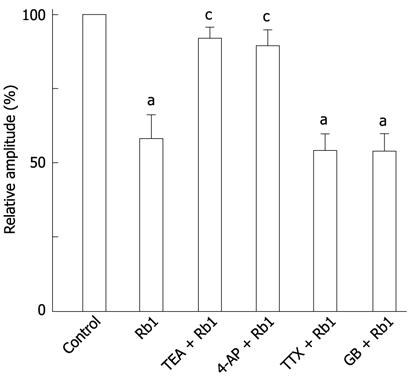
The Rb1-induced inhibitory effect on spontaneous contractions was almost completely abolished by 10 mmol/L TEA (a non-selective potassium channel blocker) and 0.5 mmol/L 4-AP (Figure 2B, C). The inhibitory percentage of Rb1 decreased from 42.03% ± 8.23% to 9.17% ± 3.54%, and the inhibitory percentage decreased from 10.90% ± 5.19% with TEA and 4-AP, respectively (Figure 3, n = 5, P < 0.05). After pre-treatment with 0.4 μmol/L TTX and 10 μmol/L glibenclamide, the inhibitory effect of Rb1 on spontaneous contraction was stable (Figure 2D, E). The inhibition percentages of Rb1 were 42.03% ± 8.23%, 46.12% ± 5.66% and 47.16% ± 3.99% in the control, TTX and glibenclamide groups, respectively (Figure 3, n = 5, P > 0.05).
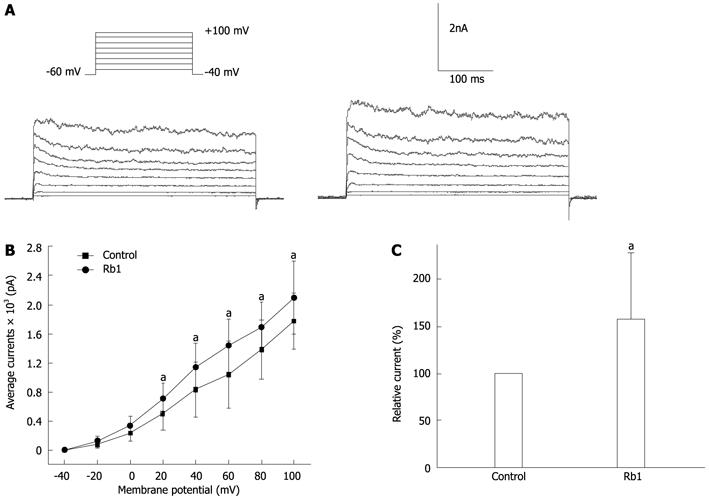
Previous experiments demonstrated that both TEA, a non-specific potassium channel blocker, and 4-AP, a specific delayed potassium channel blocker, significantly suppressed the inhibitory effect of RB1 on the spontaneous contraction of intestinal smooth muscle strips. These results indicate that Rb1-induced inhibition might be mediated by calcium-activated potassium channels and delayed repolarisation of the potassium channel. The effect of Rb1 on the IKV in intestinal smooth cells was observed in succession using the conventional whole-cell patch clamp technique. IKv was elicited by a step voltage command pulse from -40 mV to +100 mV at 20-mV increments for 400 ms at 10 s intervals. The membrane potential was clamped at -60 mV. Rb1 significantly increased IKv elicited by the step voltage command pulse (Figure 4A). Furthermore, based on the I-V relation curve, Rb1 increased IKV at all command potentials from +20 mV to +100 mV (Figure 4B). The IKv at +60 mV increased from 1137.71 ± 171.62 pA to 1449.73 ± 162.39 pA, which represented 132.11% ± 7.77% of the level in the control concentration (100%) of 50 μmol/L Rb1 (Figure 4C, n = 6, P < 0.05).
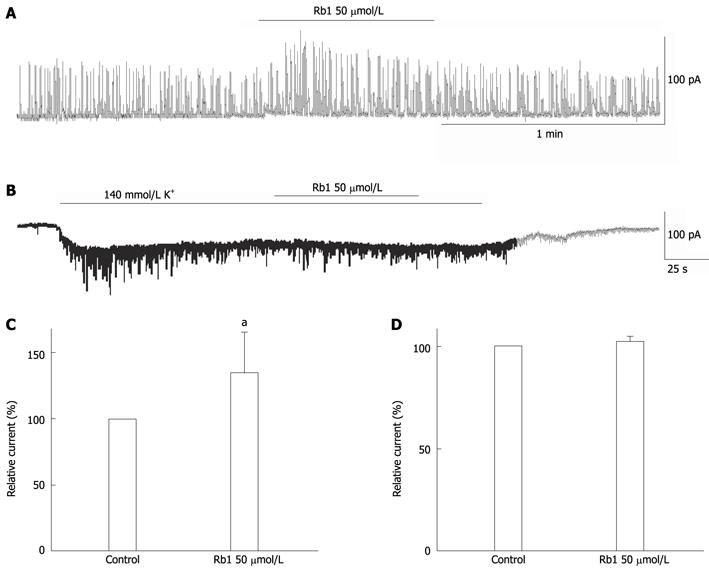
IKCa is activated by intracellular Ca2+ and can be monitored by spontaneous transient outward currents (STOCs). STOCs are believed to represent the spontaneous, sporadic release of Ca2+ from storage sites in the cell in relation to Ca2+-sensitive K+ channels[8,11]. In this study, we observed that Rb1 (50 μmol/L) enhanced the amplitude of STOCs from 582.77 ± 179.09 mV to 788.12 ± 278.34 mV, which represented a 137.76% ± 11.95% increase from the control level (100%) (Figure 5A, C, n = 5, P < 0.05) without changing the frequency.
We investigated the effect of Rb1 on KATP channels using a whole-cell patch clamp. The inward current was activated at a holding potential of -60 mV following perfusion with a symmetrical 140 mmol/L K+ solution (140 mmol/L KCl, 10 mmol/L glucose, 10 mmol/L Hepes, 1 mmol/L molgCl2, and 0.1 mmol/L CaCl2). Rb1 (50 μmol/L) did not change the KATP current (IKATP), which increased from 79.04 ± 35.88 pA to 81.32 ± 37.84 pA, representing a 102.29% ± 1.15% increase from the control level (100%) (Figure 5B, D, n = 3, P < 0.05).
Sijunzi decoction (SJZD) is widely used as a regular decoction in Chinese Traditional Medicine that can invigorate Pi viscera and replenish Qi. Conventionally, SJZD is useful for treating hypofunction of the spleen, a symptom that is partially equivalent to that of gastrointestinal motility disorders (e.g., abdominal distension and dyspepsia). The mechanism by which SJZD improves gastrointestinal disorder symptoms may relate to gastrointestinal hormones and motility. SJZD could correct deficiencies of the spleen and stomach, which are caused by digestive dysfunction to some extent[5]. Symptoms of rat models with Pi-deficiency could be significantly corrected to the normal level by SJZD treatment[4]. External nutrition plus SJZD treatment can improve and optimise cellular immune function and nutritional status in post-operative gastric cancer patients[12]. Recently, the major active components of SJZD, including ginsenoside, flavonoid, and triterpenoid, have been identified using LC/MS/MS[13]. Kim et al[14] reported that ginsenosides modulate the pacemaker activities of the ICCs. The ICCs can be targets for ginsenosides, and their interaction can affect intestinal motility. The ICCs and smooth muscle cells (SMCs) are coupled electrically, forming a multicellular syncytium. Activation of depolarising or hyperpolarizing ionic conductances in either cell type affects the total input resistance and excitability of the syncytium. For example, activation of K+ channels in ICCs reduces excitability of coupled SMCs and reduces the likelihood of reaching the action potential threshold. Responses to other stimuli, such as hormones and paracrine substances, are likely to target both ICCs and SMCs, depending upon the expression of appropriate receptors and second-messenger pathways[15]. Hashimoto et al[16] reported that Rb1 was one representative of the compounds contained in Ginseng Radix that were capable of ameliorating the accelerated transit of the small intestine. To date, the mechanism of ginsenoside action on gastrointestinal (GI) smooth muscle has not been fully studied.
In this study, we found that ginsenoside Rb1 exerted an inhibitory effect on the spontaneous contraction of intestinal smooth muscles in mice by decreasing the amplitude of spontaneous contractions in a dose-dependent manner (Figure 1). The presence of TEA (10 mmol), a non-selective potassium channel blocker, partially blocked the inhibitory effect of Rb1 on spontaneous contraction (Figure 2B). This finding suggested that the inhibitory effect of ginsenoside Rb1 on the spontaneous contraction of intestinal smooth muscle in mice might be associated with K+ channels; importantly, at least 20 species of potassium channel types are expressed by SMCs of the GI tract[15,17]. These species include voltage-gated K+ channels, ATP-dependent K+ channels, and Ca2+-activated K+ channels. We evaluated 4-AP, a voltage-gated K+ channel blocker, which partially blocked the inhibitory effect of Rb1 on spontaneous contraction (Figure 2C). In contrast, glibenclamide, an ATP-dependent K+ channel blocker, did not influence the inhibitory effect of Rb1 on spontaneous contraction (Figure 2E). In addition, the presence of TTX, a blocker of voltage-dependent Na+ channels that can block enteric nerves, did not affect the inhibitory effect of Rb1 on spontaneous contraction (Figure 2D). Thus, the results indicated that the inhibitory effect of Rb1 on spontaneous contraction was associated with activation of K+ channels in intestinal smooth muscle cells. A conventional whole-cell patch clamp configuration showed that Rb1 activated IKV and IKCa (Figures 4, 5) without any influence on IKATP (Figure 5B, D). We concluded that Rb1 inhibited the spontaneous contraction of intestinal smooth muscles via increased Ca2+-dependent K+ channel currents and voltage-dependent K+ channel currents. However, enteric nerves and KATP channels were not involved in this process. Next, to determine the Rb1-induced inhibitory effect on the spontaneous contraction of intestinal smooth muscle, the effect of Rb1 on the slow wave contraction of intestinal smooth muscle was observed. However, the amplitude and frequency of slow wave contraction was not affected by Rb1 (50 μmol/L, 100 μmol/L or 200 μmol/L, data not shown). The results indicated that the inhibitory effect of Rb1 on spontaneous contraction relies on the direct action of the compound with smooth muscle and not the ICCs themselves.
The broad ranges of resting potentials and electrical patterns of GI muscles are partly a function of the variable expression of K+ channels in SMCs. At least 20 species of K+ channels are expressed by SMCs in the GI tract[15]. The activation of potassium channels is the main determinant of cell membrane potential. Therefore, potassium channels participate in the regulation of smooth muscle tone. Activation of K+ channels in the cell membrane allows K+ efflux, causing a decrease in membrane potential and hyperpolarization. As a consequence, voltage-gated calcium channels in the cell membrane close, and the smooth muscle relaxes[18].
It has been reported that ginsenosides, including Rb1, regulate Ca2+ channels in chromaffin cells[19], sensory neurons[20] and ventricular myocytes[21]. Rb1 can alleviate cardiac hypertrophy in vitro, mediated by an inhibitive effect on elevated [Ca2+]i[3]. The ginsenoside Rb1 suppressed ventricular myocyte shortening and intracellular Ca2+ in isolated cardiac myocytes[22]. These results indicate that the primary physiological or pharmacological targets of ginsenosides are Ca2+ channels. Li et al[23]reported that ginsenosides increased IKCa activity in endothelial cells. The modulation of IKCa activity stimulated by ginsenosides was inhibited by 0.5 mmol TEA but not by 0.5 mmol glibenclamide. In our study, we first discovered that potassium channels, especially the Ca2+-dependent K+ channels and voltage-dependent K+ channels, were involved with the effects of Rb1 on the spontaneous contraction of intestinal smooth muscles in mice. This result is partially in accordance with the report of the action of ginsenosides by Li et al[23] and Kang et al[24].
In conclusion, ginsenoside Rb1 exerted an inhibitory effect on the spontaneous contraction of intestinal smooth muscles in mice by decreasing the amplitude of spontaneous contractions in a dose-dependent manner. The inhibitory effect of Rb1 is mediated by potentiating IKv and IKCa channel currents.
These experiments were performed in the Department of Physiology of the Shanghai Jiaotong University School of Medicine. We thank Professor Wen-Xie Xu for expert technical assistance concerning the experiments.
Gastrointestinal motility is a prominent research field of traditional Chinese medicine. Chinese herbal compounds, single Chinese erode drugs, Chinese herb extracts, and natural products have been experimentally investigated for their roles in promoting gastrointestinal motility.
Ginsenosides, which are isolated from the root of Panax ginseng, appear to be responsible for most of the pharmacological effects of ginseng. Ginsenosides modulate the pacemaker activities of the interstitial cells of Cajal (ICCs), and the ICCs can be targets for ginsenosides, thereby affecting intestinal motility. Rb1 was one representative of the compounds contained in Ginseng Radix that was capable of ameliorating accelerated transit in the small intestine. Until now, the mechanism by which ginsenosides affect gastrointestinal smooth muscle had not been fully studied. This study focused on the mechanism by which ginsenosides affect gastrointestinal smooth muscle.
The results suggested that the ginsenoside Rb1 exerted an inhibitory effect on the spontaneous contraction of intestinal smooth muscle in mice in a dose-dependent manner. The inhibitory effect of Rb1 is mediated by current potentiation in the voltage-gated K+ channel current (IKV), calcium-activated potassium channel currents (IKCa) channels. This effect may be involved in the mechanism by which ginseng mediates gastrointestinal motility.
This study illustrates the mechanism by which Rb1 affects spontaneous contraction of intestinal smooth muscle in mice. These findings may clarify the pharmacological action of ginseng.
This is a good descriptive study in which authors analyze the effect and the possible mechanism of ginsenoside Rb1 on small intestinal smooth muscle motility in mice. The results are interesting and suggest that ginsenoside Rb1 has an inhibitory effect on the spontaneous contraction of mouse intestinal smooth muscle mediated by the activation of IKV and IKCa, but the ATP-sensitive potassium channel current channel was not involved in this effect.
Peer reviewers: Dr. Adrian Gerard Cummins, Department of Gastroenterology and Hepatology, The Queen Elizabeth Hospital, 28 Woodville Road, Woodville South 5011, Australia; Zhao-Xiang Bian, Professor, School of Chinese Medicine, Hong Kong Baptist University, 1/F, Jockey Club School of Chinese Medicine Building, 7 Baptist University Road, Hong Kong, China
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Wu B, Xuan ZR. Progress in research on applying Sijunzi Decoction in treating digestive malignant tumor. Chin J Integr Med. 2007;13:156-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] |
| 3. | Jiang QS, Huang XN, Yang GZ, Jiang XY, Zhou QX. Inhibitory effect of ginsenoside Rb1 on calcineurin signal pathway in cardiomyocyte hypertrophy induced by prostaglandin F2alpha. Acta Pharmacol Sin. 2007;28:1149-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Shoji J, Shibata S, Ohtsuka Y, Saito H. The saponins of ginseng in recent advances in ginseng studies. Tokyo: Hirokawa Publishing Company 1990; 11-32. |
| 5. | Yi CQ, Sun JN, Zhang JJ. [Study on sijunzi decoction in rectifying digestive disorder in mice]. Zhongguo Zhong Xi Yi Jie He Zazhi. 1997;17:42-44. [PubMed] |
| 6. | Wang Z, Peng Y, Li XB. [Effect of sijunzi decoction on the intestinal flora disturbance in two rat models of Pi-deficiency syndrome]. Zhongguo Zhong Xi Yi Jie He Zazhi. 2009;29:825-829. [PubMed] |
| 7. | Zhao P, Huang X, Wang ZY, Qiu ZX, Han YF, Lu HL, Kim YC, Xu WX. Dual effect of exogenous hydrogen sulfide on the spontaneous contraction of gastric smooth muscle in guinea-pig. Eur J Pharmacol. 2009;616:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Komori S, Bolton TB. Calcium release induced by inositol 1,4,5-trisphosphate in single rabbit intestinal smooth muscle cells. J Physiol. 1991;433:495-517. [PubMed] |
| 9. | Huang X, Zhao D, Wang ZY, Zhang ML, Yan ZQ, Han YF, Xu WX, Jiang ZL. The properties of spontaneous transient inward currents of interstitial cells in rabbit portal vein. Eur J Pharmacol. 2010;643:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Yang M, Li XL, Xu HY, Sun JB, Mei B, Zheng HF, Piao LH, Xing DG, Li ZL, Xu WX. Role of arachidonic acid in hyposmotic membrane stretch-induced increase in calcium-activated potassium currents in gastric myocytes. Acta Pharmacol Sin. 2005;26:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986;371:45-67. [PubMed] |
| 12. | Cai J, Wang H, Zhou S, Wu B, Song HR, Xuan ZR. [Effect of Sijunzi Decoction and enteral nutrition on T-cell subsets and nutritional status in patients with gastric cancer after operation: a randomized controlled trial]. Zhong Xi Yi Jie He Xue Bao. 2008;6:37-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Liu Y, Yang J, Cai Z. Chemical investigation on Sijunzi decoction and its two major herbs Panax ginseng and Glycyrrhiza uralensis by LC/MS/MS. J Pharm Biomed Anal. 2006;41:1642-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Kim HS, Parajuli SP, Yeum CH, Park JS, Jeong HS, So I, Kim KW, Jun JY, Choi S. Effects of ginseng total saponins on pacemaker currents of interstitial cells of Cajal from the small intestine of mice. Biol Pharm Bull. 2007;30:2037-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20 Suppl 1:39-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Hashimoto K, Satoh K, Murata P, Makino B, Sakakibara I, Kase Y, Ishige A, Higuchi M, Sasaki H. Components of Panax ginseng that improve accelerated small intestinal transit. J Ethnopharmacol. 2003;84:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Sanders KM, Koh SD, Ward SM. Organization and electrophysiology of interstitial cells of cajal and smooth muscle cells in the gastrointestinal tract. Physiology of the gastrointestinal tract. 4th ed. San Diego, CA: Elsevier Press 2006; 533–576. |
| 18. | Baranowska M, Kozłowska H, Korbut A, Malinowska B. [Potassium channels in blood vessels: their role in health and disease]. Postepy Hig Med Dosw (. Online). 2007;61:596-605. [PubMed] |
| 19. | Nah SY, Park HJ, McCleskey EW. A trace component of ginseng that inhibits Ca2+ channels through a pertussis toxin-sensitive G protein. Proc Natl Acad Sci USA. 1995;92:8739-8743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Nah SY, McCleskey EW. Ginseng root extract inhibits calcium channels in rat sensory neurons through a similar path, but different receptor, as mu-type opioids. J Ethnopharmacol. 1994;42:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Zhang WJ, Zhong GG, Jiang Y, Wang XM, Wang ZF. [Single channel analysis on calcium channel blockade action of panaxadiol and panaxatriol saponins on cultured rat ventricular myocytes]. Zhongguo Yao Li Xue Bao. 1994;15:173-176. [PubMed] |
| 22. | Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Li Z, Nakaya Y, Niwa Y, Chen X. K(Ca) channel-opening activity of Ginkgo Biloba extracts and ginsenosides in cultured endothelial cells. Clin Exp Pharmacol Physiol. 2001;28:441-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Kang SY, Schini-Kerth VB, Kim ND. Ginsenosides of the protopanaxatriol group cause endothelium-dependent relaxation in the rat aorta. Life Sci. 1995;56:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 87] [Article Influence: 2.9] [Reference Citation Analysis (0)] |