Published online Oct 14, 2012. doi: 10.3748/wjg.v18.i38.5454
Revised: March 6, 2012
Accepted: March 20, 2012
Published online: October 14, 2012
AIM: To perform a comprehensive investigation into the potential correlation between circulating myeloid-derived suppressor cells (MDSCs) and Th17 cells in esophageal cancer (ECA).
METHODS: A total of 31 patients newly diagnosed with ECA and 26 healthy subjects were included in the current study. The frequencies of MDSCs and Th17 cells in peripheral blood were determined by flow cytometry. The mRNA expression of cytokines, arginase 1 (Arg1) and inducible NO synthase (iNOS) in peripheral blood mononuclear cells (PBMCs) and plasma Arg1 were assessed by real-time polymerase chain reaction and enzyme-linked immunosorbent assay, respectively.
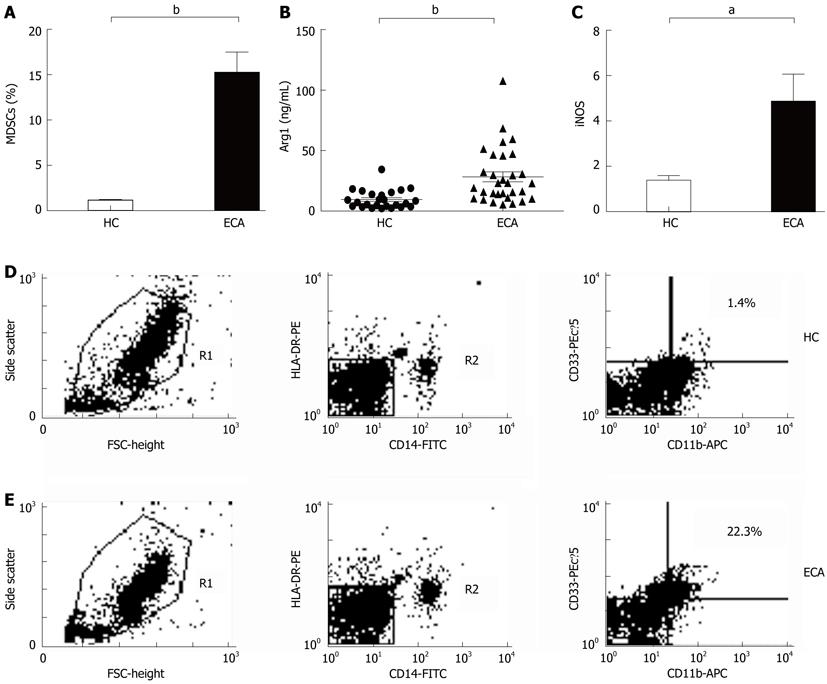
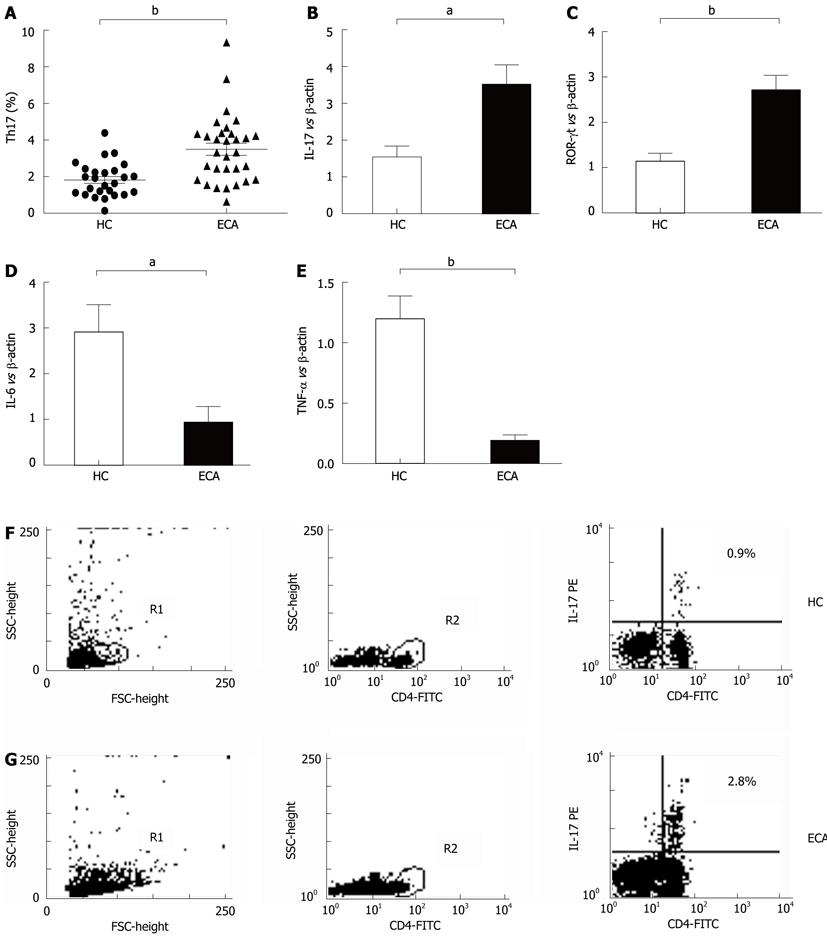
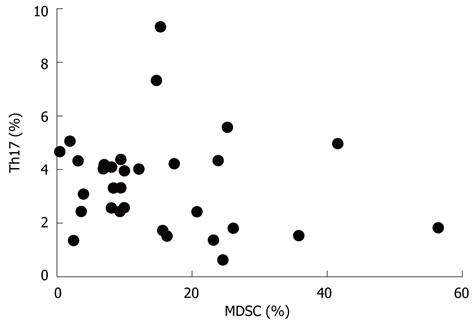
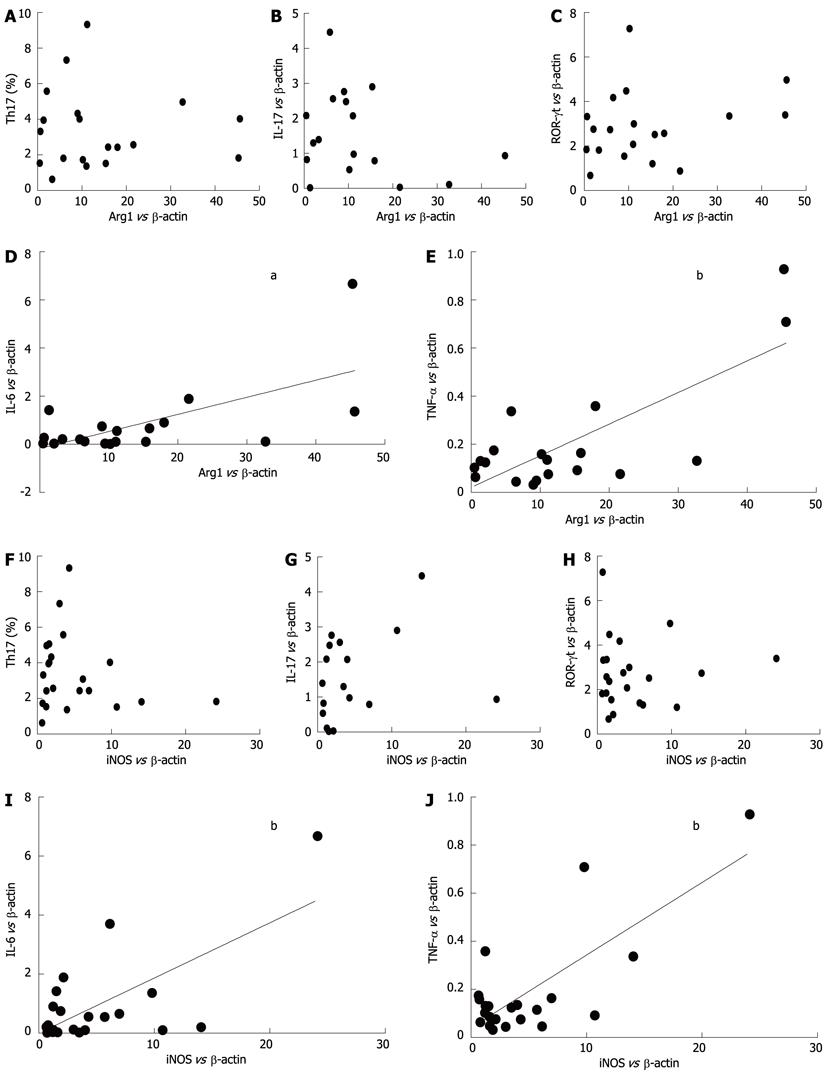
RESULTS: There was an increased prevalence of MDSCs in the peripheral blood from ECA patients (15.21% ± 2.25%) when compared with healthy control (HC) (1.10% ± 0.12%, P < 0.0001). The plasma levels of Arg1 in ECA patients were significantly higher than those in HC (28.28 ± 4.10 ng/mL vs 9.57 ± 1.51 ng/mL, P = 0.0003). iNOS mRNA levels in the peripheral blood of ECA patients also showed a threefold increase compared with HC (P = 0.0162). The frequencies of Th17 cells (CD4+IL-17A+) were significantly elevated in ECA patients versus HC (3.50% ± 0.33% vs 1.82% ± 0.19%, P = 0.0001). Increased mRNA expression of IL-17 and ROR-γt was also observed in ECA patients compared with HC (P = 0.0041 and P = 0.0004, respectively), while the mRNA expression of IL-6 and tumor necrosis factor-α (TNF-α) showed significant decreases (P = 0.0049 and P < 0.0001, respectively). No obvious correlations were found between the frequencies of MDSCs and Th17 cells in the peripheral blood from ECA patients(r = -0.1725, P = 0.3534). Arg1 mRNA levels were positively correlated with levels of IL-6 (r = 0.6404, P = 0.0031) and TNF-α (r = 0.7646, P = 0.0001). Similarly, iNOS mRNA levels were also positively correlated with levels of IL-6 (r = 0.6782, P = 0.0007) and TNF-α (r = 0.7633, P < 0.0001).
CONCLUSION: This study reveals the relationship between circulating MDSCs and Th17 cells, which may lead to new immunotherapy approaches for ECA based on the associated metabolites and cytokines.
- Citation: Jiao ZJ, Gao JJ, Hua SH, Chen DY, Wang WH, Wang H, Wang XH, Xu HX. Correlation between circulating myeloid-derived suppressor cells and Th17 cells in esophageal cancer. World J Gastroenterol 2012; 18(38): 5454-5461
- URL: https://www.wjgnet.com/1007-9327/full/v18/i38/5454.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i38.5454
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of cells comprised of myeloid progenitor cells and immature myeloid cells, which exert suppressive functions, regulating T cell responses through the production of arginase 1 (Arg1), nitric oxide and reactive oxygen species. These cells are suspected to play a crucial role in local and systemic tumor development, providing a beneficial microenvironment in which tumor cells can proliferate, expand, acquire new mutations and escape host immunosurveillance[1]. Elevated numbers of MDSCs in peripheral blood have been demonstrated in a substantial number of studies in different types of cancer, including malignant gliomas[2], head and neck cancer[3,4], invasive breast carcinomas[4], colon carcinoma[5], pancreatic cancer[6] and mesothelioma[7].
In esophageal cancer (ECA), it has recently been shown that there is a significant increase in the levels of circulating MDSCs, which is correlated with elevated numbers of regulatory T cells (Tregs) and associated with an increased expression of Arg1[6]. A recent report has also identified that MDSCs not only modulate the de novo induction of Tregs and Th17 cells from CD4+ T cells but also catalyze the transdifferentiation of Foxp3+ regulatory T cells from monocyte-induced Th17 cells[8]. These findings suggested that the interaction between MDSCs and T cell subsets may play an important role in the balance of anti- and pro-tumor immune responses. As a new member of the CD4+T-cell family, Th17 cells have been characterized as preferential producers of interleukin (IL)-17A, IL-17F, IL-21, IL-22, and tumor necrosis factor-α (TNF-α) and have been found in increased numbers in tumor-bearing hosts, including ECA[9-11]. However, it is still controversial whether these Th17 cells promote or inhibit tumor progression[12-14]. Furthermore, whether there is a correlation between circulating MDSCs and Th17 cells in ECA remains unclear. The present study was designed to evaluate the relationship between circulating MDSCs and Th17 cells by examining the cell frequencies and related cytokines and other associated products in the peripheral blood from patients with ECA.
Thirty-one patients (25 male, 6 female) newly diagnosed with ECA were included in the current study. These patients ranged from 50 to 78 years of age (average age, 61.97 ± 1.24 years). Twenty-six healthy subjects, matched for age and sex with the ECA patients, were studied as the controls. No subject was treated preoperatively or had a history of autoimmune disease, and no healthy control had a prior history of cancer. This study was approved by the research ethics committee of the Affiliated Hospital of Jiangsu University, and written informed consent was obtained from all individuals.
The MDSC population was defined as HLA-DR-/CD14-/CD33+/CD11b+. Heparinized venous blood was freshly obtained from either the ECA patients or the healthy donors. One hundred microliters of blood was mixed with 5μl of each antibody (BD Bioscience, San Jose, CA or eBioscience, San Diego, CA, United States), then incubated in a dark room for 15 min. Each sample was then mixed with 1 mL of 1 × lysing buffer (BD Biosciences). After incubation, the samples were washed with phosphate buffered saline (PBS), and the pellets were resuspended in 250 μL of PBS. Labeled cells were washed and analyzed with a FACSCalibur flow cytometer (Becton-Dickinson) using CellQuest software (Becton-Dickinson). In each case, staining was compared with that of the appropriately labeled isotype control antibody.
Following centrifugation, the plasma was removed and stored at -70 °C, while peripheral blood mononuclear cells (PBMC) were isolated by Ficoll density gradient centrifugation. PBMCs were used in two parts. Some PBMCs were frozen at -70 °C after mixing with TRIzol (Invitrogen, Carlsbad, CA, United States) for extracting total RNA, while the remaining cells was used for analysis of Th17 cells. Briefly, PBMCs were stimulated for 5 h using 50 ng/mL of phorbol myristate acetate (PMA, Sigma-Aldrich, MO, United States) and 1 g/mL ionomycin (Sigma-Aldrich, MO, United States) in the presence of 5 g/mL brefeldin A (Sigma-Aldrich, MO, United States) at 37 °C and 5% CO2. The cells were then washed in PBS and surface-labeled with CD4-FITC (eBioscience, San Diego, CA, United States). Following surface staining, the cells were fixed and permeabilized using IntraPrep Permeabilization Reagent (Beckman Coulter Inc., Fullerton, CA, United States) and then stained with IL-17A-PE (eBioscience, San Diego, CA, United States)[11]. Labeled cells were washed and analyzed with a FACSCalibur flow cytometer (Becton-Dickinson) using the CellQuest software (Becton-Dickinson).
Total RNA was extracted from individual PBMC preparations using the TRIzol reagent (Invitrogen, Carlsbad, CA, United States). cDNA was prepared by reverse transcription with oligo (dT) from total RNA extraction. Real-time polymerase chain reaction (PCR) for IL-17, IL-6, TNF-α, Arg1, iNOS and a reference gene (β-actin) was performed in a LightCycler instrument (Roche Molecular Diagnostics, Mannheim, Germany) using the SYBRgreen mastermix kit (TaKaRa, Ohtsu, Japan). The expression data for each target gene were then normalized relative to β-actin. All primer sequences are shown in Table 1.
| Target cDNA | Upper/lower | Sequence (5’ to 3’) |
| IL-17 | U | CAGATTACTACAACCGATCC |
| L | ATGTGGTAGTCCACGTTCC | |
| ROR-γt | U | GTGCTGGTTAGGATGTGCCG |
| L | GTGGGAGAAGTCAAAGATGGA | |
| IL-6 | U | AAAGAGGCACTGGCAGAAAA |
| L | TTTCACCAGGCAAGTCTCCT | |
| TNF-α | U | TAGCCCATGTTGTAGCAAACC |
| L | ATGAGGTACAGGCCCTCTGAT | |
| Arg1 | U | CAAGAAGAACGGAAGAATCAGC |
| L | TTGTGGTTGTCAGTGGAGTGTT | |
| iNOS | U | CTTTCCAAGACACACTTCACCA |
| L | TATCTCCTTTGTTACCGCTTCC | |
| β-actin | U | TGGCACCCAGCACAATGAA |
| L | CTAAGTCATAGTCCGCCTAGAAGCA |
Plasma levels of Arg1 were measured using an enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s protocols (eBioscience, San Diego, CA, United States). Hemolyzed samples were excluded. All samples were assayed in triplicate, and the mean absorbance was calculated from the standard curve.
Statistical comparisons between groups used the appropriate Student’s t-test. Statistically significant correlation between two continuous variables was analyzed by the Spearman test. A P-value < 0.05 was considered significant. Calculations were performed using GraphPad Prism, Version 5.0, software (San Diego, CA, United States).
In the present study, MDSCs was defined as HLA-DR-/CD14-/CD11b+/CD33+ cells. The frequencies of MDSCs were determined by multicolor flow cytometry and calculated as the percent (%) of total nucleated cells in whole blood samples. As shown in Figure 1A, D and E, there was an increased prevalence of MDSCs in the peripheral blood from ECA patients (15.21% ± 2.25%) when compared with healthy control (HC) (1.10% ± 0.12%, P < 0.0001).
To investigate whether the products of MDSCs were also elevated in ECA patients, we determined the plasma levels of Arg1 by ELISA and the mRNA levels of iNOS by real-time PCR. As shown in Figure 1B, the plasma levels of Arg1 in ECA patients were significantly higher than those in HC (28.28 ± 4.10 ng/mL vs 9.57 ± 1.51 ng/mL, P = 0.0003). iNOS mRNA levels in the peripheral blood of ECA patients also showed a threefold increase compared with HC (P = 0.0162, Figure 1C).
The frequencies of Th17 cells in PBMCs of ECA patients and HC were also determined by flow cytometry. As shown in Figure 2A, C and D, the frequencies of Th17 cells (CD4+IL-17A+) were significantly elevated in ECA patients when compared with HC (3.50% ± 0.33% vs 1.82% ± 0.19%, P = 0.0001).
mRNA expression analyses in peripheral blood mononuclear cells from esophageal cancer patients showed increased interleukin-17 and ROR-γt and decreased interleukin-6 and tumor necrosis factor-α
We also determined the mRNA levels of the Th17-related molecules IL-17, ROR-γt, IL-6 and TNF-α by real-time PCR. As shown in Figure 2B, increased mRNA expression of IL-17 and ROR-γt was observed in ECA patients compared with HC (P = 0.0041 and P = 0.0004, respectively), while the mRNA expression of IL-6 and TNF-α showed significant decreases (P = 0.0049 and P < 0.0001, respectively).
We next analyzed the possible correlation between the frequencies of MDSCs and Th17 cells in the peripheral blood from ECA patients and HC. As shown in Figure 3, no obvious difference was found between the two groups (r = -0.1725, P = 0.3534).
We examined the possible correlations between the mRNA levels of Arg1, iNOS and Th17-related molecules (IL-17, ROR-γt, IL-6 and TNF-α) in ECA patients. As shown in Figure 4D and E, Arg1 mRNA levels were positively correlated with levels of IL-6 (r = 0.6404, P = 0.0031) and TNF-α (r = 0.7646, P = 0.0001). Similarly, as shown in Figure 4I and J, iNOS mRNA levels were also positively correlated with levels of IL-6 (r = 0.6782, P = 0.0007) and TNF-α (r = 0.7633, P < 0.0001). However, no obvious correlations were observed with Arg1 or iNOS with IL-17 or ROR-γt (Figure 4A-C and 4F-G).
MDSCs are a heterogeneous cell population that was recently identified as a pivotal factor in the immunosuppressive network described in cancer, autoimmune disease, sepsis, infectious disease and trauma[1,15,16]. In cancer, MDSCs are responsible for T cell defects as well as angiogenesis and tumor cell motility. In this study, we evaluated the frequencies of MDSCs in the peripheral blood in ECA patients. Our study confirms previous findings about the significant increase of circulating MDSCs in a variety of cancers. An early study reported the accumulation of immature cells, including early stage myeloid cells and immature monocytes and DCs, in the blood of patients with head and neck, breast, and lung cancer[4]. Myeloid cells with immunosuppressive properties can also be found among monocytes and neutrophils circulating in the peripheral blood of patients with colon cancer and melanoma[5]. Patients with glioblastoma have increased MDSC counts (CD33+HLADR−) in their blood that are composed of neutrophilic (CD15+, > 60%), lineage-negative (CD15−CD14−, 31%), and monocytic (CD14+, 6%) subsets[2]. It has also been reported that there was an accumulation and persistence of long-lived immature granulocytic MDSC with T cell-suppressive function and impaired migratory properties in the peripheral blood of squamous cell carcinoma of the head and neck (HNSCC), lung cancer and cancers of bladder and ureter[3]. Overall, these studies demonstrated the increase of circulating MDSCs in cancers, although the levels of MDSCs in peripheral blood varied across studies. These varying levels may partly be the result of differences in the type of cancers. Another explanation for these discrepancies may be methodological differences in which different markers and cell preparations were used to identify MDSCs.
In human, MDSCs are most prevalently considered as cells expressing CD11b but lacking the expression of CD14 or more narrowly defined as HLA-DR-CD33+ cells[4,17]. In this study, we used CD14-HLA-DR-CD33+CD11b+ as specific markers for human MDSCs in lysed whole blood. A recent study also reported elevated MDSCs in pancreatic, esophageal and gastric cancer and demonstrated that these cells were an independent prognostic factor and associated with the significant elevation of the Th2 cytokine interleukin-13[6]. The authors of such study focused on circulating MDSCs defined as HLADR-Lin1low/- CD33+ CD11b+ and used a cell preparation of PBMCs rather than lysed whole blood, as was used in our study. However, both studies demonstrated elevations of plasma Arg1, which is the functional product of MDSCs, in ECA patients, compared with the controls. In addition to Arg1, we also found that iNOS mRNA levels in the peripheral blood of ECA patients showed a threefold increase compared with HC (P = 0.0162).
It is generally believed that Th17 cells and their associated cytokines IL-6, TNF, IL-1β, IL-23 and TGF-β may play important roles in promoting the growth and survival of cancer. Consistent with other previous reports, our study demonstrated that ECA patients exhibited a remarkable increase in the frequency of circulated Th17 cells. Furthermore, the mRNA levels of Th17-related cytokines, IL-17 and ROR-γt, have also been shown to be significantly increased[18]. Deans et al[19]reported that proinflammatory cytokines IL-6 and TNF-α were significantly overexpressed both at the mRNA and protein levels in cancer specimens compared with the mucosa from controls. However, in contrast to Deans’s study, we found that the mRNA levels of IL-6 and TNF-α were obviously decreased in ECA patients. One possible explanation for this discrepancy may result partly from the limited scale of ECA subjects and determination methods. Another explanation is that, as is the case for most cancers, changes in peripheral blood could reflect partly or rather poorly the changes in tumor microenvironment. Further investigation should be performed to confirm the above inferences.
Recent data demonstrated that in vivo transfer of G-MDSCs inhibited Th17 immune responses and ameliorated experimental autoimmune encephalomyelitis (EAE)[20]. In vitro, CD14+HLA-DR+ monocytes promote the generation of IL-17-secreting RORc+ Th17 cells when cocultured with naive CD4+ T cells[8]. These results suggest that the interaction of MDSC with Th17 cells would help to explain the pathogenesis of autoimmune diseases and cancers. In fact, the initial question that motivated our study was to determine whether there would be a positive correlation between circulating MDSCs and Th17 cells in individual ECA patient. As expected, there were high correlations between the mRNA levels of Arg1 or iNOS and IL-6 or TNF-α, which represent the products of MDSCs and Th17 cells, respectively. Although both the frequencies of MDSCs and Th17 cells in ECA were much higher than those in healthy controls, there was surprisingly no obvious correlation between the frequency of circulation MDSCs and Th17 cells. Consistently, there were no significant correlations between Arg1 or iNOS and Th17 cells, IL-17 or ROR-γt. There is no clear explanation for such unexpected results. Further studies are necessary to explore whether the peripheral increase of these cells could be found in local cancer tissue and whether a correlation between MDSCs and Th17 cells exist.
In conclusion, we have demonstrated a significant increase in circulating MDSCs and Th17 cells in ECA patients. The frequency of MDSCs is not correlated with Th17 cells. Arg1 and iNOS, the products of MDSCs, are consistently not associated with Th17 cells, IL-17 or ROR-γt but are positively correlated with Th17-related cytokines, including IL-6 and TNF-α. These results may bring new lines of investigation on the role of MDSCs and Th17 cells in ECA, possibly leading to new immunotherapy approaches based on the regulation of metabolites and cytokines.
Esophageal cancer (ECA) occurs when the tissue that lines the esophagus becomes malignant. Cancer of the esophagus is the sixth leading cause of cancer mortality worldwide, constituting approximately 2% of all malignant tumors. The precise mechanisms of initiation and progression of this disease are unclear. Recently, a suppressor cell population of myeloid lineage was identified, named myeloid-derived suppressor cells (MDSCs), which is capable of reducing anti-tumor as well as inflammatory immune responses. However, the characterization of MDSCs and their correlation with the newly identified pro-inflammatory Th17 cells in esophageal cancer remains unclear.
MDSCs have gained much attention in recent years, mainly in the tumor immunology community. Because MDSCs are still a very poorly defined cell population, it will be difficult to specifically target these cells in cancer patients with the aim of engaging tumor-specific immune responses. The current research priority is how to identify better markers and the interaction of MDSCs with other cell types in different clinical settings, including in esophageal cancer.
Although the distribution of MDSCs and Th17 cells in esophageal cancer has been previously reported, a comprehensive correlation between circulating MDSCs and Th17 cells in ECA remains unclear. The present study was designed to evaluate the relationship between circulating MDSCs and Th17 cells by examining the cell frequencies and related cytokines and other products in the peripheral blood from patients with ECA. The authors confirmed previous findings about significant increases in circulating MDSCs and Th17 cells in ECA patients. However, the frequency of MDSCs is not correlated with Th17 cells. Arginase I (Arg1) and inducible NO synthase (iNOS), the products of MDSCs, are consistently not associated with Th17 cells, IL-17 or ROR-γt but are positively correlated with Th17-related cytokines, including interleukin (IL)-6 and tumor necrosis factor-α (TNF-α). The results reveal the association between circulating MDSCs and Th17 cells, which may lead to new immunotherapy approaches for ECA based on the regulation of metabolites and cytokines.
Although it is difficult to specifically target these cells for immunotherapy, the results suggest that the regulation of metabolites and cytokines would be a potential therapeutic approach for esophageal cancer.
MDSCs: Myeloid-derived suppressor cells (MDSCs), a heterogeneous population of cells that consists of immature myeloid cells, immature granulocytes, monocytes-macrophages, dendritic cells and myeloid progenitor cells, is capable of reducing anti-tumor as well as inflammatory immune responses; Th17 cells: a new member of the CD4+T cell family that have been characterized as preferential producers of interleukin-17A (IL-17A), IL-17F, IL-21, IL-22, and TNF-α and have been found in increased numbers in tumor-bearing hosts.
This paper reported on the relationship between MDSCs and Th17 cells in ECA patients. The presented data are preliminary but novel.
Peer reviewer: Dr. Satoru Motoyama, Department of Surgery, Akita University Graduate School of Medicine, 1-1-1 Hondo, Akita 010-8543, Japan
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5480] [Cited by in RCA: 5311] [Article Influence: 331.9] [Reference Citation Analysis (0)] |
| 2. | Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, Garcia J, Vogelbaum MA, Finke J. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13:591-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 3. | Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 4. | Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678-689. [PubMed] |
| 5. | Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562-6568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 247] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 6. | Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother. 2011;60:1419-1430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 479] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 7. | Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 229] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 8. | Hoechst B, Gamrekelashvili J, Manns MP, Greten TF, Korangy F. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532-6541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Chen D, Hu Q, Mao C, Jiao Z, Wang S, Yu L, Xu Y, Dai D, Yin L, Xu H. Increased IL-17-producing CD4(+) T cells in patients with esophageal cancer. Cell Immunol. 2012;272:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Lee JJ, Chang YL, Lai WL, Ko JY, Kuo MY, Chiang CP, Azuma M, Chen CW, Chia JS. Increased prevalence of interleukin-17-producing CD4(+) tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2011;33:1301-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Wang J, Cai D, Ma B, Wu G, Wu J. Skewing the balance of regulatory T-cells and T-helper 17 cells in breast cancer patients. J Int Med Res. 2011;39:691-701. [PubMed] |
| 12. | Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7:651-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 489] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 13. | Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 620] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 14. | Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457-1464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 569] [Cited by in RCA: 666] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185:5828-5834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Haile LA, von Wasielewski R, Gamrekelashvili J, Krüger C, Bachmann O, Westendorf AM, Buer J, Liblau R, Manns MP, Korangy F. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871-881, 881.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 248] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s-726s. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 368] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 18. | Wang WW, Wang ZM, Liu YY, Qin YH, Shen Q. [Increased level of Th17 cells in peripheral blood correlates with the development of hepatocellular carcinoma]. Zhonghua Zhong Liu Zazhi. 2010;32:757-761. [PubMed] |
| 19. | Deans DA, Wigmore SJ, Gilmour H, Paterson-Brown S, Ross JA, Fearon KC. Elevated tumour interleukin-1beta is associated with systemic inflammation: A marker of reduced survival in gastro-oesophageal cancer. Br J Cancer. 2006;95:1568-1575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Ioannou M, Alissafi T, Lazaridis I, Deraos G, Matsoukas J, Gravanis A, Mastorodemos V, Plaitakis A, Sharpe A, Boumpas D. Crucial role of granulocytic myeloid-derived suppressor cells in the regulation of central nervous system autoimmune disease. J Immunol. 2012;188:1136-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 182] [Article Influence: 13.0] [Reference Citation Analysis (0)] |