Published online Oct 14, 2012. doi: 10.3748/wjg.v18.i38.5434
Revised: June 4, 2012
Accepted: June 5, 2012
Published online: October 14, 2012
AIM: To investigate the expression of myofibrillogenesis regulator-1 (MR-1) in relation to clinicopathological parameters and postoperative survival in a group of Chinese patients with gastric cancer.
METHODS: In our previous study of human whole-genome gene expression profiling, the differentially expressed genes were detected in the gastric cancer and its adjacent noncancerous mucosa. We found that MR-1 was associated with the location and differentiation of tumors. In this study, MR-1 protein expression was determined by immunohistochemistry in specimens of primary cancer and the adjacent noncancerous tissues from gastric cancer patients. A set of real-time quantitative polymerase chain reaction assays based on the Universal ProbeLibrary-a collection of 165 presynthesized, fluorescence-labeled locked nucleic acid hydrolysis probes-was designed specifically to detect the expression of MR-1 mRNA. The correlation was analyzed between the expression of MR-1 and other tumor characteristics which may influence the prognosis of gastric cancer patients. A retrospective cohort study on the prognosis was carried out and clinical data were collected from medical records.
RESULTS: MR-1 mRNA and protein could be detected in gastric cancer tissues as well as in matched noncancerous tissues. MR-1 was up-regulated at both mRNA (5.459 ± 0.639 vs 1.233 ± 0.238, P < 0.001) and protein levels (34.2% vs 13.2%, P = 0.003) in gastric cancer tissues. Correlation analysis demonstrated that high expression of MR-1 in gastric cancer was significantly correlated with clinical stage (P = 0.034). Kaplan-Meier analysis showed that the postoperative survival of the MR-1 positive group tended to be poorer than that of the MR-1 negative group, and the difference was statistically significant (P = 0.002). Among all the patients with stage I-IV carcinoma, the 5-year survival rates of MR-1 positive and negative groups were 50.40% and 12.70%, respectively, with respective median survival times of 64.27 mo (95%CI: 13.41-115.13) and 16.77 mo (95%CI: 8.80-24.74). Univariate and multivariate analyses were performed to compare the impact of MR-1 expression and other clinicopathological parameters on prognosis. In a univariate analysis on all 70 specimens, 6 factors were found to be significantly associated with the overall survival statistically: including MR-1 expression, depth of invasion, distant metastasis, lymph node metastasis, vascular invasion and the tumor node metastasis (TNM) stage based on the 7th edition of the International Union against Cancer TNM classification. To avoid the influence caused by univariate analysis, the expressions of MR-1 as well as other parameters were examined in multivariate Cox analysis. Clinicopathological variables that might affect the prognosis of gastric cancer patients were analyzed by Cox regression analysis, which showed that MR-1 expression and TNM stage were independent predictors of postoperative survival. The best mathematical multivariate Cox regression model consisted of two factors: MR-1 expression and TNM stage. Our results indicated that MR-1 protein could act as an independent marker for patient overall survival [Hazard ratio (HR): 2.215, P = 0.043].
CONCLUSION: MR-1 is an important variable that can be used to evaluate the outcome, prognosis and targeted therapy of gastric cancer patients.
- Citation: Guo J, Dong B, Ji JF, Wu AW. Myofibrillogenesis regulator-1 overexpression is associated with poor prognosis of gastric cancer patients. World J Gastroenterol 2012; 18(38): 5434-5441
- URL: https://www.wjgnet.com/1007-9327/full/v18/i38/5434.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i38.5434
Gastric cancer remains the fourth most common malignancy, and the second leading cause of cancer-related death worldwide. It is estimated that one million new cases of gastric cancer occur each year[1,2], and most of them come from Asia (China, Japan and South Korea) and parts of Central and South America (Costa Rica, El Salvador and Columbia)[1]. More new cases of gastric cancer are diagnosed in China than in any other countries each year[3]. Many patients are found at an advanced stage with lymph node invasion and metastasis at their initial diagnosis. Despite a curative surgery and postoperative adjuvant therapy, nearly 60% of those patients succumb to the disease[4-6]. Cancer progression and metastasis is a highly complex multi-stage process. It involves increased cell adhesion, alterations in gene expression, and changes in cell motility. During invasion and metastasis, cancer cells move within tissues, and the invasion involves multiple processes regulated by various molecules[7]. As gastric cancer is featured as a heterogeneous disease in both histology and genetics, it is hard to predict patient outcome using the classic histological classifications. Gastric carcinogenesis is thought to be a multifactorial and multistep process involving the activation of oncogenes and the inactivation of tumor suppressor genes at different stages of gastric cancer progression. However, promising molecules that have clinicopathological/prognostic significance in gastric cancer are substantially limited. It is crucial to further understand the molecular mechanisms of cancer progression and the development involved in gastric cancer and to identify more valuable prognostic markers in order to improve patient prognosis as well as to provide novel promising therapy targets[8-11].
In our recent study, we utilized human whole-genome gene expression BeadChip of Illumina Company (Human 6-V2) to compare the differentially expressed genes between the adenocarcinoma of the esophagogastric junction (AEG) group and the distal gastric cancer group, and analyzed the difference of the genes related to gastric cancer and its adjacent noncancerous mucosa. There are 1121 differentially expressed genes from the BeadChip. By further analyzing the cDNA microarray data, we found that 15 genes were differentially expressed in AEG and distal gastric cancer, 90 genes were related to the differentiation of tumors. Myofibrillogenesis regulator-1 (MR-1) was associated with the location and differentiation of tumors simultaneously. In this study, we focused on the expression of MR-1 mRNA and protein in gastric cancers.
MR-1, which is mapped to 2q35, was first cloned from a human skeletal muscle cDNA library using polymerase chain reaction (PCR) and rapid amplification of cDNA ends (Genbank™ accession no. AF417001). MR-1 is composed of three distinct exons, in which exon 3 is unique when compared with other two genes, and encodes a protein of 142 amino acids with a hydrophobic transmembrane structure from 75 to 92 amino acids[12-15]. The transcription level of MR-1 in human tissues is especially high in myocardium and skeletal muscles as revealed by Northern blot and serial analysis of gene expression[12]. Overexpression of MR-1 could promote cancer cell proliferation and migration in human hepatoma G2 (HepG2) cells[16]. MR-1 might promote cancer cell proliferation by binding to specific proteins, such as eukaryotic initiation factor 3 that is highly correlated with tumor cell growth and invasion regulation[17]. Also, overexpression of MR-1 can activate the nuclear factor κB signaling pathway, which is correlated with a wide variety of diseases, including cancer, inflammation, and autoimmune diseases[18].
Taking all the evidences listed above into account, we hypothesized that MR-1 may take part in the development and progression of gastric cancer. On the basis of these studies, we used real-time quantitative reverse transcriptase-polymerase chain reaction (qPCR) and immunohistochemistry to examine the expression of MR-1 in gastric cancer samples and adjacent normal tissues. Our study was the first attempt to investigate the relationship between MR-1 expression and prognosis of gastric cancer patients with complete clinical and follow-up data. We analyzed MR-1 protein expression and studied the relationship between MR-1 expression and survival. We also evaluated the possible associations between MR-1 protein expression and clinicopathological characteristics.
In this retrospective study, a consecutive series of 70 paired tissue specimens were collected from the patients with gastric cancer who received subtotal or total gastrectomy at the Peking University Cancer Hospital in Beijing between January 2004 and December 2005. Written informed consent was obtained before sample collection and this study was approved by the Ethics Committee of Peking University. There were 45 males and 25 females with a mean age of 56 years (range: 26-81 years). None of the patients had undergone either chemotherapy or radiotherapy before surgery and there was no other co-occurrence of diagnosed cancers. A number of clinicopathological variables such as gender, age, tumor location, histological type, tumor-node-metastasis (TNM) stage, depth of tumor invasion, lymph node metastasis, distant metastasis and vascular invasion were obtained from the histopathological records and included for survival analysis.
We classified the postoperative staging of gastric cancer according to 7th American Joint Committee on Cancer (AJCC) TNM staging classification for carcinoma of the stomach[19]. There were 6 patients with stage I, 15 patients with stage II, 43 patients with stage III, and 6 patients with stage IV carcinoma. After gastrectomy, resected specimens were processed routinely for histopathological assessment; necrotic hemorrhage and connective tissues were removed and each paired bulk sample [tumor samples (T)/matched normal samples (N)] was immediately put into liquid nitrogen and stored at -80 °C until processed. The resected specimens of gastric cancer were also routinely subjected to macroscopic pathological assessment and fixed with 10% formalin in phosphate buffered saline (PBS) for immunohistochemistry. All tissue specimens were formalin-fixed and paraffin-embedded. Formalin fixed tissue sections were stained with haematoxylin and eosin and classified by a pathologist. These results were compared with the histopathologial records from Peking University Cancer Hospital. Final pathology was determined by consensus and reviewed if necessary. The patients were followed up from a period of 2.23 to 89.07 mo (mean, 30.78 mo). Follow-up was managed through correspondence, over the telephone or in the clinic every 3 to 6 mo for 5 years and half a year thereafter. In the clinic, history enquiry, physical examination, complete blood count, biochemical tests, imaging studies and endoscopy were routinely completed. All gastric cancer patients in our study were followed up regularly and follow-up information was complete. The primary endpoint of the follow-up was death of gastric cancer patients. Patients who did not die as a result of gastric cancer were excluded.
Total RNA was extracted according to the manufacturer’s instructions (TRIzol, Invitrogen, United States). The integrity of the RNA samples was determined by electrophoresis through agarose gels and staining with ethidium bromide, and the 18S and 28S RNA bands were visualized under ultraviolet (UV) light. The RNA was stored at -80 °C in RNase-free water until reverse transcription or fluorescence labeling.
Reverse transcription (RT) was performed in a 25 μL reaction volume with 2 μg total RNA treated with 0.5 μg of Oligo (dt), 200 U Moloney murine leukemia virus reverse transcriptase, 25 U RNase inhibitor and 2.5 mmol dNTP to synthesize the first-strand cDNA (Promega, United States), according to the manufacturer’s recommendations. The reaction system was incubated at 70 °C for 5 min (primer annealing), 42 °C for 1 h (synthesis) and resulting cDNA was stored at -20 °C. The resulting cDNA was subjected to PCR for the evaluation of the relative expression levels of β-actin (as an internal control) and MR-1. PCR was done using 1 unit HotMaster Taq DNA polymerase (Qiagen, Germany) and 1:20 of the reverse transcription reaction, with an initial hot start of 5 min at 95 °C followed by 30 s denaturation. Primers, annealing and extension temperatures, and number of cycles used (chosen for the exponential phase of amplification) were as follows: (1) β-actin forward primer: CATGCCATCCTGCGTCTGGAC, reverse primer: CACGGAGTACTTGCGCT CAGGAGG; 55 °C, 45 s, 72 °C, 45 s, 28 cycles, 72 °C, 5-min bands of 275 bp; and (2) MR-1 forward primer: GCTTTGCAGGTGTGGTGGAG, reverse primer: AGGAACGGGTTGTAGGAGCG; 52 °C, 40 s, 72 °C,
45 s, 35 cycles, 72 °C, 5-min bands of 133 bp. Two bands were detected at 133 bp and 275 bp corresponding to the molecular weight marker. PCR products were electrophoresed on 1.5% agarose gels with 0.01% ethidium bromide. Band intensities were measured under UV light using Gel Analyst software (UVP, Upland, United States).
Real-time qPCR was performed in an ABI Prism 7500 HT (Applied Biosystems, Foster City, CA) with a Universal ProbeLibrary (UPL) probe (Roche). Primers and probe were designed by online Roche Assay Design Center (https://http://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp?id=uplct_030000). The primers for real-time qPCR were: MR-1 forward: 5’-CTTCTCAGGGGACCTGCTCT-3’, reverse: 5’-TCAGCATGGTCTCTGCAT TG-3’; β-actin forward: 5’-CCAACCGCGAGAAGATGA-3’, reverse: 5’-CCAGAGGCGTACAGGGATAG-3’. UPL probe #76 was designed for MR-1 and UPL probe #64 for β-actin. All other reaction conditions were as described by the manufacturer. The cDNA was denatured and the Taq DNA polymerase was activated for 10 min at 95 °C, and the cycling conditions were set as follows: 40 cycles of denaturation at 95 °C for 30 s, annealing at 52 °C for 30 s, extension at 72 °C for 30 s and a final step at 42 °C for 2 min. Standard curves were determined by running a dilution series on the housekeeping gene (β-actin) and target gene. The experiments were repeated three times independently.
Immunohistochemical analysis was done to study the altered protein expression in the 70 specimens of human gastric cancer tissues and non-cancerous gastric tissue controls. Four μm sections from formalin-fixed and paraffin-embedded tissues were mounted on poly-L-lysine-coated slides, baked overnight at 50 °C and then deparaffinized in xylene and rehydrated through alcohol to distilled water. After hydration, endogenous peroxidase activity was blocked by incubation with 3% (v/v) hydrogen peroxidase (H2O2) for 20 min at room temperature. Standard antigen retrieval was then performed with heat-induced epitope retrieval (HIER) by heating the slides immersed in retrieval solution (pH 6.0) in a pressure boiler. After boiling, the slides remained in the pressure boiler for 3 min and then gradually cooled at room temperature. For the detection of MR-1, after washing with PBS three times, the sections were incubated with the polyclonal goat anti-human MR-1 antibody (HPA017068-100UL, Sigma, Germany) at 4 °C overnight. Then, the slides were incubated with peroxidase-labeled polymer conjugated to poly peroxidase-anti-mouse/rabbit IgG (PV-9000, Zhongshan Biotechnology Company, Beijing, China) at 37 °C for 30 min followed by a gentle rinse with washing buffer three times. 3,3’-diaminobenzidine tetrahydrochloride (DAB) staining reaction was then performed and followed by Meyer hematoxylin counterstaining. The slides were then dehydrated, cleared and mounted as usual. For negative controls, the primary antibody was replaced by non-immune rabbit serum to confirm the specificity. Internal positive control was used for quality assurance. MR-1 staining was principally evaluated according to the scoring criteria. The information recorded was: subcellular location (nuclear and/or cytoplasmic), intensity of staining (negative, weak, moderate or strong) and percentage of positive immunoreactive cells. The positive group referred to the cases with > 10% cells having positive immunoreactivity. The rest was defined as negative. The slide evaluation was performed by two pathologists, and both pathologists gave almost identical reports with only minor differences. A consensus regarding controversial cases was reached after discussion.
All statistical analyses were performed using SPSS statistical analysis software, version 16.0 (SPSS, Chicago, IL, United States). A paired-samples t test was used to compare the MR-1 mRNA levels in the tumor tissue samples and their paired adjacent non-tumor tissue samples. Regarding MR-1 expression and the clinicopathological variables, data were cross-tabulated and a χ2 test was performed. Cumulative survival was estimated by the Kaplan-Meier method and comparisons between groups were made with a log-rank test. Postoperative survival was measured from the date of first surgery to the date of death of gastric cancer, or the last date of information collection if no end event was documented. A multivariate analysis of Cox proportional hazards regression model (backward, stepwise) was performed to assess the influence of each variable on survival. P < 0.05 was considered statistically significant.
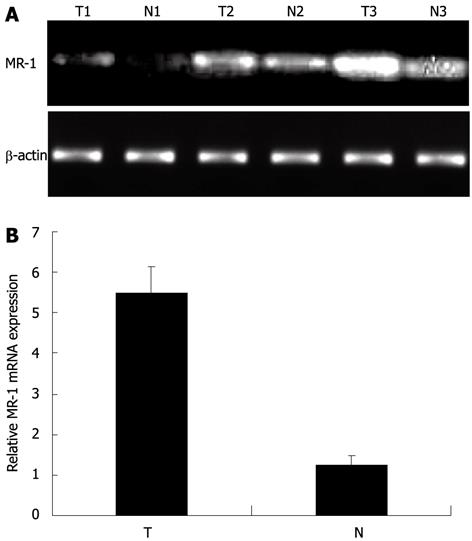
We randomly selected 60 specimens from the total samples, including 30 gastric cancer tissues and 30 matched noncancerous tissues to investigate the MR-1 mRNA expression level using semiquantitative RT-PCR. As shown in Figure 1A, MR-1 mRNA could be detected in both gastric cancer tissues and matched noncancerous tissues. However, a significant increase in the levels of MR-1 mRNA expression was observed in primary gastric cancer compared with matched normal tissues.
To validate the results of semiquantitative RT-PCR, we examined the MR-1 mRNA expression level with real-time qPCR in the 30 paired clinical samples chosen randomly from the total cases. By real-time qPCR analysis, we found that the level of MR-1 mRNA was increased remarkably in gastric cancer tissues. Expression of MR-1 mRNA was measured in triplicate, and then normalized relative to the reference gene β-actin (divided by the expression level of human β-actin). The average ratios of MR-1 mRNA to β-actin mRNA in gastric cancer tissues and noncancerous gastric tissues were 5.459 ± 0.639 and 1.233 ± 0.238, respectively (Figure 1B), which suggested that the expression level of MR-1 mRNA was significantly higher in gastric cancer tissues than in the corresponding noncancerous gastric tissues (P < 0.001).
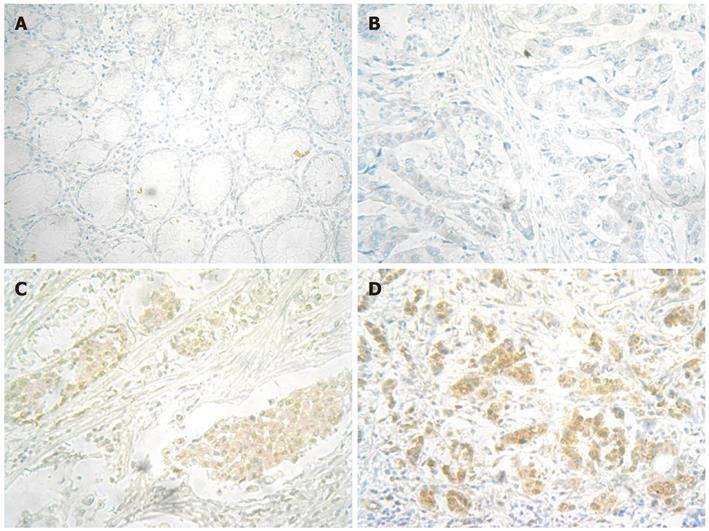
MR-1 protein expression was detected in the nuclei and cytoplasm of both adjacent noncancerous mucosa and gastric cancer cells. However, the positive rate of MR-1 expression in gastric cancer cells was much higher than that in adjacent noncancerous mucosa (34.2% vs 13.2%, P = 0.003, Figure 2).
We investigated the association of MR-1 protein expression with clinicopathological variables and postoperative survival. Correlation analysis demonstrated that high expression of MR-1 in gastric cancer was significantly correlated with TNM stage (P = 0.034). It suggested that MR-1 protein expression had no obvious association with other clinicopathological variables except TNM stage (Table 1).
| Variables | Cases | MR-1 negativen = 43 (%) | MR-1 positiven = 27 (%) | P value |
| Gender | 0.855 | |||
| Male | 45 | 28 (62.22) | 17 (37.78) | |
| Female | 25 | 15 (60.00) | 10 (40.00) | |
| Location | 0.246 | |||
| Upper | 8 | 3 (37.50) | 5 (62.50) | |
| Lower | 62 | 40 (64.52) | 22 (35.48) | |
| Age (yr) | 0.400 | |||
| < 55 | 25 | 17 (68.00) | 8 (32.00) | |
| ≥ 55 | 45 | 26 (57.78) | 19 (42.22) | |
| TNM stage | 0.034 | |||
| I+ II | 21 | 17 (80.95) | 4 (19.05) | |
| III + IV | 49 | 26 (53.06) | 23 (46.94) | |
| Depth of invasion | 0.809 | |||
| T1 + T2 | 12 | 7 (58.33) | 5 (41.67) | |
| T3 + T4 | 58 | 36 (62.07) | 22 (37.93) | |
| Lymph node metastasis | 0.347 | |||
| Negative | 12 | 9 (75.00) | 3 (25.00) | |
| Positive | 58 | 34 (58.62) | 24 (41.38) | |
| Distant metastasis | 0.196 | |||
| M0 | 64 | 41 (64.06) | 23 (35.94) | |
| M1 | 6 | 2 (33.33) | 4 (66.67) | |
| Vascular invasion | 0.887 | |||
| Negative | 41 | 25 (60.98) | 16 (39.02) | |
| Positive | 27 | 16 (59.26) | 11 (40.74) | |
| Differentiation | 0.278 | |||
| Well and moderately | 3 | 1 (33.33) | 2 (66.67) | |
| Poorly | 60 | 36 (60.00) | 24 (40.00) | |
| Others | 5 | 4 (80.00) | 1 (20.00) | |
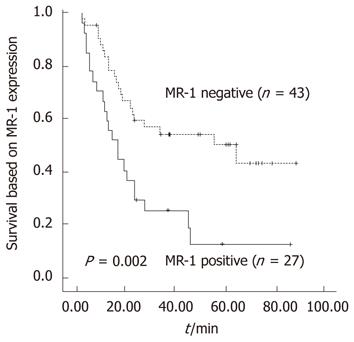
Kaplan-Meier analysis showed that the postoperative survival of the MR-1 positive group tended to be poorer than that of the MR-1 negative group, and the difference was statistically significant (P = 0.002). For all the patients with stages I-IV carcinoma, the 5-year survival rates of MR-1 positive and negative groups were 50.40% and 12.70%, respectively, with respective median survival times of 64.27 mo (95%CI: 13.41-115.13) and 16.77 mo (95%CI: 8.80-24.74, Figure 3).
We performed univariate and multivariate analyses to compare the impact of MR-1 expression and other clinicopathological parameters on prognosis. In the univariate analysis on all 70 specimens, 6 factors were found to have statistically significant associations with overall survival: MR-1 expression, depth of invasion, distant metastasis, lymph node metastasis, vascular invasion and the TNM stage based on the 7th edition of the International Union Against Cancer TNM classification (Table 2). To avoid the influence caused by univariate analysis, the expressions of MR-1 as well as other parameters were examined in multivariate Cox analysis. Clinicopathological variables that might affect the prognosis of gastric cancer patients were analyzed by Cox regression analysis, which showed that MR-1 expression and TNM stage were independent predictors of postoperative survival (Table 2). Therefore, the best mathematical multivariate Cox regression model consisted of two factors: MR-1 expression and TNM stage. In the testing set, MR-1 was again found to be a significant independent prognostic factor for poor prognosis (hazard ratio, 2.125; 95%CI: 1.023-4.410, P = 0.043; Table 2) in Chinese gastric cancer patients.
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| MR-1 expression (positive vs negative) | 2.502 (1.365-4.586) | 0.003 | 2.125 (1.023-4.410) | 0.043 |
| TNM stage (stages III-IV vsI-II) | 12.608 (3.845-41.345) | < 0.001 | 5.214 (1.155-23.526) | 0.032 |
| Depth of invasion (T3 + T4 vs T1 + T2) | 7.094 (1.709-29.441) | 0.007 | 3.340 (0.612-18.212) | 0.164 |
| Distant metastasis (M1 vs M0) | 0.395 (0.166-0.943) | 0.036 | 0.579 (0.199-1.686) | 0.316 |
| Gender (male vs female) | 1.180 (0.635-2.192) | 0.601 | 0.783 (0.376-1.631) | 0.514 |
| Age (yr) (< 55 vs≥ 55) | 0.603 (0.324-1.122) | 0.110 | 0.717 (0.340-1.514) | 0.383 |
| Location (upper vs lower) | 1.740 (0.726-4.168) | 0.214 | 1.136 (0.449-2.876) | 0.787 |
| Vascular invasion (positive vs negative) | 0.517 (0.267-1.001) | 0.046 | 0.964 (0.456-2.038) | 0.924 |
| Lymph node metastasis (positive vs negative) | 4.973 (1.529-16.176) | 0.008 | 1.158 (0.249-5.382) | 0.852 |
| Differentiation (well differentiated vs poorly differentiated) | 0.774 (0.478-1.254) | 0.298 | 0.831 (0.485-1.426) | 0.502 |
Gastric cancer is reported to be the second most common cause of cancer-related death worldwide. For most cases, at the advanced stage when diagnosed, surgery is the only curative procedure for localized gastric cancer. A high recurrence rate, a low survival rate, and a poor prognosis were found in the advanced gastric cancer patients because currently available agents are not very effective. Thus, treatment of gastric tumors remains a challenge for physicians. To identify more effective approaches for cancer treatment, new targeted therapies for advanced gastric cancers are needed. Right now, targeted therapy for advanced gastric carcinoma relies on target gene status evaluation[20,21]. Because of the cellular migration and metastasis, the mortality rate of gastric cancer remains high. The development of gastric cancer is a comprehensive action associated with multiple factors, such as inhibition of tumor suppressor genes, overexpression of related genes, and a failure to regulate cell proliferation. Therefore, it is urgently needed to find a sensitive biomarker for the detection of gastric cancer at the curative stage.
Li et al [12] identified a novel human gene, MR-1, from a human skeletal muscle cDNA library. MR-1 is located on human chromosome 2q35, having three alternatively spliced forms[22]. Based on our recent findings that MR-1 was associated with the location and differentiation of tumors simultaneously, and that MR-1 played a role in promoting the proliferation and invasion in some types of human cancers[14,18], we designed this study to comprehensively investigate the association between MR-1 and gastric cancer and the roles it may play in the pathogenesis or disease progression.
Real-time qPCR procedure combined with the Universal ProbeLibrary (UPL) technology has been successfully employed for both detection and quantification of expression levels of numerous genes, and mammalian and human viral pathogens[23-27]. UPL is a collection of 165 presynthesized, fluorescence-labeled DNA/locked nucleic acid (LNA) hybrid hydrolysis probes, which have been selected carefully to detect 8- and 9-mer motifs that are very prevalent in the transcriptomes, ensuring optimal coverage of all transcripts in a given transcriptome. On the basis of the MR-1 and β-actin sequences, UPL probe #76 and #64 with each specific PCR primer pairs were obtained from universal probe library database (Roche Diagnostics). In the present study, the prognostic relevance of MR-1 expression in gastric cancer was reported for the first time. It has been shown that MR-1 mRNA was overexpressed in gastric tumors compared with adjacent normal gastric tissues from the same individuals, in agreement with the data obtained from our microarray analysis.
In this study, immunohistochemistry was used to analyze the levels of MR-1 expression in 70 clinicopathologically characterized gastric cancer cases. The positive rate of MR-1 protein expression in gastric cancer cells was found much higher than in adjacent noncancerous mucosa. The results indicate that there are certain functions of MR-1 protein that are highly expressed in gastric cancer cells, which was confirmed by postoperative survival analysis in our study. To the best of our knowledge, there has been no report examining the role of MR-1 in gastric cancer. This is the first study showing that expression of MR-1 is increased in gastric cancer tissues compared with benign control tissues.
The clinical significance of MR-1 protein expression was studied. The result reveals that high expression of MR-1 in gastric cancer is significantly correlated with TNM stage (P = 0.034), but has no obvious association with other clinicopathological variables related to the prognosis of gastric cancer patients, such as depth of invasion and vascular invasion[28,29].
AEG has a different clinicopathological feature and poor prognosis compared with the distal gastric cancer. In this study, 5 (62.50%) of 8 cases of AEG highly expressed MR-1 protein, whereas weak expression was detected only in 22 (35.48%) of 62 cases of the distal gastric cancer. Expression was detected in two (66.67%) of three cases of well and moderately differentiated gastric cancers, whereas 24 (40.00%) of 60 cases of the poorly differentiated gastric cancer expressed MR-1 protein. These findings were in accordance with the data obtained from our microarray analysis. However, data derived from these studies were statistically not significant, which may be because of the small sample size. Therefore, the accuracy of this first explorative result should be further tested in a larger validation study.
With respect to the results of survival analysis, in patients with stages I-IV gastric cancer, the 5-year survival rate for those with high MR-1 expression was significantly lower than that of patients with low MR-1 expression. The results showed that MR-1 was up-regulated in gastric cancer tissues compared with normal gastric tissues and correlated significantly with prognosis. Multivariate analysis suggested that MR-1 expression and TNM stage were independent prognostic indicators for gastric cancer. The relative risk of death in patients with MR-1-positive tumors was 2.125 times higher than that in the patients with MR-1-negative tumors [hazard ratio (HR) = 2.125, 95%CI: 1.023-4.410]. Depth of invasion, distant metastasis, lymph node metastasis and vascular invasion, which were significant prognostic factors in the univariate analysis, showed no significant influence on survival in the multivariate analysis, possibly because their prognostic value was overlapped by the TNM stage. Thus, MR-1 is a potentially novel therapeutic target for the treatment of gastric cancer.
The mechanism by which MR-1 promotes tumorigenesis and cancer progression has not been well elucidated. The interaction of MR-1 with sarcomeric structural proteins involved in muscle contraction and its presence in human myocardial myofibrils indicate that MR-1 could regulate contractile proteins in the myocardium and might be associated with cardiac hypertrophy. Myosin light chain-2 (MLC-2) plays an important role in cell migration from solid cancers such as ovarian tumor, and its dephosphorylation could induce apoptosis[30]. A study showed that MLC-2 may regulate cell proliferation and migration by interacting with MR-1[31]. Knockdown of MR-1 expression in human hepatoma HepG2 cells inhibits cell migration and proliferation both in vitro and in vivo. The mechanism underlying this action is that MR-1 induces MLC-2 activation, subsequently stimulates stress fiber formation, and indirectly activates the focal adhesion kinase/protein kinase B (FAK/Akt) signaling pathway to promote cell migration and proliferation[15]. Further studies are needed to define the molecular mechanisms that govern the potential role of MR-1 expression in gastric cancer progression, clarify whether MR-1 is an early diagnostic marker for gastric cancer and to assess its full therapeutic potential.
In conclusion, our data show that a subset of patients with gastric cancer have MR-1 overexpression, which is associated with an aggressive clinical course and poor overall survival. Thus, MR-1 may be a novel biological marker and potential therapeutic target for the treatment of gastric cancer. It could also be used to monitor the effect of anti-cancer therapies. The results of our study suggest that overexpression of MR-1 in gastric cancer tissues might play an important role in the progression and metastases of the disease and that MR-1 may be a useful prognostic and survival indicator. These findings may help us explore novel therapeutic modalities and prognostic predictors for gastric cancer patients, thus improving the treatment outcomes. This is the first report to suggest a relationship between MR-1 and prognosis of patients with gastric cancer, and further prospective investigations would be worth doing in clinical settings.
Gastric cancer is one of the commonest malignant tumors in the alimentary tract and is characterized by delayed clinical presentation, rapid progression, and poor survival. Although this neoplasm is a serious public health problem due to its high incidence and mortality, little is known about the molecular events involved in gastric carcinogenesis.
Gastric cancer, similar to other neoplasms, is a multifactorial disease that results from a combination of environmental factors and the accumulation of generalized and specific genetic and epigenetic alterations. Myofibrillogenesis regulator-1 (MR-1) protein family has been recently found associated with carcinogenesis, but not in the stomach. In this study, the authors evaluated mRNA and protein expression of MR-1 in gastric neoplasms and corresponding non-neoplastic samples.
No previous study has evaluated the gene and protein expression of MR-1 in gastric carcinogenesis. The findings from this study suggested that MR-1 may play an important role in the development of gastric cancer and it is a potential indicator that could be used to evaluate the outcome of gastric cancer treatment.
These results suggest that overexpression of MR-1 is associated with clinical stage and serves as a prognostic factor in patients with gastric cancer.
MR-1, which is mapped to 2q35, was first cloned from a human skeletal muscle cDNA library using PCR and rapid amplification of cDNA ends (Genbank™ accession no. AF417001). It encodes a protein of 142 amino acids with a hydrophobic transmembrane structure from 75 to 92 amino acids.
The study discovered some differential expression genes through bioinformatics and elucidated the expression level of MR-1 in gastric cancer and evaluated the link between MR-1 and the poor outcome of gastric cancer. The major finding of this study was that MR-1, which up-regulated in gastric cancer tissues comparing with matched non-tumor tissues, might be a novel prognostic marker for gastric cancer.
Peer reviewers: Islam Khan, Professor, Department of Biochemistry, Faculty of Medicine, Kuwait University, Jabrya Safat-13110, Kuwait; Dr. Guang-Wen Cao, MD, Professor, Department of Microbiology, Second Military Medical University, 800 Xiang Yin Road, Shanghai 200433, China
S- Editor Gou SX L- Editor Kerr C E- Editor Lu YJ
| 1. | Thun MJ, DeLancey JO, Center MM, Jemal A, Ward EM. The global burden of cancer: priorities for prevention. Carcinogenesis. 2010;31:100-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 602] [Cited by in RCA: 626] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 2. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 3. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7953] [Cited by in RCA: 8103] [Article Influence: 506.4] [Reference Citation Analysis (2)] |
| 4. | de Maat MF, van de Velde CJ, Umetani N, de Heer P, Putter H, van Hoesel AQ, Meijer GA, van Grieken NC, Kuppen PJ, Bilchik AJ. Epigenetic silencing of cyclooxygenase-2 affects clinical outcome in gastric cancer. J Clin Oncol. 2007;25:4887-4894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Macdonald JS. Gastric cancer--new therapeutic options. N Engl J Med. 2006;355:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Hundahl SA, Wanebo HJ. Changing gastric cancer treatment in the United States and the pursuit of quality. Eur J Surg Oncol. 2005;31:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Yamazaki D, Kurisu S, Takenawa T. Regulation of cancer cell motility through actin reorganization. Cancer Sci. 2005;96:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 470] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 8. | Oue N, Hamai Y, Mitani Y, Matsumura S, Oshimo Y, Aung PP, Kuraoka K, Nakayama H, Yasui W. Gene expression profile of gastric carcinoma: identification of genes and tags potentially involved in invasion, metastasis, and carcinogenesis by serial analysis of gene expression. Cancer Res. 2004;64:2397-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Chen CN, Lin JJ, Chen JJ, Lee PH, Yang CY, Kuo ML, Chang KJ, Hsieh FJ. Gene expression profile predicts patient survival of gastric cancer after surgical resection. J Clin Oncol. 2005;23:7286-7295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Lee HS, Cho SB, Lee HE, Kim MA, Kim JH, Park do J, Kim JH, Yang HK, Lee BL, Kim WH. Protein expression profiling and molecular classification of gastric cancer by the tissue array method. Clin Cancer Res. 2007;13:4154-4163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Yasui W, Oue N, Sentani K, Sakamoto N, Motoshita J. Transcriptome dissection of gastric cancer: identification of novel diagnostic and therapeutic targets from pathology specimens. Pathol Int. 2009;59:121-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Li TB, Liu XH, Feng S, Hu Y, Yang WX, Han Y, Wang YG, Gong LM. Characterization of MR-1, a novel myofibrillogenesis regulator in human muscle. Acta Biochim Biophys Sin (Shanghai). 2004;36:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Rainier S, Thomas D, Tokarz D, Ming L, Bui M, Plein E, Zhao X, Lemons R, Albin R, Delaney C. Myofibrillogenesis regulator 1 gene mutations cause paroxysmal dystonic choreoathetosis. Arch Neurol. 2004;61:1025-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Ghezzi D, Viscomi C, Ferlini A, Gualandi F, Mereghetti P, DeGrandis D, Zeviani M. Paroxysmal non-kinesigenic dyskinesia is caused by mutations of the MR-1 mitochondrial targeting sequence. Hum Mol Genet. 2009;18:1058-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Chen DH, Matsushita M, Rainier S, Meaney B, Tisch L, Feleke A, Wolff J, Lipe H, Fink J, Bird TD. Presence of alanine-to-valine substitutions in myofibrillogenesis regulator 1 in paroxysmal nonkinesigenic dyskinesia: confirmation in 2 kindreds. Arch Neurol. 2005;62:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Ren K, Jin H, Bian C, He H, Liu X, Zhang S, Wang Y, Shao RG. MR-1 modulates proliferation and migration of human hepatoma HepG2 cells through myosin light chains-2 (MLC2)/focal adhesion kinase (FAK)/Akt signaling pathway. J Biol Chem. 2008;283:35598-35605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Li HL, She ZG, Li TB, Wang AB, Yang Q, Wei YS, Wang YG, Liu DP. Overexpression of myofibrillogenesis regulator-1 aggravates cardiac hypertrophy induced by angiotensin II in mice. Hypertension. 2007;49:1399-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Clemens MJ, Bushell M, Jeffrey IW, Pain VM, Morley SJ. Translation initiation factor modifications and the regulation of protein synthesis in apoptotic cells. Cell Death Differ. 2000;7:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | AJCC Cancer Staging Manual. 7th ed. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. New York, NY: Springer-Verlag 2010; 143-164. |
| 20. | Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30:1415-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 21. | Yu GZ, Chen Y, Wang JJ. Overexpression of Grb2/HER2 signaling in Chinese gastric cancer: their relationship with clinicopathological parameters and prognostic significance. J Cancer Res Clin Oncol. 2009;135:1331-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Liu X, Li T, Sun S, Xu F, Wang Y. Role of myofibrillogenesis regulator-1 in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2006;290:H279-H285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Ferraresso S, Vitulo N, Mininni AN, Romualdi C, Cardazzo B, Negrisolo E, Reinhardt R, Canario AV, Patarnello T, Bargelloni L. Development and validation of a gene expression oligo microarray for the gilthead sea bream (Sparus aurata). BMC Genomics. 2008;9:580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Mikeska T, Dobrovic A. Validation of a primer optimisation matrix to improve the performance of reverse transcription - quantitative real-time PCR assays. BMC Res Notes. 2009;2:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Rosen O, Manor R, Weil S, Gafni O, Linial A, Aflalo ED, Ventura T, Sagi A. A sexual shift induced by silencing of a single insulin-like gene in crayfish: ovarian upregulation and testicular degeneration. PLoS One. 2010;5:e15281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007;3:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 863] [Cited by in RCA: 872] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 27. | Wenzel JJ, Walch H, Bollwein M, Niller HH, Ankenbauer W, Mauritz R, Höltke HJ, Zepeda HM, Wolf H, Jilg W. Library of prefabricated locked nucleic acid hydrolysis probes facilitates rapid development of reverse-transcription quantitative real-time PCR assays for detection of novel influenza A/H1N1/09 virus. Clin Chem. 2009;55:2218-2222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Zhang M, Zhang H, Ma Y, Zhu G, Xue Y. Prognosis and surgical treatment of gastric cancer invading adjacent organs. ANZ J Surg. 2010;80:510-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Santoro R, Carboni F, Lepiane P, Ettorre GM, Santoro E. Clinicopathological features and prognosis of gastric cancer in young European adults. Br J Surg. 2007;94:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Fazal F, Gu L, Ihnatovych I, Han Y, Hu W, Antic N, Carreira F, Blomquist JF, Hope TJ, Ucker DS. Inhibiting myosin light chain kinase induces apoptosis in vitro and in vivo. Mol Cell Biol. 2005;25:6259-6266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Gutjahr MC, Rossy J, Niggli V. Role of Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Exp Cell Res. 2005;308:422-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |