Published online Oct 7, 2012. doi: 10.3748/wjg.v18.i37.5197
Revised: June 18, 2012
Accepted: June 28, 2012
Published online: October 7, 2012
AIM: To study the effect of H2 gas on liver injury in massive hepatectomy using the Intermittent Pringle maneuver in swine.
METHODS: Male Bama pigs (n = 14) treated with ketamine hydrochloride and Sumianxin II as induction drugs followed by inhalation anesthesia with 2% isoflurane, underwent 70% hepatotectomy with loss of bleeding less than 50 mL, and with hepatic pedicle occlusion for 20 min, were divided into two groups: Hydrogen-group (n = 7), the pigs with inhalation of 2% hydrogen by the tracheal intubation during major hepatotectomy; Contrast-group (n = 7), underwent 70% hepatotectomy without inhalation of hydrogen. Hemodynamic changes and plasma concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid (HA), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and malondialdehyde (MDA) in liver tissue were measured at pre-operation, post-hepatotectomy (PH) 1 h and 3 h. The apoptosis and proliferating cell nuclear antigen (PCNA) expression in liver remnant were evaluated at PH 3 h. Then we compared the two groups by these marks to evaluate the effect of the hydrogen in the liver injury during major hepatotectomy with the Pringle Maneuver in the swine.
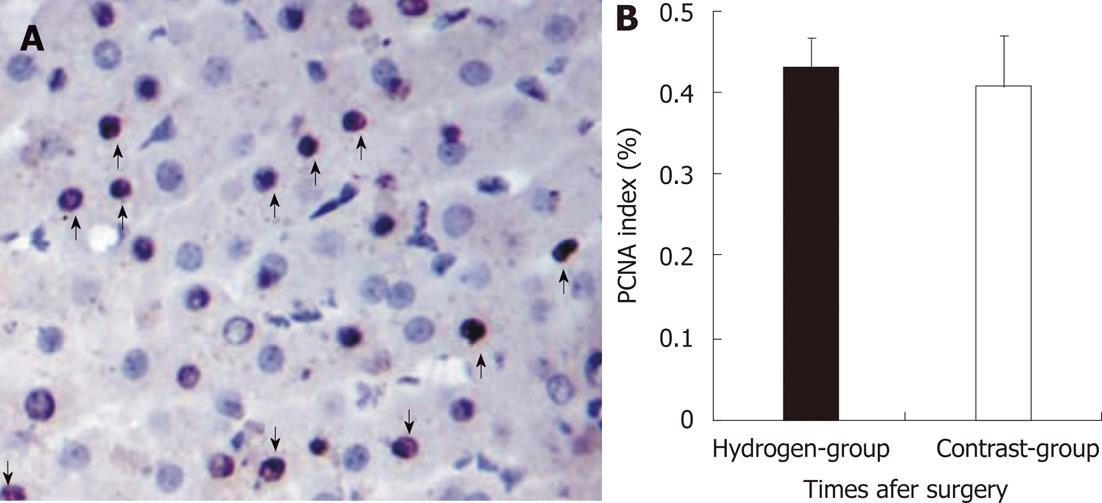
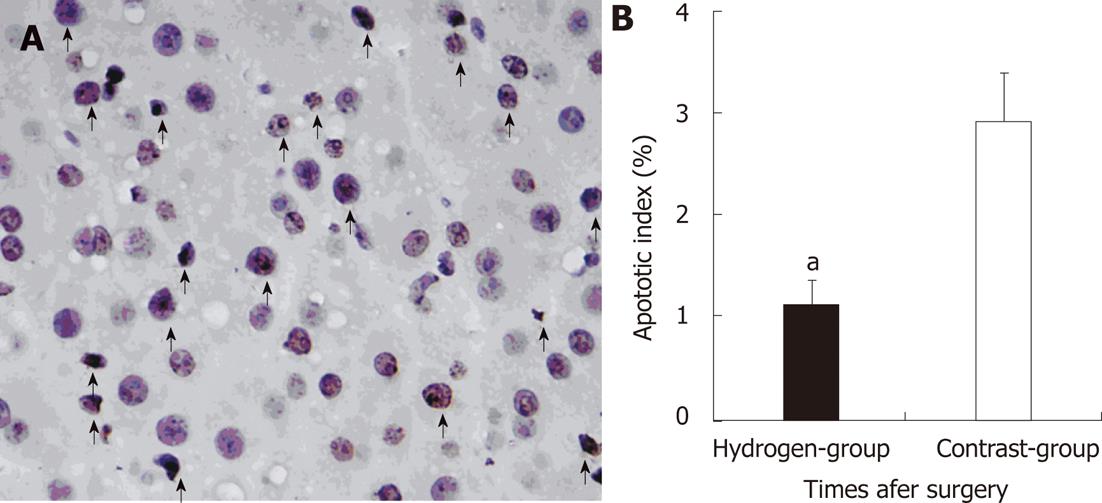
RESULTS: There were no significant differences in body weight, blood loss and removal liver weight between the two groups. There was no significant difference in changes of portal vein pressure between two groups at pre-operation, PH 30 min, but in hydrogen gas treated-group it slightly decrease and lower than its in Contrast-group at PH 3 h, although there were no significant difference (P = 0.655). ALT and AST in Hydrogen-group was significantly lower comparing to Contrast-group (P = 0.036, P = 0.011, vs P = 0.032, P = 0.013) at PH 1 h and 3 h, although the two groups all increased. The MDA level increased between the two group at PH 1 h and 3 h. In the hydrogen gas treated-group, the MDA level was not significantly significant at pre-operation and significantly low at PH 1 h and 3 h comparing to Contrast-group (P = 0.0005, P = 0.0004). In Hydrogen-group, the HA level was also significantly low to Contrast-group (P = 0.0005, P = 0.0005) although the two groups all increased at PH 1 h and 3 h. The expression of cluster of differentiation molecule 31 molecules Hydrogen-group was low to Contrast-group. However, PCNA index (%) was not statistically significant between the two groups (P = 0.802). Microphotometric evaluation of apoptotic index (AI) in terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling-stained tissue after hepatotectomy for 3h, the AI% level in the hydrogen was significantly low to Contrast-group (P = 0.012). There were no significant difference between Hydrogen-group and Contrast-group at pre-operation (P = 0.653, P = 0.423), but after massive hepatotectomy, the TNF-α and IL-6 levels increase, and its in Hydrogen-group was significantly low compared with Contrast-group (P = 0.022, P = 0.013, vs P = 0.016, P = 0.012), respectively. Hydrogen-gas inhalation reduce levels of these markers and relieved morphological liver injury and apoptosis.
CONCLUSION: H2 gas attenuates markedly ischemia and portal hyperperfusion injury in pigs with massive hepatotectomy, possibly by the reduction of inflammation and oxidative stress, maybe a potential agent for treatment in clinic.
- Citation: Xiang L, Tan JW, Huang LJ, Jia L, Liu YQ, Zhao YQ, Wang K, Dong JH. Inhalation of hydrogen gas reduces liver injury during major hepatotectomy in swine. World J Gastroenterol 2012; 18(37): 5197-5204
- URL: https://www.wjgnet.com/1007-9327/full/v18/i37/5197.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i37.5197
In hepatotectomy, it is often needed to occlude the portal inflow in order to reducing bleeding, causing liver ischemic injury, the Pringle maneuver, interrupts the blood flow to the liver, produces profound hepatic ischemia and intestinal congestion, it has been used clinically during hepatectomy[1-3]. However, ischemia-reperfusion injury (I/R-I) resulting from the Pringle maneuver is one of the pathogenetic factors involved in postoperative liver dysfunction and hepatic failure, especially when the liver is steatotic and cirrhotic[3-5]. The risk of post-operative liver failure (PLF) or "small of size" syndrome (SFSS) is the central problem in the field of liver resection[3,6]. Oxidative stress is regarded as a major contributor to the development of various hepatic disorders including acute hepatic failure, hepatic fibrosis, and hepatic cancer[7-9]. Protective effect of H2 gas on liver ischemia reperfusion (I/R) injury and toxic liver injury in rodents has been demonstrated. Previously through ameliorating oxidative stress, H2 becomes an important potential anti-oxygen spices agent in clinic[10-12]. However, all experiments about H2 gas focus on small animal[11,13], and lack of the study in big animal which provide a much more clinically relevant means of investigating the pathophysiology of a disease process. Protective effect of H2 in big animal can provid more treatment options that can be more readily applied in the human setting. In this study, we investigated firstly the effect of H2 gas on liver remnant injury in major hepatectomy using the Pringle maneuver in swine, and its feasibility in clinic.
Fourteen pigs male Bama miniature pigs (15-20 kg) were obtained from the Pig and Poultry Production Institute (Guangxi Zhuang Autonomous Region, China). The swine were raised from a closed herd and kept under strict quarantine protocol. The study was approved by the Hospital Clinic Committee on Ethics in Animal Experimentation. All animals in this study were treated humanely and in accordance with institutional and national guide lines for ethical animal.
An upper midline incision with right or bilateral subcostal extensions (inverse "L" shape or Mercedesb incision) was performed. The subtotal hepatotectomy with loss of bleeding less than 50 mL, and with hepatic pedicle occlusion for 20 min were performed according to the previous introduce[13]. A 16-gauge catheter was inserted into the main portal vein via the gastroduodenal vein to measure the portal vein pressure (PVP).
Hydrogen-group (n = 7) inhaled with 2% and H2 98% oxygen supplied through trachea cannula, gas inhalation started once trachea cannula accomplished, ontrast-group (n = 7), only inhale oxygen though tracheal tube. In the two groups, the intraoperative PVP and flow were respectively monitored at the proctectomy, 1 h and 3 h after finishing the hepatectomy.
Blood samples were obtained before laparotomy, posthepatectomy 60 min and 180 min. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), hyaluronic acid (HA), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) were evaluated. Serum AST, ALT and HA were measured using standard clinical methods for automated analysis (Model 7170; Hitachi Inc, Tokyo, Japan). Plasma TNF-α, IL-6 levels were examined by enzyme-linked immunosorbent assay (ELISA) using a commercial porcine TNF-α/TNFSF2 immunoassay kit (Shanghai Yi Hua Scientific, Inc. China). Serum HA levels reflect sinusoidal endothelial damage. HA was measured by a radiometric assay with the Pharmacia HA test (Shanghai Yi Hua Scientifi c, Inc. China) in prereperfusion and postreperfusion serum samples.
Tissue samples were obtained at 180 min post-hepatotectomy (PH) and were divided into two parts. One was immediately cut into cubes 1 mm and fixed in 2.5% glutaraldehyde in cacodylate buffer (0.1 mol/L sodium cacodylate-HCl buffer, pH 7.4) at 4 °C, prior to sectioning for transmission electron microscopy. Another was fixed with 10% formalin for 24 h and embedded in paraffin. Three-micrometer-thick sections were stained with hematoxylin and eosin and analyzed by the in situ terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) method using an apoptosis in situ detection kit (Shanghai Yi Hua Scientifi c, Inc. China) according to the manufacturer’s instructions[14]. The percentage of TUNEL-positive cells of total cell (apoptosis index, AI) nuclei in 10 high power fields were calculated for the 2 groups then compared. Proliferating cell nuclear antigen (PCNA) is a stable cell cycle nuclear. PCNA protein expression was detected by immunostaining using monoclonal anti-PCN-antibody (Jingmei Biotech Co. Ltd, Shenzhen, China). Data were expressed as the percentage of PCNA-stained hepatocytes per total number of hepatocytes (PCNA index). The mean numbers of PCNA-stained hepatocytes per 10 high power fields were calculated for the 2 groups, divided by the total cell number and then compared.
Tissue samples were obtained at pre-hepatotectomy, 60 min, 180 min after hepatectomy for hepatic malondialdehyde (MDA) measurement. Hepatic MDA levels were determined using a agents were purchased from the Nanjing Jiangcheng Bioengineering Institute (Nanjing, China), measured according to the manufacturer’s instructions. MDA levels were normalized against protein (pmol/mg).
Serum TNF-α and IL-6 measurement reagents were purchased from the Nanjing Jiangcheng Bioengineering Institute (Nanjing, China). TNF-α and IL-6 ELISA kits (Shanghai Yi Hua Scientifi c Inc., China). TNF-α and IL-6 were measured according to the manufacturer’s instructions.
Values of parameters are presented as mean ± SD. Statistical significance was determined by Student’s t-test. Fisher’s exact test was used for comparison of adhesions. P < 0.05 was considered significant.
There were no significant difference between two groups in body weight, removal liver weight, estimated loss of bleeding and operating time (Table 1).
| Hydrogen-group | Contrast-group | P value | |
| BW (kg) | 19.2 ± 2.7 | 18.8 ± 3.1 | NS |
| RLW (g) | 302 ± 21 | 296 ± 23 | NS |
| ELB (mL) | 43 ± 12 | 53 ± 16 | NS |
| OT (h) | 2.9 ± 0.3 | 3.3 ± 0.3 | NS |
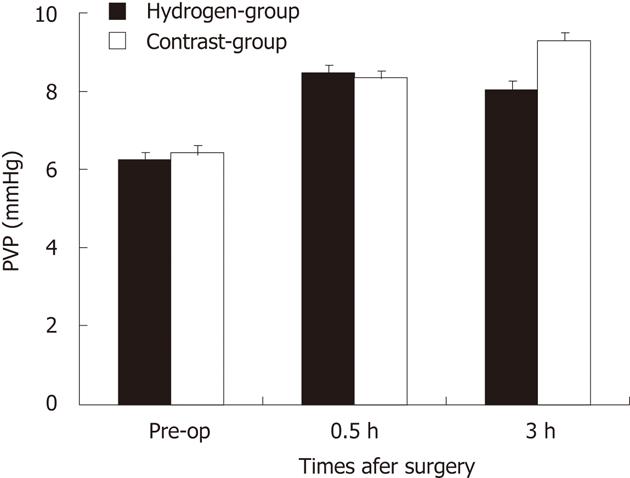
There was no significant difference in changes of PVP between two groups at pre-operation, PH 30 min (Figure 1). The PVP in hydrogen gas treated-group and moderately increased beyond that measured at laparotomy. The PVP in Contrast-group continue to rise at 3 h of posthepatotectomy, but in hydrogen gas treated-group it slightly decrease and lower than its in Contrast-group, although there no statistical significant differernce (P = 0.06).
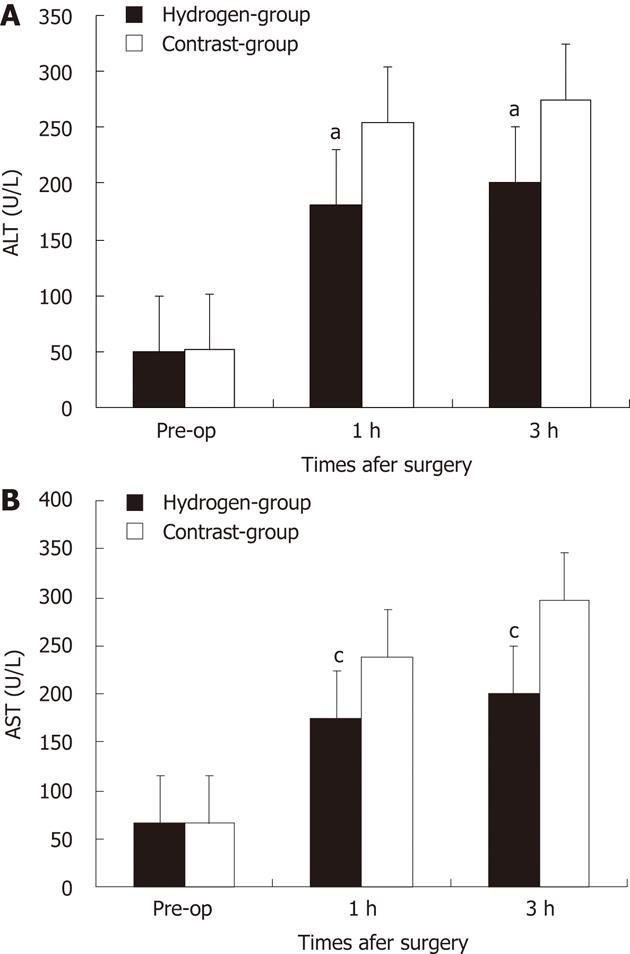
The preoperative and serial postoperative measurements of serum ALT and AST, are shown in Figure 2, on which significant differences are noted. There were no significant difference between two groups at pre-operation. After hepatotectomy ALT and AST increased in all of the animals and its in hydrogen gas treated-group was significantly lower comparing to Contrast-group (P = 0.036, P = 0.011).
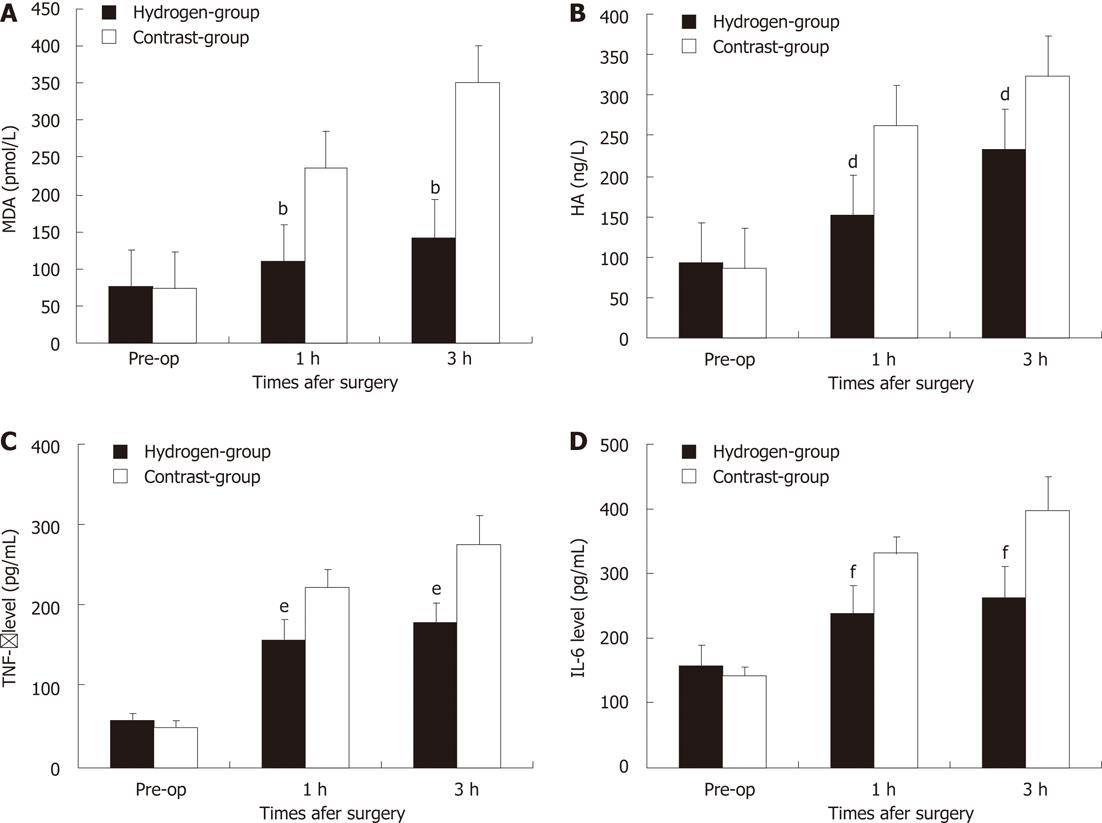
The serial change of hepatic MDA level in two groups was shown in Figure 3A. Baseline of hepatic MDA between two groups were no significant difference (P = 0.747). One hour after massive hepatotectomy, the MDA concentration increased in all the swines. In hydrogen gas treated-group, H2 gas significantly decreased levels of hepatic MDA, a marker of oxidative stress, the MDA level was significantly low to Contrast-group (P = 0.0005, P = 0.0004).
The serial change of serum HA level in two groups were showed in Figure 3B. Baseline of HA between two groups were no significant difference (P = 0.488). One hour after subtotal hepatotectomy, the HA concentration in serum increased in all the pigs. In Hydrogen-group, the HA level was significantly low to Contrast-group (P = 0.0051, P = 0.0052).
The risk of PLF is the central problem in the field of liver resection[2,3]. This is principally due to the PLF or SFSS, an excessive and destructive portal flow through a remnant liver that is too small, which becomes functionally insufficient[3,4,15], the intraoperative injury including ischemia and inflammatory response is another important pathogenic factors involved in postoperative liver dysfunction and hepatic failure. In studies of extended hepatectomy in dogs, severe damage to the sinusoidal endothelial cells (SECs) of the remnant liver 3 h after the operation was one of the main factors responsible for the high mortality rates[16,17]. Therefore, to reducing the intraoperative injury sometimes is determinant to prevention the PLF or SFSS, when the intraoperative damage is irreversible. It is well known oxidative stress is a major contributor to the development of various hepatic disorders including acute hepatic failure, at present, there are no effective agents to alleviate the oxidative stress during clinically operation. However, molecular H2, has recently been defined as a novel antioxidant, which selectively quenches detrimental the reactive oxygen species (ROS), while maintaining metabolic oxidation reduction reaction and other less potent ROS[10,11,18], indicating it is promising strategies to alleviate intraoperative injury.
In massive hepatotectomy, the intrahepatic vascular space in the remnant liver experiences a drastic reduction, and this leads to portal congestion and hemodynamic instability[4,5,9], the ischemia attenuate the instability, increases the metabolic burden, mitochondria produce more oxygen radicals. During the hepatic inflow occlusion, the intestinal congestion causing the damage of intestine barrier function and the increase of endotoxin absorbtion or bacterial translocation, however, the function of reticuloendothelial system decrease due to the removing of most of liver masss, which contained a lot of phagocyte[6,9,17]. Each individual Kupffer cell in a small-remnant is exposed to higher amounts of endotoxin than that in a whole liver, and triggers Kupffer cells to release a large quantity of free radicals. Lipid peroxidation, which plays a significant role in oxidative damage[18,19], was measured indirectly by assessing the increases in the levels of a lipid peroxidation product, MDA[19-21]. The MDA level is widely used as an indicator of free radical-mediated lipid peroxidation injury. In the present study, serum levels of MDA in contra-group increased rapidly during hepatotectomy (Figure 3A). The observed increase of liver MDA levels was an indicator of lipid peroxidation, which also verified the oxidative damage in the liver tissue in this animal model. While H2 inhalation inhibited this increase significantly, inhalation of H2 gas dramatically decreased MDA levels almost to the normal level.
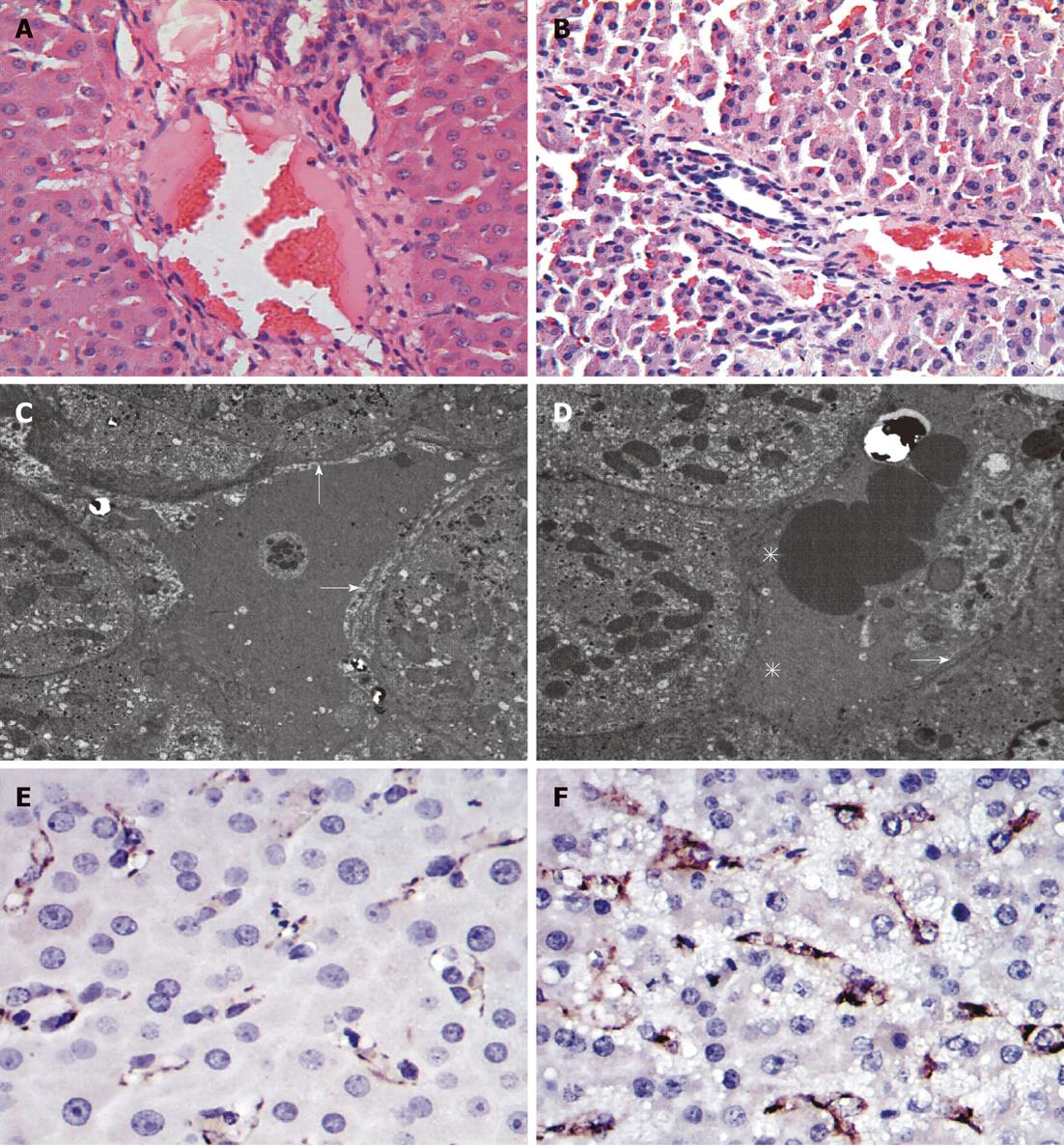
In the Contrast-group and Hydrogen-group, there were significant endothelial denudation in the medium-sized portal vein branches, sinusoidal dilation, hydropic changes of hepatocytes and hemorrhage into perivenular connective tissue, which extended into the hepatic parenchyma (Figure 4B), and there was no intraparenchymal hemorrhage present in H2 group (Figure 4A). Transmission electron microscopic photographs of the sinusoid was shown (Figure 4C and D). In the Hydrogen-group, the SECs (arrows) and hepatocytes were well preserved, and the structure of the endothelial lining can also be perceived (Figure 4C), in contrast, the sinusoidal endothelial lining was partially destroyed and detached into the sinusoidal space with enlargement of the Disse’s spaces (asterisks). Cluster of differentiation molecule 31 (CD31) immunostaining was notable for destruction of the endothelial lining among animals in Contrast-group (Figure 4F) and, in contrast to mild sinusoidal microarchitecture injury in Hydrogen-group (Figure 4E).
Many studies had demonstrated that the high shear-stress or hyperperfusion, due to small liver remnant could cause the sinusoidal endothelial injury, and hepatocyte injury, swelling degeneration of hepatocytes[22,23]. In Contrast-group, the portal overflow damage the 30% liver remnant underwent 20 min ischemia, causing the endothelial denudation, sinusoidal dilation, hydropic changes of hepatocytes and hemorrhage into perivenular connective tissue, in contrast, the H2 inhalation alleviated the hyper reperfusion, make the rise in PVP low to Contrast-group, attenuate markedly these injuries (Figure 4A and B). It also identified by the measurement of HA level. HA is synthesized by mesenchymal cells and eliminated in the hepatic sinusoidal endothelium; increased serum HA levels reflect sinusoidal endothelial damage[24,25]. In the present study, an elevation in serum HA level,caused by liver hyperfusion is also significantly low to Contrast-group, indicating the effect of H2 on hyperfusion injury in the hepatic sinusoidal endothelial (Figure 3B). CD31 immunoglobulin helps maintain endothelial stability by interdigitating with other CD31 molecules at the extracellular border of adjacent cells[26]. The study also showed that hydrogen-inhalation decreased the expression of CD31 molecules (Figure 4E and F), it means the H2 can reduce the injury of hyperperfusion,and was further demonstrated by the observation of transmission electron microscope examination (Figure 4C and D).
Normal liver has vigorous regenerative potential, portal hyperperfusion is likely to be an important physiologic trigger that stimulates liver regeneration[27,28]. The strength of the regenerative stimulus is proportional to the increase in portal blood flow, as previous shown in experimental animals[29]. The results in the study revealed also there were no significantly difference between the two group at PH 3 h (Figure 5). This means the liver regeneration in the early stage was determined to the portal shear stress, although H2 decrease free radicals injury. On the other hand, apoptotic cell death is an important contributor to the organ failure common to ALF, even for etiologies thought traditionally to involve mainly hepatocyte necrosis[25,26]. The free radicals may exert a strong cytotoxic effect, and played an important role in inducing apoptosis in the postoperative liver insufficient. The study demonstrated the AI in the hydrogen gas treated-group significantly decrease comparing to the Contrast-group (Figure 6A), this probably attribute to the protection of the H2 against injury of ROS. Therefore, H2 play an important role in deceasing the injury of SFSS with decreasing the apoptosis of hepatocyte, without increasing the regeneration.
Even though free radical scavengers have been demonstrated to reduce liver I/R damage[29,30], this is the first observation that the H2 decreases PVP or hyperperfusion injury in an animal model, which is determinant factor to PLF or SFSS. We observed that H2 inhalation reduced not only morphological injury, but also serum ALT, AST, IL-1, TNF-α (Figures 2 and 3C, D). We especially investigated the effect of hydrogen on oxidative stress PVP, and injury/regeneration in this liver hypefusion injury model. The use of gas inhalation to treat diseases has become increasingly popular. There are three endogenous gas include nitric oxide, carbon monoxide and H2 sulfate. The increased production of these gases under stress conditions may reflect the active involvement of these gases in the protective response[31-33]. However, the inherent toxicity of these gases must be investigated for gas inhalation to be considered an effective therapeutic strategy. H2 is not produced endogenously in mammalian cells since the hydrogenase activity responsible for the formation of H2 gas has not been identified.
In conclusion, intraoperative H2 inhalation in massive hepatotectomy was feasible and can protected the liver injury from hyperperfusion, by reduction of inflammation and oxidative stress, liver remnant apoptosis or necrosis, although it didn’t increase the regeneration. However, the exact mechanism and signalling pathway involved in the protection role of H2 in the small liver remnant injury need to be studied in the future. It is required to fully exploit inhalation of H2 gas as a therapeutic strategy.
Hydrogen selectively reduce levels of hydroxyl 1 radicals and alleviates acute oxidative stress in many animal models. But most of these study were used in small animal models and lack of the study in big animal which provide a much more clinically relevant means of investigating the pathophysiology of a disease process. In this study, the authors investigated firstly the effect of H2 gas on small liver remnant injury or “small of size” syndrome (SFSS) after massive hepatectomy, and its feasibility in clinic.
It is well known oxidative stress is a major contributor to the development of various hepatic disorders including acute hepatic failure, at present, there are no effective agents to alleviate the oxidative stress during clinically operation. However, molecular H2, has recently been defined as a novel antioxidant, which selectively quenches detrimental the reactive oxygen species (ROS), while maintaining metabolic oxidation reduction reaction and other less potent ROS, indicating it is promising strategies to alleviate intraoperative injury. Oxidative stress is regarded as a major contributor to the development of various hepatic disorders including acute hepatic failure, hepatic fibrosis, and hepatic cancer. Through ameliorating oxidative stress, H2 becomes an important potential anti-oxygen spices agent in clinic.
Even though free radical scavengers have been demonstrated to reduce liver ischemia reperfusion damage, this is the first observation that the H2 decreases portal vein pressure or hyperperfusion injury in an animal model, which is determinant factor to post-operative liver failure or SFSS. It was also firstly demonstrated the feasibility of intraoperative inhalation in big animals. As a kind of gas, intraoperative inhalation was convenient and safe.
The present study demonstrated firstly the protective effect of H2 gas on liver ischemia-reperfusion injury (I/R-I), toxic liver injury, and portal hyperperfusion injury in swine, that the physiology is similar to human, indicating intraoperative H2 gas inhalation will be a treatment modality as potential anti-inflammation response agent in clinic .
In this study, many biochemical markers mostly investigated in the study of hepatic I/R-I were evaluated and showed that inhaled hydrogen gas attenuated the I/R-I. This study gives a new insight into the pivotal role of hydrogen gas toward the hepatic I/R-I in clinical settings because hydrogen gas was used in swine model.
Peer reviewers: Ezio Laconi, MD, PhD, Professor of General Pathology, Department of Sciences and Biomedical Technologies, Unit of Experimental Pathology, University of Cagliari, Via Porcell, 4, IV Piano, 09125 Cagliari, Italy; Dr. Assy Nimer, MD, Assistant Professor, Liver Unit, Ziv Medical Centre, BOX 1008, 13100 Safed, Israel; Takashi Kobayashi, MD, PhD, Department of Surgery, Showa General Hospital, 2-450 Tenjincho, Kodaira, Tokyo 187-8510, Japan
S- Editor Lv S L- Editor A E- Editor Xiong L
| 1. | Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704-711; discussion 711-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 334] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 2. | Man K, Fan ST, Ng IO, Lo CM, Liu CL, Yu WC, Wong J. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999;134:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | van den Broek MA, Olde Damink SW, Dejong CH, Lang H, Malagó M, Jalan R, Saner FH. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767-780. [PubMed] |
| 4. | Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Nishizaki T, Ikegami T, Hiroshige S, Hashimoto K, Uchiyama H, Yoshizumi T, Kishikawa K, Shimada M, Sugimachi K. Small graft for living donor liver transplantation. Ann Surg. 2001;233:575-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 155] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Ben-Haim M, Emre S, Fishbein TM, Sheiner PA, Bodian CA, Kim-Schluger L, Schwartz ME, Miller CM. Critical graft size in adult-to-adult living donor liver transplantation: impact of the recipient's disease. Liver Transpl. 2001;7:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Ferret PJ, Hammoud R, Tulliez M, Tran A, Trébéden H, Jaffray P, Malassagne B, Calmus Y, Weill B, Batteux F. Detoxification of reactive oxygen species by a nonpeptidyl mimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001;33:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Aram G, Potter JJ, Liu X, Wang L, Torbenson MS, Mezey E. Deficiency of nicotinamide adenine dinucleotide phosphate, reduced form oxidase enhances hepatocellular injury but attenuates fibrosis after chronic carbon tetrachloride administration. Hepatology. 2009;49:911-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Sakurai T, He G, Matsuzawa A, Yu GY, Maeda S, Hardiman G, Karin M. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 414] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 10. | Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1346] [Cited by in RCA: 1680] [Article Influence: 93.3] [Reference Citation Analysis (1)] |
| 11. | Fukuda K, Asoh S, Ishikawa M, Yamamoto Y, Ohsawa I, Ohta S. Inhalation of hydrogen gas suppresses hepatic injury caused by ischemia/reperfusion through reducing oxidative stress. Biochem Biophys Res Commun. 2007;361:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 319] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 12. | Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol. 2009;64:753-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 13. | Court FG, Wemyss-Holden SA, Morrison CP, Teague BD, Laws PE, Kew J, Dennison AR, Maddern GJ. Segmental nature of the porcine liver and its potential as a model for experimental partial hepatectomy. Br J Surg. 2003;90:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Daemen MA, van 't Veer C, Denecker G, Heemskerk VH, Wolfs TG, Clauss M, Vandenabeele P, Buurman WA. Inhibition of apoptosis induced by ischemia-reperfusion prevents inflammation. J Clin Invest. 1999;104:541-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 439] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Sugawara Y, Makuuchi M, Takayama T, Imamura H, Dowaki S, Mizuta K, Kawarasaki H, Hashizume K. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001;192:510-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 164] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Niiya T, Murakami M, Aoki T, Murai N, Shimizu Y, Kusano M. Immediate increase of portal pressure, reflecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepatobiliary Pancreat Surg. 1999;6:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Sato Y, Koyama S, Tsukada K, Hatakeyama K. Acute portal hypertension reflecting shear stress as a trigger of liver regeneration following partial hepatectomy. Surg Today. 1997;27:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Bautista AP, Spitzer JJ. Superoxide anion generation by in situ perfused rat liver: effect of in vivo endotoxin. Am J Physiol. 1990;259:G907-G912. [PubMed] |
| 19. | Zhang F, Tong L, Qiao H, Dong X, Qiao G, Jiang H, Sun X. Taurine attenuates multiple organ injury induced by intestinal ischemia reperfusion in rats. J Surg Res. 2008;149:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2652] [Cited by in RCA: 2721] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 21. | Zhong Z, Connor HD, Froh M, Bunzendahl H, Lind H, Lehnert M, Mason RP, Thurman RG, Lemasters JJ. Free radical-dependent dysfunction of small-for-size rat liver grafts: prevention by plant polyphenols. Gastroenterology. 2005;129:652-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Sato Y, Tsukada K, Hatakeyama K. Role of shear stress and immune responses in liver regeneration after a partial hepatectomy. Surg Today. 1999;29:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Man K, Lo CM, Ng IO, Wong YC, Qin LF, Fan ST, Wong J. Liver transplantation in rats using small-for-size grafts: a study of hemodynamic and morphological changes. Arch Surg. 2001;136:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Itasaka H, Suehiro T, Wakiyama S, Yanaga K, Shimada M, Sugimachi K. Significance of hyaluronic acid for evaluation of hepatic endothelial cell damage after cold preservation/reperfusion. J Surg Res. 1995;59:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Eriksson S, Fraser JR, Laurent TC, Pertoft H, Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983;144:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 213] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Couvelard A, Scoazec JY, Feldmann G. Expression of cell-cell and cell-matrix adhesion proteins by sinusoidal endothelial cells in the normal and cirrhotic human liver. Am J Pathol. 1993;143:738-752. [PubMed] |
| 27. | Fondevila C, Hessheimer AJ, Taurá P, Sánchez O, Calatayud D, de Riva N, Muñoz J, Fuster J, Rimola A, García-Valdecasas JC. Portal hyperperfusion: mechanism of injury and stimulus for regeneration in porcine small-for-size transplantation. Liver Transpl. 2010;16:364-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2466] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 29. | Cardinal JS, Zhan J, Wang Y, Sugimoto R, Tsung A, McCurry KR, Billiar TR, Nakao A. Oral hydrogen water prevents chronic allograft nephropathy in rats. Kidney Int. 2010;77:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 136] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q, Chang Y, Liu Q, Sun X, Wu M. The protective role of hydrogen-rich saline in experimental liver injury in mice. J Hepatol. 2011;54:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Kaizu T, Nakao A, Tsung A, Toyokawa H, Sahai R, Geller DA, Murase N. Carbon monoxide inhalation ameliorates cold ischemia/reperfusion injury after rat liver transplantation. Surgery. 2005;138:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 77] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 32. | Kaizu T, Ikeda A, Nakao A, Tsung A, Toyokawa H, Ueki S, Geller DA, Murase N. Protection of transplant-induced hepatic ischemia/reperfusion injury with carbon monoxide via MEK/ERK1/2 pathway downregulation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G236-G244. [PubMed] |
| 33. | Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |