INTRODUCTION
Endoscopic mucosal resection (EMR) is one of the standard techniques for removing superficial early-stage cancers in the gastrointestinal tract with low invasiveness compared with open surgery[1,2]. In the case of the esophagus, EMR is a useful operation for removing m1 (epithelial cancers restricted to the intraepithelial layer) and m2 (mucosal cancers with invasion of intermediate depth) carcinomas without esophageal reconstruction. To permit large en bloc resection of cancerous lesions without technical restriction of EMR, endoscopic submucosal dissection (ESD) was developed as a new mucosal resection technique with development of endoscopic devices[3-5]. Early-stage esophageal cancers are removed using a hook-knife, which is an endoscopic device used to perform ESD[6]. ESD is a more impressive operative procedure than EMR for reducing the recurrence of esophageal squamous cell carcinoma[7]. Although these developments of endoscopic surgery contribute low invasive cancer resection for patients suffering from esophageal cancer, there are some postoperative complications after ESD. Esophageal stenosis is a major complication caused by endoscopic resection, and the stenosis is significantly associated with the mucosal defect involving over three-fourths circumference of the esophagus lumen[8]. The esophageal stenosis caused by aggressive ESD considerably affects the patient’s quality of life, since the patient has to receive treatment with balloon dilation or temporary stents to expand the esophageal stricture with further inflammation and postoperative pain. These physical dilations carry a risk of perforation[9]. Treatment with anti-inflammatory drugs after endoscopic resection may be an effective therapy for preventing stricture after ESD[10,11].
With recent progression of tissue engineering and regenerative medicine, there are some reports proposing new technologies using biological scaffolds[12] or cell suspensions[13,14] for preventing the esophageal stenosis caused by mucosal defects. We have developed a novel method of endoscopic transplantation of autologous epithelial cell sheets immediately after ESD to prevent the postoperative complications[15]. Transplantable tissue-like epithelial cell grafts are fabricated by cell sheet technology. On the basis of results obtained with canine and porcine models, we have used this technology with human patients since 2008.
CELL SHEET TECHNOLOGY FOR REGENERATION OF ESOPHAGEAL MUCOSA
Tissue engineering by using cell sheet technology
The concept of tissue engineering was originally proposed by Langer et al[16]. Conventionally, biodegradable polymer scaffolds have been used to reconstruct tissue architecture, and cells are seeded on them. The technique should be useful to reconstruct bone and cartilage, having a large amount of extracellular matrices (ECM) and few cells. However, scaffold-based tissue engineering would not be optimal for the regeneration of parenchymal tissues filled with a huge amount of cells and faint ECM. Therefore, we have proposed an alternative method of tissue reconstruction by using transplantable cell sheets to eliminate biodegradable scaffolds.
In order to fabricate transplantable cell sheets without any scaffolds, we employ temperature-responsive culture surfaces, onto which poly (N-isopropylacrylamide) is covalently immobilized to control cell adhesion/detachment with a simple temperature change[17]. Cells adhere, spread, and proliferate on temperature-responsive surfaces at 37 °C, which is the normal temperature for mammalian cell culture. By reducing temperature below 32 °C, cells spontaneously detach from the surfaces without proteolytic enzyme such as trypsin, since the grafted polymer becomes hydrophilic. When the temperature is reduced after cells reach confluence, all the cells are harvested as a single contiguous cell sheet. Because this technique eliminates trypsin for cell harvest, all the cell membrane proteins including growth factor receptors, ion channels and cell-to-cell junction proteins are intact even after cell and cell sheet harvest. Furthermore, ECM deposited during cell culture is retained under cell sheets[18]. Therefore, cell sheets easily integrate to transplanted sites. With this technique, many types of epithelial cell sheets are fabricated and subjected to regenerative medicine of skin[19], cornea[20], urinary bladder[21], and trachea[22].
Cultured epithelial cells for clinical application
Cultured autologous epidermal keratinocytes have been used as cell grafts to treat burns as the first therapy using cultured cells[23]. A transplantable epidermal cell graft is fabricated using murine 3T3 feeder layer cells[24]. Human keratinocytes co-cultured with 3T3 feeder cells proliferate and show stratification as in vivo with characteristics of native epithelial tissues including tonofilaments and desmosomes. Interestingly, human epidermal keratinocytes can be serially cultured to more than 150th passages in vitro[25]. The cultivation of human keratinocytes achieved clinical treatments for severe burns[26] and giant congenital nevi[27] by transplantation of cultured autologous keratinocyte grafts. Moreover, allogeneic keratinocyte grafts are also used for skin ulcers[28]. In these cases, keratinocyte sheets are harvested with bacteria-derived dispase treatment.
Oral mucosal epithelium is also stratified squamous epithelium, and on the basis of the feeder layer method, the epithelial cells have been used as a cell source to fabricate transplantable epithelial cell grafts for treating oral mucosa[29-31]. Moreover, since extraction of oral mucosal tissue is easy, cultured human oral mucosal epithelial cells are selected as useful cell grafts for ectopic transplantation, such as skin[30,32]. We have successfully applied cultured autologous oral mucosal epithelial cell sheets fabricated on temperature-responsive culture inserts to treat human patient cornea suffering from limbal epithelial stem cell deficiency[33,34]. Furthermore, we utilized cultured autologous oral mucosal epithelial cell sheets for bladder reconstruction by the transplantation onto a demucosalized gastric flap in a canine model[35]. We believe that cultured oral mucosal epithelial cell sheets fabricated on temperature-responsive surfaces are useful for treatment of many types of epithelial diseases as well as reconstruction of defective tissues.
Fabrication of transplantable oral mucosal epithelial cell sheets in the clinical setting
To promote healing of esophageal ulcers after ESD for the prevention of postoperative complications, we performed endoscopic transplantation of cultured autologous oral mucosal epithelial cell sheets in the clinical setting. From the safety view point, animal-derived materials, such as bovine serum and 3T3 feeder cells should be eliminated as much as possible. For example, human embryonic stem cells cultured under a typical culture condition using animal-derived serum replacements and mouse feeder layer express an immunogenic nonhuman sialic acid[36]. Moreover, cells co-cultured with a mouse feeder layer are classified as xenogeneic products by United States Food and Drug Administration.
We reported that oral mucosal epithelial cells make transplantable stratified cell sheets on temperature-responsive cell culture inserts having micropores to supply culture medium from the basal cell surfaces even without a 3T3 feeder layer and allogeneic serum[37]. The method can, in principle, permit the cultured human oral mucosal epithelial cell sheets to be used as an autologous epithelial cell sheet graft for human patients[38]. Therefore, the autologous epithelial cell sheets are not, in principle, rejected after transplantation onto the esophageal ulcer and may be replaced by native epithelium in the esophagus. Moreover, the culture medium for the preparation of cultured oral mucosal epithelial cell sheets is modified to exclude laboratory reagents as much as possible for the clinical use[39].
Transplantable epithelial cell sheets are fabricated in a cell-processing center (CPC) with clean rooms according to the good manufacturing practice guideline in the clinical study. The cleanliness of the CPC is controlled by air conditioning systems using high efficiency particulate air filters, and standard operation procedures (SOP) are documented to keep a sterile environment of cell culture rooms in the CPC. The environment of the CPC is continuously monitored with various monitors including aerosol particles, temperature, and humidity, and various sterilization tests are performed to validate the cleanliness of the environment. In these tests culture rooms in the CPC were successfully kept sterile[40,41]. SOP of the culture method was also documented for the prevention of human error of operators to fabricate cell sheets highly reproducibly. Consequently, transplantable human epithelial cell sheets were successfully fabricated in a clinical study to treat ten human patients, and validation tests to demonstrate the quality assurance were performed one day before the ESD and transplantation.
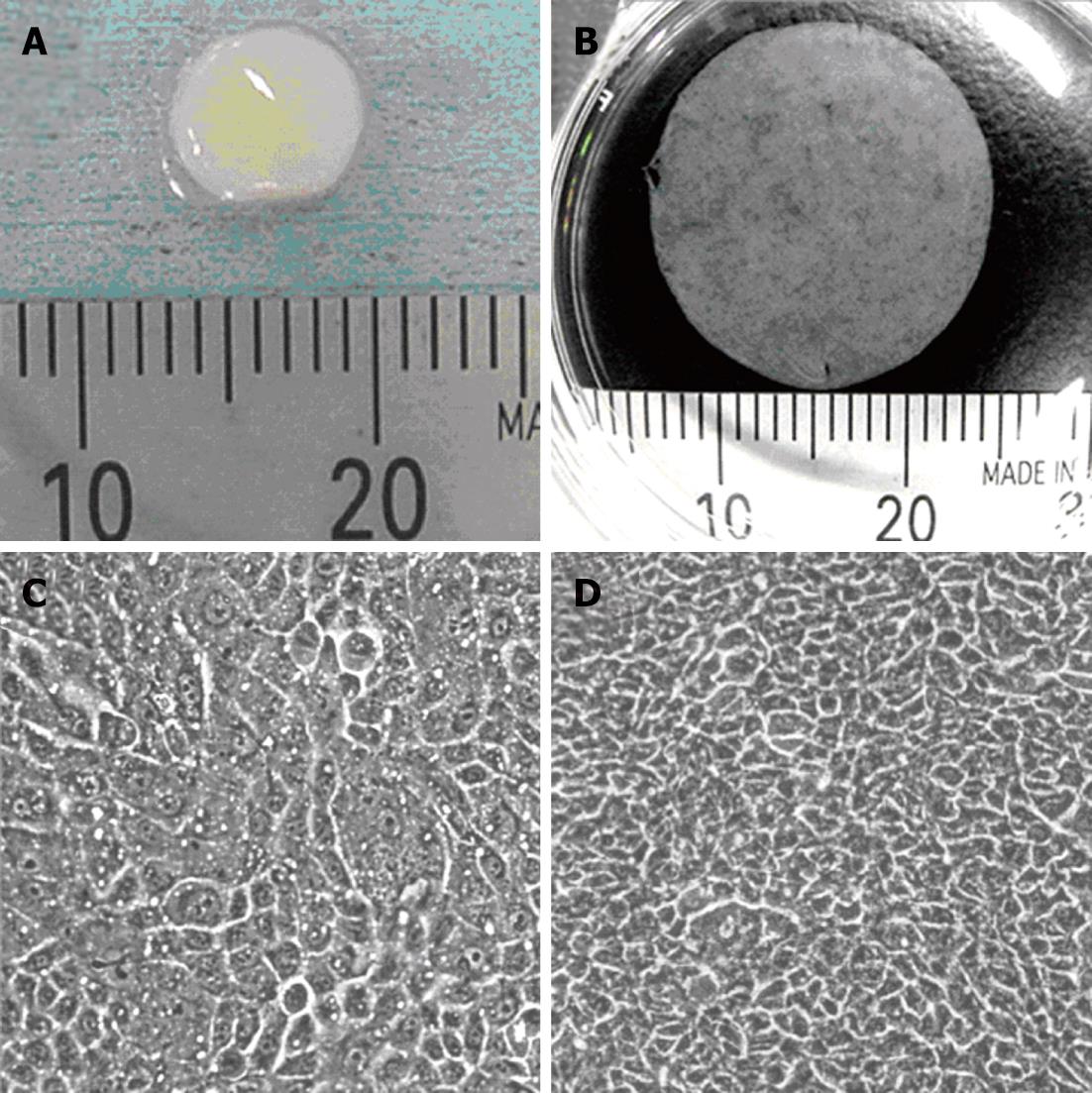
From the average yield of the cells isolated from excised human oral mucosal tissues of healthy volunteer donors, we concluded that approximately 0.3 cm2 of oral mucosal tissue is needed to fabricate one transplantable epithelial cell sheet having an area of 2.8 cm2 (Figure 1A and B). The degree of cell stratification is an important point to enable reproducible cell sheet harvesting from temperature-responsive culture inserts. Figure 1C and D show the growth of seeded human oral mucosal epithelial cells. These cells became confluent on the surface of temperature-responsive culture inserts, and elevation of cell density accompanied with cell stratification was observed after a further 5 d.
Figure 1 Transplantable oral mucosal epithelial cell sheet.
A: Swine buccal oral mucosal tissue taken by punch biopsy; B: Human oral mucosal epithelial cell sheet harvested from temperature-responsive cell culture insert; C: Cultured human oral mucosal epithelial cells just become confluent on the surface of temperature responsive culture insert; D: The oral mucosal epithelial cells cultured for more than 5 d.
Endoscopic transplantation of epithelial cell sheet onto esophageal ulcer immediately after ESD
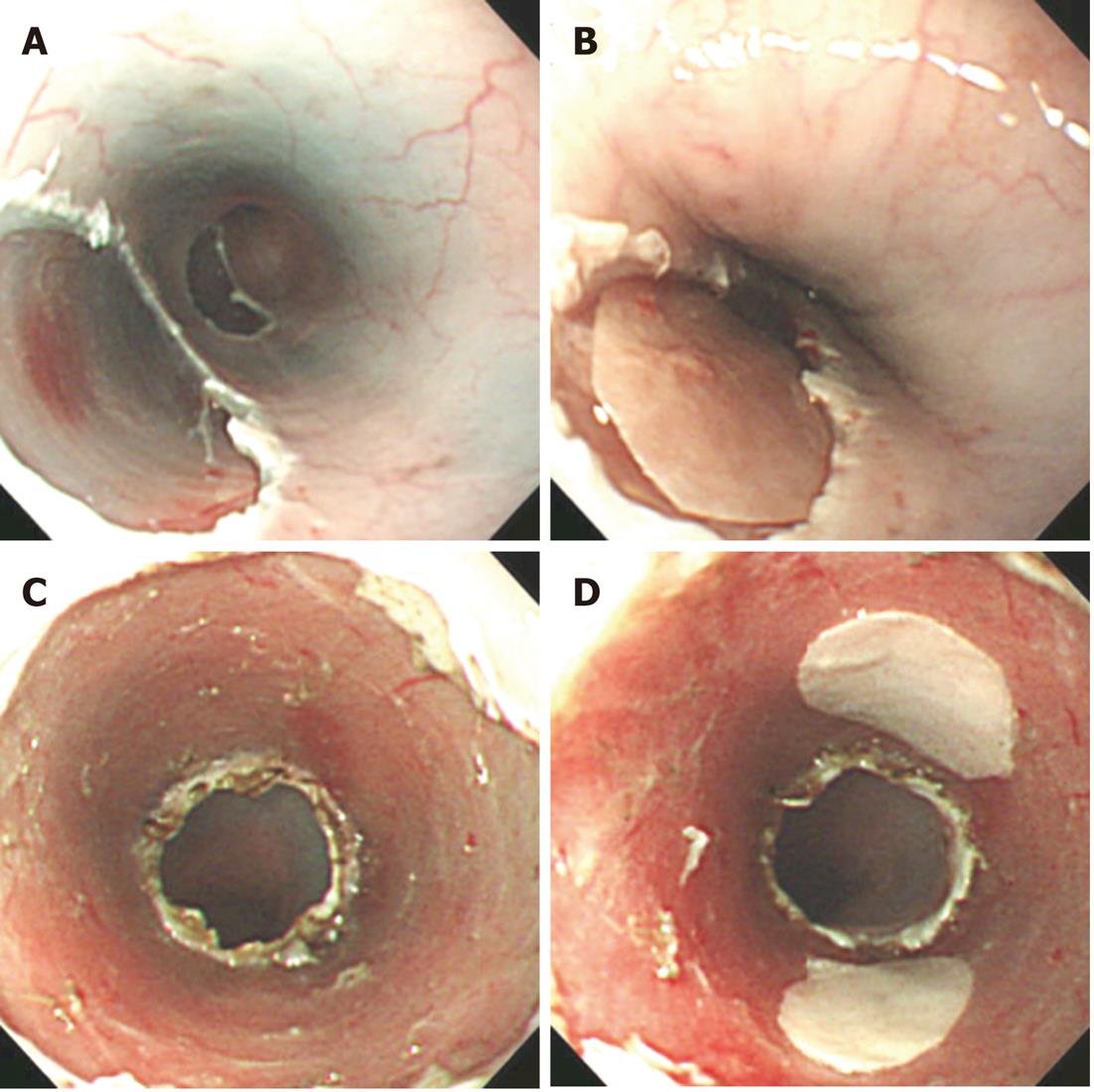
Since 2008, we have performed a clinical study using cultured autologous human oral mucosal epithelial cell sheets to treat esophageal ulcer after endoscopic resection of a mucosal neoplasm. Endoscopic transplantation of the cultured epithelial cell sheets is performed using a support membrane to grasp by endoscopic forceps[42]. Epithelial cell sheets transplanted onto esophageal ulcer with a support membrane in a swine model are shown in Figure 2. One epithelial cell sheet fabricated on temperature-responsive culture insert (23 mm in diameter) covers the ulcer surface involving approximately one-third circumference of the esophagus lumen (Figure 2A and B), and three or four epithelial cell sheets were needed to cover the full circumferential ulcer (Figure 2C and D).
Figure 2 Transplantation of oral mucosal epithelial cell sheets on esophageal ulcer surface in animal model.
A: Swine esophageal ulcer after endoscopic mucosal resection (EMR); B: Swine oral mucosal epithelial cell sheet transplanted on esophageal ulcer after EMR; C: Swine esophageal ulcer after endoscopic submucosal dissection (ESD); D: Two swine oral mucosal epithelial cell sheets transplanted on esophageal ulcer after ESD.
Although more research is needed for understanding the mechanism and correlation between ulcer size and number of transplanted epithelial cell sheets for preventing inflammation and postoperative stenosis, serial cultivation is a useful method of preparation of epithelial cells from small tissue for fabricating cell grafts to transplant onto a large ulcer after aggressive ESD. Serial culture using low calcium concentration medium (LCM) is a useful method to amplify a cell number of stratified squamous epithelial cells without using 3T3 feeder cells[43,44]. Interestingly, although normal human epidermal keratinocytes cultured in LCM show some mesenchymal-like phenotype, the keratinocytes can express the differentiation marker and stratify in differentiation-inducing culture conditions[45]. Since the conventional culture medium for serial cultivation of epithelial cells typically includes animal-derived material, the medium is not useful for clinical use. The modification of the LCM would be important for fabricating epithelial cell sheets to transplant onto large esophageal ulcers after aggressive ESD without any complications.
ACKNOWLEDGMENTS
The author thanks Mr. Mizutani M, Mr. Ichikura S, Mr. Sugiyama H, Ms. Nohmi Y, Izawa Y and Oguri S (CellSeed Inc., Tokyo, Japan) for their useful comments and technical criticism.