Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5260
Revised: May 29, 2012
Accepted: June 8, 2012
Published online: October 7, 2012
AIM: To investigate the value of adenosine deaminase (ADA) for early detection of tuberculous peritonitis (TBP) among cirrhotic patients.
METHODS: We retrospectively analyzed 22 patients with TBP from July 1990 to June 2010. Twenty-five cirrhotic patients with uninfected ascites were prospectively enrolled as the cirrhosis control group from July 2010 to June 2011. An additional group of 217 patients whose ascites ADA levels were checked in various clinical conditions were reviewed from July 2008 to June 2010 as the validation group.
RESULTS: The mean ascites ADA value of cirrhotic patients with TBP (cirrhotic TBP group, n = 8) was not significantly different from that of non-cirrhotic patients (non-cirrhotic TBP group, n = 14; 58.1 ± 18.8 U/L vs 70.6 ± 29.8 U/L, P = 0.29), but the mean ascites ADA value of the cirrhotic TBP group was significantly higher than that of the cirrhosis control group (58.1 ± 18.8 U/L vs 7.0 ± 3.7 U/L, P < 0.001). ADA values were correlated with total protein values (r = 0.909, P < 0.001). Using 27 U/L as the cut-off value of ADA, the sensitivity and specificity were 100% and 93.3%, respectively, for detecting TBP in the validation group.
CONCLUSION: Even with lower ADA activity in ascites among cirrhotic patients, ADA values were significantly elevated during TBP, indicating that ADA can still be a valuable diagnostic tool.
- Citation: Liao YJ, Wu CY, Lee SW, Lee CL, Yang SS, Chang CS, Lee TY. Adenosine deaminase activity in tuberculous peritonitis among patients with underlying liver cirrhosis. World J Gastroenterol 2012; 18(37): 5260-5265
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5260
Tuberculous peritonitis (TBP) is one of the most frequent extra-pulmonary locations of tuberculosis, and the mortality rate may exceed 50% without prompt treatment[1,2]. The gold standard for diagnosing TBP is culture of Mycobacterium in ascites fluid or peritoneal biopsy, but the cultures are time-consuming and have low positivity rates, with a mean sensitivity of 43%-83% depending on the quality of samples cultured and methods utilized[3,4]. In addition, caseous granulomas of peritoneal biopsies obtained by either laparoscopy or laparotomy are alternative methods for rapid primitive diagnosis, but the procedures are invasive and may increase rates of morbidity and mortality[4-6]. The high mortality rate in untreated patients warrants a quick and noninvasive test for diagnosing TBP[1,3,7], and adenosine deaminase (ADA) in ascites is an available test that has been proposed to be useful for rapid diagnosis[1,4,6,8,9]. By analyzing ADA level in ascites, the sensitivity and specificity for diagnosing TBP have been reported to be as high as 100% and 97%, respectively[2,8,10-12].
The risk of TBP is increased in patients with underlying liver cirrhosis[4,13], and the percentage of underlying cirrhosis among patients with TBP could be as high as 50% in the United States[14]. However, concerns regarding the sensitivity of ascites ADA in detecting TBP have been raised among patients with underlying liver cirrhosis[3,15], and the low sensitivity is thought to be caused by the concomitant immunocompromised status and dilutional phenomenon in advanced liver disease[3]. In addition, cirrhotic patients always have a low protein level in ascites, and the correlation between total protein and ADA activity has been discussed in previous studies[3,10,15]. However, the laboratory analysis of ascites might be confounded by heterogenous clinical conditions in the control groups, and previous studies show conflicting results for cirrhotic patients with TBP[4]. Therefore, in the present study, we employed a prospectively-enrolled cirrhosis control group in order to investigate the laboratory changes in ascites and the clinical utility of measuring ADA activity in cirrhotic patients with TBP.
All patients diagnosed with TBP at Taichung Veterans General Hospital, a tertiary referral center in central Taiwan, from July 1990 to June 2010 were retrospectively analyzed and formed the TBP group. The patients’ medical records were reviewed and demographic, laboratory, microbiological, histological and laparoscopic features were collected. A definite diagnosis of TBP was based on one or more of the following: (1) positive TB cultures of ascites or peritoneal biopsy; (2) characteristic finding of caseous granulomas on histology of peritoneal biopsy; and (3) clinical judgment of TBP by a physician based on ascites data followed by a good response to anti-tuberculous treatment. A good response to therapy was defined as complete resolution or clinical improvement during the follow-up period. Exclusion criteria included (1) ascites ADA data unavailable; (2) ascites ADA analyzed after anti-tuberculous treatment; and (3) patients with concomitant end-stage renal disease under continuous ambulatory peritoneal dialysis, whose ascites might be diluted by dialysate. In addition, for further analysis of the role of liver cirrhosis, patients were evaluated to determine whether or not they had concurrent liver cirrhosis. Liver cirrhosis was defined as typical morphologic change such as blunted edge of liver, irregular liver surface and highly coarse liver parenchyma[16], with or without evidence of portal hypertension, such as previous history of ascites before TBP, splenomegaly or esophageal/gastric varices recognized by imaging techniques or endoscopy.
Patients with liver cirrhosis and uninfected ascites were prospectively enrolled as the cirrhosis control group from July 2010 to June 2011, and medical history, etiology of cirrhosis, and Child-Pugh’s classification, as well as imaging features were recorded. Patients with primary or metastatic liver malignancy, peritoneal carcinomatosis, congestive heart failure, spontaneous bacterial peritonitis, nephritic syndrome, renal failure under dialysis, or evidence of peritonitis were excluded. Ascites was obtained and analyzed with regard to parameters including ADA, albumin, total protein, lactate dehydrogenase, glucose, cell counts, cytology, ordinary and anaerobic culture, acid-fast stain, bacteria and tuberculosis cultures.
Patients whose ascites ADA levels were detected by a clinical need for differential diagnosis from July 2008 to June 2010 comprised the validation group and their medical records were retrospectively reviewed. The patients in the cirrhosis control group were not included in the validation cohort. Further analysis of subgroups according to etiology of ascites was performed.
ADA activity was determined using a method similar to that described by Slaats et al[17] with an autoanalyzer (Hitachi 7170, Japan) and ADA-N kit (Denka Seiken Co. Ltd, Japan). The kinetic method estimated ADA activity by coupling the liberated NH3 to oxoglutarate with glutamate dehydrogenase, which leads to a decrease in the reduced form of nicotinamide adenine dinucleotide phosphate absorbance at 340 nm[3,17]. The ratio of decreased absorbance reflects the activity of ADA.
This work has been carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was approved ethically by the Institutional Review Board of Taichung Veterans General Hospital (C10124).
The discrete variables are presented with number and percentage; continuous variables are presented with mean ± SD. The continuous variables were compared by Mann-Whitney U test. The discrete variables were compared by χ2 test and Fisher’s exact test. Diagnostic utility of ADA for TBP was evaluated at various cutoff values by the sensitivity and specificity. These were assessed based on comparisons of relative operating characteristic curves. Spearman’s linear regression was used to evaluate the correlation between total protein of ascites and serum. P < 0.05 was considered to be statistically significant.
A computerized database search identified 29 consecutive patients who were diagnosed with TBP from July 1988 to June 2010 and who met the inclusion criteria. Seven patients (6 patients without ADA data and 1 patient under continuous ambulatory peritoneal dialysis) were excluded according to the exclusion criteria of this study, and none had concurrent liver cirrhosis. A total of 22 patients with TBP were included in the final analysis and formed the TBP group, which was further divided into two subgroups: (1) cirrhotic TBP group: concomitant cirrhosis was diagnosed in 8 of 22 patients; and (2) non-cirrhotic TBP group: no evidence of cirrhosis among the other 14 of 22 patients. The demographic and ascites data of the two TBP subgroups were compared (Table 1), and there were no significant differences in ascites ADA, white blood cell (WBC) count and lymphocyte count between the two subgroups. However, the mean total protein concentration of ascites in the non-cirrhotic TBP group was significantly greater than that in the cirrhotic TBP group (P < 0.05).
| Demographic data | TBP without cirrhosis (n = 14) | TBP with cirrhosis (n = 8) | P value |
| Age (yr) | 63.3 ± 22.8 | 66.5 ± 9.7 | 0.65 |
| Sex | 0.64 | ||
| Male | 10 (71.4) | 5 (62.5) | |
| Female | 4 (28.6) | 3 (37.5 | |
| Ascites data | |||
| ADA (U/L) | 70.6 ± 29.8 | 58.1 ± 18.8 | 0.29 |
| SAAG (g/dL) | 0.9 ± 0.3 | 1.3 ± 0.6 | 0.10 |
| Total protein (mg/dL) | 4781.8 ± 1645.5 | 3400.0 ± 1000.0 | 0.04 |
| WBC (/mm3) | 1411.1 ± 1291.2 | 1489.9 ± 759.3 | 0.42 |
| Lymphocytes (/mm3) | 1078.3 ± 935.6 | 1135.0 ± 696.1 | 0.71 |
The demographic and ascites data of the cirrhotic TBP group and the cirrhosis control group were compared (Table 2), and there were no significant differences in demographic data between the two groups. However, the mean values of ADA, total protein, WBC counts and lymphocyte counts in ascites of the cirrhotic TBP group were significantly higher than those of the cirrhosis control group (P < 0.05).
| Demographic data | TBP with cirrhosis (n =8) | Cirrhosis control (n =25) | P value |
| Age (yr) | 66.5 ± 9.7 | 59.8 ± 13.5 | 0.65 |
| Sex | 1.00 | ||
| Male | 5 (62.5) | 15 (60.0) | |
| Female | 3 (37.5) | 10 (40.0) | |
| Child’s classification | 0.99 | ||
| A | 1 (12.5) | 3 (12.0) | |
| B | 4 (50.0) | 12 (48.0) | |
| C | 3 (37.5) | 10 (40.0) | |
| Ascites data | |||
| ADA (U/L) | 58.1 ± 18.8 | 7.0 ± 3.7 | < 0.001 |
| SAAG (g/dL) | 1.3 ± 0.6 | 2.1 ± 0.5 | 0.01 |
| Total protein (mg/dL) | 3400.0 ± 1000.0 | 1176.0 ± 636.6 | < 0.001 |
| WBC (/mm3) | 1489.9 ± 759.3 | 174.7 ± 159.6 | < 0.001 |
| Lymphocytes (/mm3) | 1135.0 ± 696.1 | 113.3 ± 127.1 | < 0.001 |
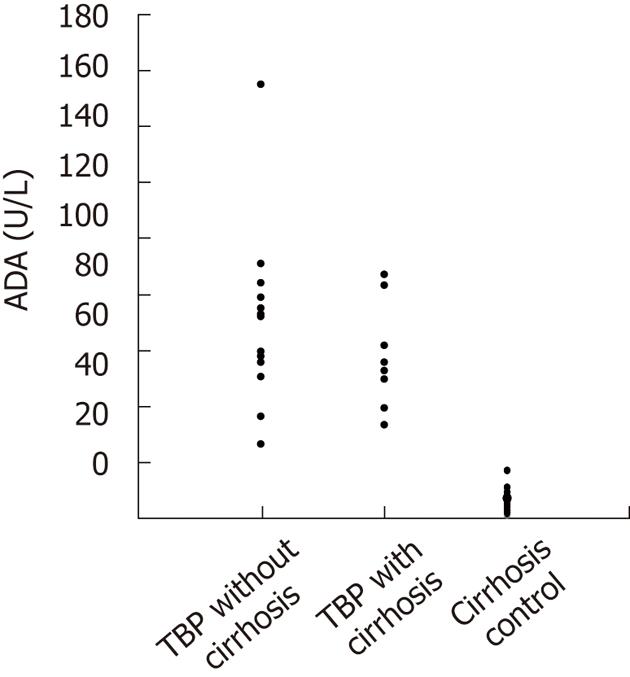
The distribution of ascites ADA values among the patient groups were compared (Figure 1), and the values of ascites ADA in the cirrhosis control group were markedly different from those of the other two groups with no overlapping area. Using 27 U/L as a cut-off value of ADA in ascites (the lowest value in the TBP group), no patient in the cirrhosis control group was found to have an ascites ADA concentration higher than this value.
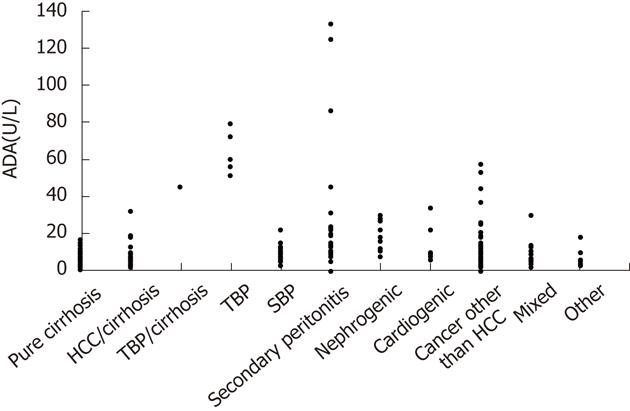
The validation group included 217 patients, and subgroup analysis was performed according to the different etiologies of ascites (Figure 2). Using 27 U/L as a cut-off value of ascites ADA in the validation group, the sensitivity in detecting TBP was 100% and specificity was 93.3%. There were fifteen (6.9%) patients (one hepatocellular carcinoma, four malignancy other than hepatoma, five intra-abdominal infection, three nephrogenic ascites, one cardiogenic ascites and one hepatocellular carcinoma mixed with spontaneous bacterial peritonitis) who had ascites ADA levels higher than 27 U/L but did not have TBP.
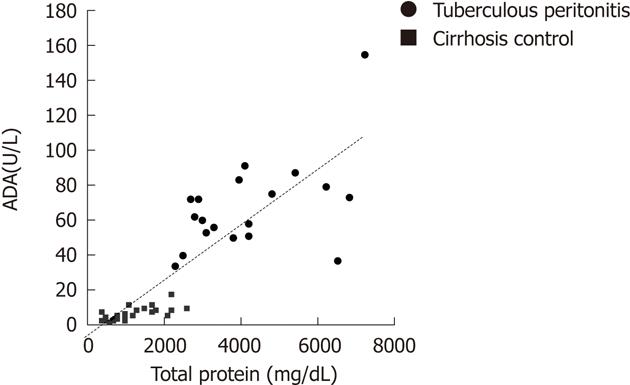
Spearman’s correlation analysis revealed that ascites ADA levels of all patients were strongly correlated with ascites total protein amounts (r = 0.909, P < 0.001) (Figure 3).
The utility of ascites ADA in the differential diagnosis of TBP for patients with underlying liver cirrhosis remains controversial due to conflicting results in previous studies. This is the first study to investigate cirrhotic patients with TBP involving a prospectively-enrolled cirrhosis control group, which was included to avoid the potential confounding effects of heterogeneous clinical conditions. In this study, even though lower levels of ascites ADA were found in the cirrhotic TBP group, their mean ascites ADA value was not significantly different from that of the non-cirrhotic TBP group. Moreover, ascites ADA values of cirrhotic patients with TBP were notably higher than those of the cirrhosis control group, and every patient in the cirrhosis control group had an ascites ADA level lower than the lowest value in the TBP group (27 U/L). Therefore, these data provide convincing evidence that ascites ADA may be significantly raised in TBP patients with underlying cirrhosis, and thus TBP should be considered in the differential diagnosis.
Previous studies showed high sensitivity and specificity in detecting TBP by checking ADA activity in ascites, based on ADA cut-off values of 36-40 IU/L[2,10,11,18]. However, Hillebrand et al[3] used only 7 IU/L as the cut-off value of ADA, and they found the sensitivity of ADA was only 30% in the setting of cirrhosis. There was considerable overlap in the ADA activity between TBP and sterile ascites among cirrhotic patients. Furthermore, Hillebrand et al[3] postulated that lower sensitivity and cut-off value might be caused by the higher proportion of cirrhosis (59%) in their patients with TBP. In contrast, Burgess et al[4] reported a sensitivity of 94% and a specificity of 92% for cirrhotic patients with TBP using a cut-off value for ADA of 30U/L. In the current study, there was no overlapping phenomenon in the ADA activity between TBP and sterile ascites among cirrhotic patients, and the sensitivity and specificity in detecting TBP were also high (100% and 93.3%, respectively) in the validation group using a cut-off value for ADA of 27 U/L. In addition, Hillebrand et al[3] determined ADA activity by detecting the decrease in adenosine concentration under the action of ADA, but this method was different from the measurement techniques applied in most previous studies. The methods employed by Slaats et al[17] or Giusti et al[19] have been extensively described in the literature[2,4,9,18], and involve determination of ADA activity by changes of NH3 or NADH, respectively, after interacting ADA with adenosine. The different techniques used might affect the sensitivity and specificity of the ADA test, so the correlation of ADA values obtained from different methods need further investigation.
As shown in our validation group and in previous studies[2-4], false positive findings of ascites ADA are still possible. Peritoneal carcinomatosis and secondary bacterial peritonitis are the two most likely etiologies after TBP. ADA plays an important role in regulating the level of adenosine, and its primary function in humans is development and maintenance of the immune system[20,21]. ADA is involved in proliferation and differentiation of T lymphocytes, and diseases such as malignant conditions, collagen vascular diseases and some microorganism infections that are associated with lymphocytosis may increase ADA levels[22]. Therefore, for patients with elevated ascites ADA, further differential diagnosis by ascites cytological examination or radiological imaging studies should be performed, and laparoscopic visualization and biopsy may be considered for equivocal cases. Furthermore, due to the high negative predictive value (100%) found in this study, invasive procedures such as laparoscopic peritoneum biopsy or laparotomy, which are relatively high risk in cirrhosis patients, may be unnecessary and should be avoided when ascites ADA activity is low. In addition, the mean time to develop positive tuberculosis culture from ascites was 36.4 ± 18.2 d in our study, but the mean time in previous studies on ascites ADA was only 3.0 ± 0.5 d. Testing for ADA in ascites has high sensitivity and specificity, and may therefore be useful as a rapid test for diagnosis. Although TB polymerase chain reaction (PCR) has been used as a rapid diagnostic tool for pulmonary TB, PCR cannot be suggested for diagnosing TBP due to its low sensitivity rate[9,23]. In endemic area of tuberculosis, DNA from dead TB bacilli may also give a false-positive result[9]. In addition, testing for ADA may also be currently more widely available than other valuable clinical tests such as interferon-gamma[24].
In this study, ADA activity was found to be strongly correlated with total protein in ascites in the TBP and control groups, and this finding was compatible with the simultaneously elevated total protein and ADA found in cirrhotic patients suffering from TBP. It is interesting to note that Fernandez-Rodriguez et al[15] found ADA was correlated with total protein in ascites among patients with TBP (r = 0.842), but the correlation was non-significant when the control group was included in the analysis. This inconsistent finding might be explained by heterogeneous etiologies and complex clinical conditions in the control group. Elevated total protein in the body fluid has been one of the diagnostic markers for inflammatory exudate[25], and it is also an important finding in ascites of TBP[5,15]. ADA is related to activation and differentiation of mononuclear leukocytes, and it is secreted during immune responses[3,21,26]. Lymphocytes are the predominant cells in ascites of TBP, and simultaneously elevated total protein and ADA can be explained by lymphocytic inflammation. Moreover, although mean total protein in the cirrhotic TBP group was significantly lower than that of the non-cirrhotic TBP group in this study, mean ADA levels were not significantly different. Compared with the cirrhosis control group, the WBC and lymphocyte counts were significantly higher in the cirrhotic TBP group, and the immune responses were predominant. Even in cirrhotic patients with a relatively immunocompromised status, TBP still activated strong immune reactions, which resulted in a sharp elevation of ADA in ascites, and this phenomenon indicates that ascites ADA may still be a valuable tool in the differential diagnosis of TBP.
We acknowledge several limitations in this study. Firstly, this study reflects the experience of a single medical center with a relatively small sample size, but the statistical power of the analysis was adequate. Secondly, cases with TBP were obtained from a computerized database search and retrospectively analyzed, so some TBP cases may have been missed due to incorrect coding in this long-term cohort study. Thirdly, we compared a historical TBP cohort with a prospectively-enrolled cirrhosis control group, and different time periods of study might produce some bias. However, patients were consecutively recruited, and the modalities for diagnosing TBP were not changed during the study period. The possible bias should be minimized.
In conclusion, even with lower ascites ADA activity in liver cirrhosis, ascites ADA levels could be significantly elevated due to strong immune responses when cirrhotic patients suffer from TBP. Owing to its high sensitivity and specificity, ascites ADA may be a valuable tool in the differential diagnosis of TBP in patients with underlying cirrhosis, and concomitant cirrhosis should not limit its clinical utility.
We thank the Biostatistics Task Force of Taichung Veterans General Hospital (Taichung, Taiwan, China) for statistical assistance.
The risk of tuberculous peritonitis (TBP) is increased in patients with underlying liver cirrhosis. Adenosine deaminase (ADA) in ascites has been proposed to be a useful test for early detection of TBP, but its value among patients with underlying cirrhosis is uncertain.
The utility of ascites ADA in the differential diagnosis of TBP in patients with underlying liver cirrhosis remains controversial due to conflicting results in previous studies. In addition, the relationship between ascites ADA and other parameters such as total protein has not been well discussed, and the mechanisms of ADA elevation among cirrhotic patients with TBP need further investigation.
This is the first study to investigate cirrhotic patients with TBP which includes a prospectively-enrolled cirrhosis control group, and ADA activity was strongly correlated with total protein in ascites. Even with lower ascites ADA activity in liver cirrhosis, ascites ADA levels could be significantly elevated due to strong immune responses when cirrhotic patients suffer from TBP.
Owing to its high sensitivity and specificity, ascites ADA may be a valuable tool in the differential diagnosis of TBP in patients with underlying cirrhosis, and concomitant cirrhosis should not limit its clinical utility. Furthermore, due to the high negative predictive value, invasive procedures such as laparoscopic peritoneum biopsy or laparotomy may be unnecessary when ascites ADA activity is low.
ADA is an enzyme involved in purine metabolism, and it can be a product of immune responses relating to T lymphocyte activity in humans.
This is a good case-control study and the message is clear. The authors examined ADA activity in TBP among patients with underlying liver cirrhosis, and a prospectively-enrolled cirrhosis control group was conducted to avoid the potential confounding effects of heterogeneous clinical conditions. Due to convincing data in this study, ascites ADA can still be a valuable tool in the differential diagnosis of TBP.
Peer reviewers: Dr. Nimer Assy, Liver Unit, Ziv Medical Centre, 13100 Safed, Israel; A Ibrahim Amin, MD, Department of Surgery, Queen Margaret Hospital, Dunfermline, Fife KY12 0SU, United Kingdom
S- Editor Gou SX L- Editor Logan S E- Editor Xiong L
| 1. | Chow KM, Chow VC, Hung LC, Wong SM, Szeto CC. Tuberculous peritonitis-associated mortality is high among patients waiting for the results of mycobacterial cultures of ascitic fluid samples. Clin Infect Dis. 2002;35:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 132] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Riquelme A, Calvo M, Salech F, Valderrama S, Pattillo A, Arellano M, Arrese M, Soza A, Viviani P, Letelier LM. Value of adenosine deaminase (ADA) in ascitic fluid for the diagnosis of tuberculous peritonitis: a meta-analysis. J Clin Gastroenterol. 2006;40:705-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 149] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Hillebrand DJ, Runyon BA, Yasmineh WG, Rynders GP. Ascitic fluid adenosine deaminase insensitivity in detecting tuberculous peritonitis in the United States. Hepatology. 1996;24:1408-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 4. | Burgess LJ, Swanepoel CG, Taljaard JJ. The use of adenosine deaminase as a diagnostic tool for peritoneal tuberculosis. Tuberculosis (Edinb). 2001;81:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Shakil AO, Korula J, Kanel GC, Murray NG, Reynolds TB. Diagnostic features of tuberculous peritonitis in the absence and presence of chronic liver disease: a case control study. Am J Med. 1996;100:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Adenosine deaminase and tuberculous peritonitis. Lancet. 1989;1:1260-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Yeh HF, Chiu TF, Chen JC, Ng CJ. Tuberculous peritonitis: analysis of 211 cases in Taiwan. Dig Liver Dis. 2012;44:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Voigt MD, Kalvaria I, Trey C, Berman P, Lombard C, Kirsch RE. Diagnostic value of ascites adenosine deaminase in tuberculous peritonitis. Lancet. 1989;1:751-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis--presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22:685-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 239] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Bhargava DK, Gupta M, Nijhawan S, Dasarathy S, Kushwaha AK. Adenosine deaminase (ADA) in peritoneal tuberculosis: diagnostic value in ascitic fluid and serum. Tubercle. 1990;71:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Ribera E, Martínez Vásquez JM, Ocaña I, Ruiz I, Jimínez JG, Encabo G, Segura RM, Pascual C. Diagnostic value of ascites gamma interferon levels in tuberculous peritonitis. Comparison with adenosine deaminase activity. Tubercle. 1991;72:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Dwivedi M, Misra SP, Misra V, Kumar R. Value of adenosine deaminase estimation in the diagnosis of tuberculous ascites. Am J Gastroenterol. 1990;85:1123-1125. [PubMed] |
| 13. | Aguado JM, Pons F, Casafont F, San Miguel G, Valle R. Tuberculous peritonitis: a study comparing cirrhotic and noncirrhotic patients. J Clin Gastroenterol. 1990;12:550-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Harlan WR, Grimm IS. Tuberculous peritonitis: can ADA keep the laparoscope away? Gastroenterology. 1997;113:687-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Fernandez-Rodriguez CM, Perez-Arguelles BS, Ledo L, Garcia-Vila LM, Pereira S, Rodriguez-Martinez D. Ascites adenosine deaminase activity is decreased in tuberculous ascites with low protein content. Am J Gastroenterol. 1991;86:1500-1503. [PubMed] |
| 16. | Xie J, Yu BF, Xu J, Zhang YH, Cheng NL, Niu B, Hu XN, Xiang Q, Zhang ZG. Protein transduction domain of membrane penetrating peptide can efficiently deliver DNA and protein into mouse liver for gene therapy. Hepatobiliary Pancreat Dis Int. 2005;4:90-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Slaats EH, Asberg EG, van Keimpema AR, Kruijswijk H. A continuous method for the estimation of adenosine deaminase catalytic concentration in pleural effusions with a Hitachi 705 discrete analyser. J Clin Chem Clin Biochem. 1985;23:677-682. [PubMed] |
| 18. | Martinez-Vazquez JM, Ocaña I, Ribera E, Segura RM, Pascual C. Adenosine deaminase activity in the diagnosis of tuberculous peritonitis. Gut. 1986;27:1049-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Giusti G. Adenosine deaminase. Methods of Enzymatic Analysis. New York: Academic Press 1974; 1092–1099. |
| 20. | Wilson DK, Rudolph FB, Quiocho FA. Atomic structure of adenosine deaminase complexed with a transition-state analog: understanding catalysis and immunodeficiency mutations. Science. 1991;252:1278-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 318] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 21. | Zavialov AV, Gracia E, Glaichenhaus N, Franco R, Zavialov AV, Lauvau G. Human adenosine deaminase 2 induces differentiation of monocytes into macrophages and stimulates proliferation of T helper cells and macrophages. J Leukoc Biol. 2010;88:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Pettersson T, Ojala K, Weber TH. Adenosine deaminase in the diagnosis of pleural effusions. Acta Med Scand. 1984;215:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | American Thoracic Society Workshop. Rapid diagnostic tests for tuberculosis: what is the appropriate use? American Thoracic Society Workshop. Am J Respir Crit Care Med. 1997;155:1804-1814. [PubMed] |
| 24. | Saleh MA, Hammad E, Ramadan MM, Abd El-Rahman A, Enein AF. Use of adenosine deaminase measurements and QuantiFERON in the rapid diagnosis of tuberculous peritonitis. J Med Microbiol. 2012;61:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Light RW. Clinical practice. Pleural effusion. N Engl J Med. 2002;346:1971-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 405] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 26. | Zavialov AV, Engström A. Human ADA2 belongs to a new family of growth factors with adenosine deaminase activity. Biochem J. 2005;391:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |