Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5084
Revised: May 9, 2012
Accepted: May 13, 2012
Published online: September 28, 2012
AIM: To determine whether alteration of the mitochondria DNA (mtDNA) copy number and its oxidative damage index (mtDNA∆CT) can be detected by analysis of peripheral blood cells in hepatitis C virus (HCV)-infected patients.
METHODS: This study enrolled two groups of patients aged 40-60 years: a control group and an HCV-infected group in Department of Gastroenterology and Hepatology in Changhua Christian Hospital. Patients with co-infection with hepatitis B virus or human immunodeficiency virus, autoimmune disease, malignant neoplasia, pregnancy, thyroid disease, or alcohol consumption > 40 g/d were excluded. HCV-infected patients who met the following criteria were included: (1) positive HCV antibodies for > 6 mo; (2) alanine aminotransferase (ALT) levels more than twice the upper limit of normal on at least two occasions during the past 6 mo; and (3) histological fibrosis stage higher than F1. The mtDNA copy number and oxidative damage index of HCV mtDNA (mtDNA∆CT) were measured in peripheral blood leukocytes. The association between mtDNA copy number and mtDNA∆CT was further analyzed using clinical data.
RESULTS: Forty-seven normal controls (male/female: 26/21, mean age 50.51 ± 6.15 years) and 132 HCV-infected patients (male/female: 76/61, mean age 51.65 ± 5.50 years) were included in the study. The genotypes of HCV-infected patients include type 1a (n = 3), type 1b (n = 83), type 2a (n = 32), and type 2b (n = 14). Liver fibrosis stages were distributed as follows: F1/F2/F3/F4 = 1/61/45/25 and activity scores were A0/A1/A2/A3 = 7/45/55/25. There were no age or gender differences between the two groups. HCV-infected patients had higher hepatitis activity (aspartate transaminase levels 108.77 ± 60.73 vs 23.19 ± 5.47, P < 0.01; ALT levels 168.69 ± 93.12 vs 23.15 ± 9.45, P < 0.01) and lower platelet count (170.40 ± 58.00 vs 251.24 ± 63.42, P < 0.01) than controls. The mtDNA copy number was lower in HCV-infected patients than in controls (173.49 vs 247.93, P < 0.05). The mtDNA∆CT was higher in HCV-infected patients than in controls (2.92 vs 0.64, P < 0.05). To clarify the clinical significance of these results in HCV-infected patients, their association with different clinical parameters among HCV-infected patients was analyzed. A negative association was found between mtDNA copy number and elevated aspartate transaminase levels (r = -0.17, P < 0.05). Changes in mtDNA copy number were not associated with HCV RNA levels, HCV genotypes, liver fibrosis severity, or inflammatory activity in the liver biopsy specimen. However, a correlation was observed between mtDNA∆CT and platelet count (r = -0.22, P < 0.01), HCV RNA level (r = 0.36, P < 0.01), and hepatitis activity (r = 0.20, P = 0.02). However, no difference in the change in mtDNA∆CT was observed between different fibrosis stages or HCV genotypes.
CONCLUSION: Oxidative stress and mtDNA damage are detectable in patient’s peripheral leukocytes. Increased leukocyte mtDNA∆CT correlates with higher HCV viremia, increased hepatitis activity, and lower platelet count.
- Citation: Yen HH, Shih KL, Lin TT, Su WW, Soon MS, Liu CS. Decreased mitochondrial deoxyribonucleic acid and increased oxidative damage in chronic hepatitis C. World J Gastroenterol 2012; 18(36): 5084-5089
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5084.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5084
Mitochondria play an important role in energy metabolism of cells[1,2]. Human mitochondrial DNA (mtDNA) is prone to oxidative injury because mitochondria generate reactive oxygen species during adenosine triphosphate (ATP) synthesis[3]. Unlike genomic DNA, mitochondria are unique in that they possess multiple copies of mtDNA. Alteration or depletion of mtDNA copy number in tissues or serum has been linked to various diseases[4-7] associated with different oxidative stress conditions.
Hepatitis C virus (HCV) is an important cause of chronic viral hepatitis. Without treatment, patients progress to cirrhosis and eventually develop hepatocellular carcinoma. Several mechanisms have been postulated for HCV-induced liver injury, including immunological hepatocellular damage, cytotoxicity of different viral products, and increased oxidative stress[8]. In previous studies, HCV infection (especially by HCV genotype 1) has also been found to be associated with decreased hepatocyte mtDNA[9]. Some studies suggested hepatocyte mtDNA depletion in HCV/human immunodeficiency virus (HIV) co-infected patients[7,10-12]. However, these studies were limited to a small number of cases and did not include a group of normal controls. Alterations of mtDNA and oxidative stress in HCV-infected patients have been mainly studied in tissues; however, recent observations suggested that use of peripheral blood cells may also be informative[4,5]. Cardin et al[13] observed increased oxidative DNA damage in peripheral lymphocytes of HCV-infected patients, and Levent et al[8] observed increased oxidative stress in erythrocytes of chronic HCV-infected patients. Thus, we hypothesized that alteration of mtDNA copy number and oxidative damage of mtDNA may be detected by analyzing peripheral blood leukocytes of chronic HCV-infected patients.
The control group comprised 47 healthy persons aged 40-60 years who had been included in our previous study of mtDNA copy number in human leukocytes[5]. The 132 HCV-infected patients included in this study were aged 40-60 years, had undergone liver biopsy, and had received interferon (IFN)-based therapy at the Department of Gastroenterology and Hepatology of Changhua Christian Hospital between October 2003 and July 2009. Exclusion criteria were coinfection with hepatitis B virus or HIV, autoimmune disease, malignant neoplasia, pregnancy, thyroid disease, alcohol consumption > 40 g/d, intravenous drug abuse, and any contraindication for pegylated IFN-α or ribavirin therapy. The study was approved by the Institutional Review Board of Changhua Christian Hospital and conducted in accordance with institutional guidelines.
Criteria for chronic hepatitis C that required combined IFN-based therapy were as follows: (1) patients demonstrating positive HCV antibodies for > 6 mo; (2) patients with alanine aminotransferase (ALT) levels more than twice the upper limit of normal on at least two occasions during the 6 mo prior to enrolment into the study; and (3) patients with a histological fibrosis stage of more than F1 (F0: no fibrosis; F1: portal fibrosis without septa; F2: portal fibrosis with rare septa; F3: numerous septa without cirrhosis; and F4: liver cirrhosis) in the METAVIR scoring system[14].
Any history of hypertension, diabetes, and hyperlipidemia was recorded. Hypertension was defined as systolic blood pressure > 140 mmHg or use of antihypertensive drugs; diabetes mellitus as hemoglobin A1c > 6.5% or use of oral antidiabetic agents/insulin injection; and hyperlipidemia as total cholesterol level of > 200 mg/dL, low-density lipoprotein cholesterol level of > 130 mg/dL, or triglyceride level of > 200 mg/dL.
Biochemical and hematological tests were performed prior to IFN therapy using commercially available assays. HCV genotype was assigned at baseline by sequencing using a Beckman CEQ 8000 Sequencer (Beckman Coulter, Fullerton, CA, United States). Serum HCV RNA was measured using a quantitative assay (COBAS AMPLICOR Hepatitis C Virus Test, version 2.0; Roche, Branchburg, NJ, United States) with a detection limit of 50 IU/mL. The degree of liver fibrosis (F0: no fibrosis; F1: portal fibrosis without septa; F2: portal fibrosis with rare septa; F3: numerous septa without cirrhosis; and F4: liver cirrhosis) and the intensity of liver necroinflammation (A0: no activity, A1: mild activity, A2: moderate activity, and A3: severe activity) were graded according to the METAVIR scoring system[14] by a single expert pathologist.
Leukocytes were separated from the blood sample taken prior to IFN therapy, and DNA was isolated as described previously[5]. Fluorescence-based quantitative polymerase chain reaction (FQ-PCR) was performed using the LightCycler FastStart DNA Master Kit (Roche Molecular Biochemicals, Penzberg, Germany) to measure total copy number in leukocyte mtDNA[15]. In brief, the threshold cycle number of mitochondrial ND1 and β-globin genes was determined in leukocytes of HCV-infected patients. mtDNA copy number was calculated according to a previously described method[5]. The total amount of mtDNA in leukocytes was expressed as copy number/cell. In addition, the mtDNA oxidative damage index (mtDNA∆CT) was evaluated as described previously[15]. In brief, human 8-oxoguanine DNA glycosylase (hOGG1) was used to remove 8-hydroxydeoxyguanosine (8-OHdG) from the damaged DNA template in which lesion sites were formed. Amplification in FQ-PCR was then blocked, and the cycle threshold (Ct) value increased. Thus, ∆Ct value was determined by calculating the difference between values before and after treatment with hOGG1. A higher mtDNA∆CT value indicated the presence of more 8-OHdG in mtDNA, and thus greater oxidative damage.
Continuous variables in the groups were compared using the Student’s t test or Mann-Whitney U test as appropriate. Categorical variables were evaluated using the χ2 test. Relationships between two continuous variables were evaluated by Pearson correlation. Differences between the two groups were considered significant when the P value was < 0.05. Statistical analyses were performed using MedCalc software version 11.5 (MedCalc Software bvba, Broekstraat 52, 9030 Mariakerke, Belgium).
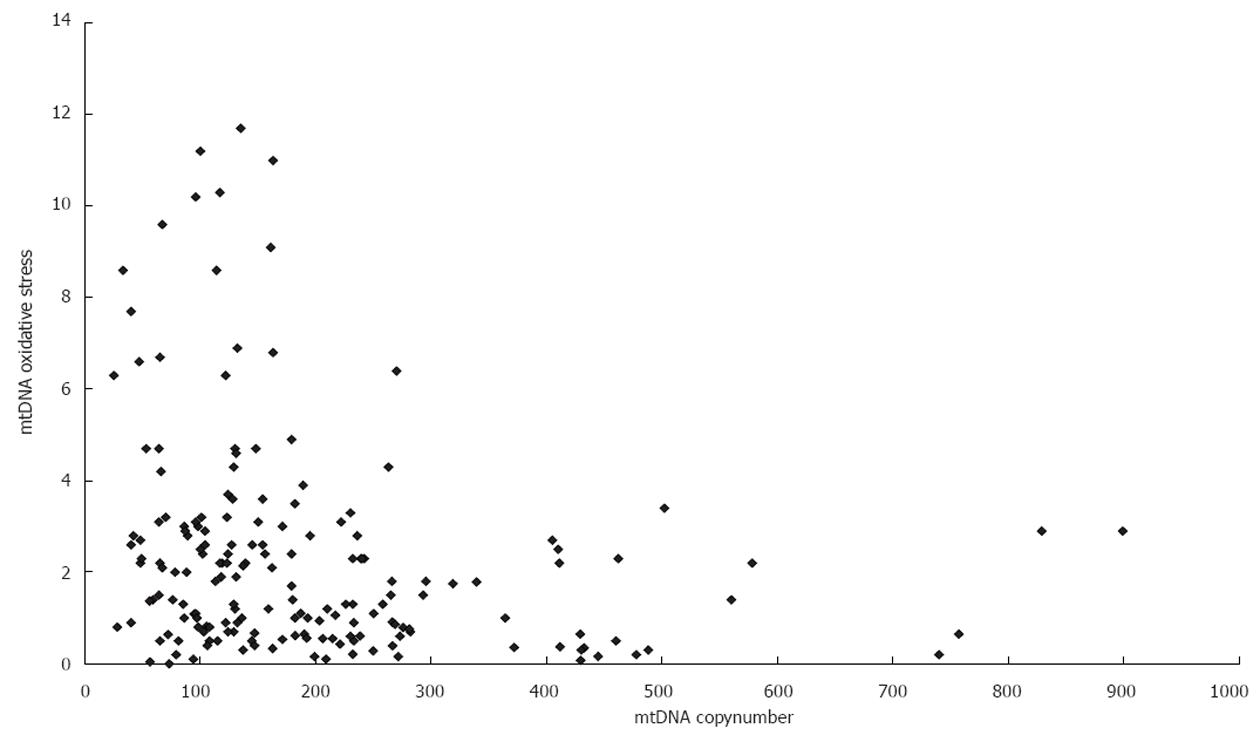
Clinical features of the controls and HCV-infected patients are shown in Table 1. Genotypes of HCV-infected patients include type 1a (n = 3), type 1b (n = 83), type 2a (n = 32), and type 2b (n = 14). The distribution of liver fibrosis stages was as follows: F1/F2/F3/F4 (n = 1/61/45/25) and activity score A0/A1/A2/A3 (n = 7/45/55/25). A negative association (r = -0.2277, P < 0.01) was found between mtDNA content and the extent of mtDNA oxidative stress (measured as mtDNA∆CT) (Figure 1). There were no age or gender differences between the patient and control groups. A lower platelet count and higher hepatitis activity were found in HCV-infected patients (P < 0.01). In addition, the peripheral blood lymphocyte mtDNA copy number in HCV-infected patients was lower (173.5 vs 247.93, P < 0.01) and mtDNA∆CT was higher (2.92 vs 0.67, P < 0.01) than those in the control group.
| Normal (n = 47) | HCV infection (n = 132) | P value | |
| Age (yr) | 50.51 ± 6.15 | 51.65 ± 5.50 | 0.24 |
| Gender (male/female) | 26/21 | 71/61 | 0.99 |
| Smoking (n) | 9 | 28 | 0.93 |
| Hypertension (n) | 1 | 30 | < 0.01 |
| Hyperlipidemia (n) | 4 | 21 | 0.31 |
| DM (n) | 0 | 35 | < 0.01 |
| WBC (103/mL) | 5.82 ± 1.48 | 5.75 ± 1.57 | 0.85 |
| Hb (g/dL) | 13.67 ± 1.77 | 14.49 ± 1.71 | 0.75 |
| Platelet (103/mL) | 251.24 ± 63.42 | 170.40 ± 58.00 | < 0.01 |
| AST (IU/L) | 23.19 ± 5.47 | 108.77 ± 60.73 | < 0.001 |
| ALT (IU/L) | 23.15 ± 9.45 | 168.69 ± 93.12 | < 0.001 |
| mtDNA | 247.93 ± 135.46 | 173.49 ± 147.40 | < 0.01 |
| mtDNA∆CT | 0.67 ± 0.48 | 2.92 ± 2.50 | < 0.01 |
To clarify further the clinical significance of alterations in mtDNA copy number in HCV-infected patients, their association with different clinical parameters was analyzed (Table 2). A negative association was found with elevated aspartate transaminase (AST) levels (r = -0.17, P < 0.05). However, alterations in mtDNA copy number were not associated with the HCV viremia level, HCV genotypes, severity of liver fibrosis, or inflammatory activity in the liver biopsy specimen.
| Characteristics | Correlation statistics | |
| r | P value | |
| Age (yr) | -0.07 | 0.40 |
| Laboratory data | ||
| AST (IU/L) | -0.17 | < 0.05 |
| ALT (IU/L) | -0.07 | 0.38 |
| Hb (g/dL) | 0.14 | 0.09 |
| Platelet count (103/mL) | 0.03 | 0.71 |
| WBC count (103/mL) | 0.08 | 0.34 |
| HCV RNA | -0.10 | 0.47 |
| Liver histology | ||
| Inflammatory activity | -0.06 | 0.45 |
| Fibrosis staging | 0.04 | 0.59 |
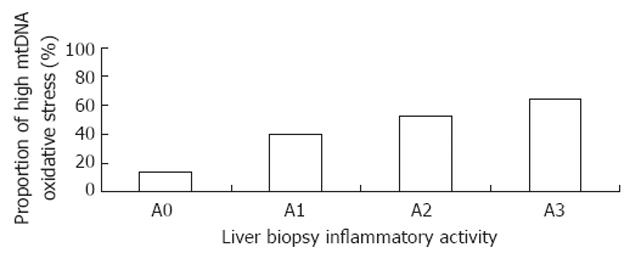
To evaluate the clinical significance of alterations in 8-OHdG content in mitochondrial DNA (measured as mtDNA∆Ct) in HCV-infected patients, their association with different clinical parameters was analyzed (Table 3). Higher mtDNA∆Ct suggested higher mitochondrial oxidative stress. A negative association with mtDNA∆Ct was observed for decreased platelet count (r = -0.22, P < 0.01). Increased mtDNA∆Ct was associated with increased inflammatory activity in liver tissue (r = 0.20, P = 0.02) and the HCV RNA level (r = 0.36, P < 0.01). The trend of mtDNA oxidative damage (low damage: mtDNA∆Ct≤ median mtDNA∆Ct) increased with increased hepatic inflammatory activity (Cochran-Armitage test for trend, P < 0.01) (Figure 2).
| Characteristics | Correlation statistics | |
| r | P value | |
| Age (yr) | 0.16 | 0.06 |
| Laboratory data | ||
| AST (IU/L) | 0.15 | 0.08 |
| ALT (IU/L) | 0.09 | 0.25 |
| Hb (g/dL) | -0.06 | 0.47 |
| Platelet count (103/mL) | -0.22 | < 0.01 |
| WBC count (103/mL) | -0.13 | 0.13 |
| HCV RNA | 0.36 | < 0.01 |
| Liver histology | ||
| Inflammatory activity | 0.20 | 0.02 |
| Fibrosis staging | 0.05 | 0.61 |
This was the first study to detect and compare differences in mtDNA copy number and mtDNA∆CT between a normal population and HCV-infected patients.
Oxidative stress and mitochondrial injury have been postulated as mechanisms in the pathogenesis of HCV-related liver injury and subsequent development of cirrhosis or hepatocellular carcinoma. Previous studies have suggested that protein products of hepatitis C, for example, core, NS5A, and NS3 proteins, may directly induce oxidative injury in hepatocytes[2,16]. These proteins cause Ca2+ uptake into the mitochondria, oxidation of mitochondrial glutathione, and subsequent production of free radicals by the mitochondrial electron transport complex, and induce apoptosis of HCV-infected hepatocytes[2,17]. Consequently, the extent of mitochondrial injury and the severity of oxidative injury exerted in the liver tissue are correlated with the severity of HCV infection[9]. Barbaro et al[9] first studied liver biopsy specimens from 75 HCV-infected patients and 22 normal subjects. Hepatic mtDNA levels were decreased in HCV-infected patients, especially in those with HCV genotype 1, with increased free radical production. These findings were also observed in subsequent studies[18,19]. However, direct measurement of such changes in the liver tissue is difficult and invasive, whereas measurements in peripheral blood are more attractive because they are easier and less invasive. HCV is also lymphocytotropic[20], which accounts for the many immunological manifestations in HCV-related disease. Peripheral blood leukocytes can therefore be used as surrogates for hepatocytes.
Cardin et al[13] measured 8-OHdG levels as a marker of DNA oxidative damage in circulating leukocytes from 110 HCV-infected patients and 20 normal subjects and found increased levels in the former, showing that increased oxidative DNA can also be detected in peripheral blood leukocytes. In our previous studies[5,21,22], we found that measurement of the peripheral blood mtDNA copy number was a useful marker of oxidative stress and was associated with differences in disease status. The present study suggests that measurement of the peripheral blood leukocyte mtDNA copy number and oxidative stress can be used as a noninvasive marker of mitochondrial damage in HCV-infected patients. Whether or not this information can be utilized as a biomarker to evaluate the activity or severity of HCV-related liver disease remains unclear.
Blood ALT levels and severity of liver inflammation have been found to correlate with 8-OHdG levels in liver tissue, reflecting oxidative DNA damage in hepatocytes[18,19,23]. However, Chuma et al[23] found that 8-OHdG levels in serum tended to correlate with those in liver tissue. In the present study, all HCV-infected patients underwent liver biopsy for pretreatment evaluation, thereby facilitating comparison of mtDNA copy number and oxidative stress in different HCV-infected patient groups in terms of the degree of inflammation or fibrosis. The mtDNA copy number was negatively associated with AST levels, but was not correlated with the severity of liver fibrosis or inflammation. In contrast, mtDNA∆CT was correlated with inflammatory activity and HCV viremia levels. This finding suggested that mtDNA∆CT in peripheral blood leukocytes can be used as a biomarker to evaluate the severity of hepatocyte injury.
This study had several limitations. First, HCV-infected patients eligible for IFN-based treatment were cases with ALT levels two-fold higher than the normal limit or with a biopsy-proven liver fibrosis score more than F1 determined on the basis of national treatment guidelines that were in effect before 2009. Therefore, use of mtDNA copy number and changes in oxidative markers may not be extrapolated to HCV-infected patients with less severe disease (i.e., ALT levels less than two-fold higher than the normal limit or a fibrosis score lower than F1). A recent report[20] suggested that mtDNA damage can be observed even in occult HCV-infected patients. Further studies are still required to evaluate and compare the clinical significance of mtDNA damage between occult and overt HCV-infected patients. Second, subjects in this study were not followed for subsequent measurement of alterations in mtDNA or liver biopsy following IFN-based therapy. Therefore, changes in mtDNA damage over time among different HCV-infected patient groups remain to be investigated. Mutation or depletion of mtDNA has been implicated in development of several cancers[24-26] through changes in the microenvironment of nuclear DNA that lead to progression of cancer[26]. Several reports have suggested that such oxidative DNA damage in the liver tissue can be a risk factor for the subsequent development of hepatocellular carcinoma[4,23,27]. This also supports the finding that antiinflammatory therapy[28,29], in addition to IFN, can decrease the risk of hepatocellular carcinoma. Further studies are still required to evaluate changes in peripheral leukocyte mtDNA damage over time and the risk of the subsequent development of hepatocellular carcinoma.
In conclusion, oxidative stress and mtDNA damage is detectable in peripheral leukocytes of HCV-infected patients. The degree of peripheral leukocyte mtDNA damage correlated with increased hepatic inflammation. However, further studies are required to explore the long-term clinical significance of these findings in HCV patients.
The authors thank Enago (http://www.enago.tw) for the English language review.
The alteration of mitochondria DNA (mtDNA) copy number and its oxidative damage index (mtDNA∆CT) is associated a different disease status. It is not clear whether such changes can be detected by analysis of peripheral blood cells in hepatitis C virus (HCV)-infected patients.
Previous studies utilize the liver tissue to evaluate the mitochondria injury in chronic hepatitis C infected patients. In this study, the authors studied the change of mitochondrial injury of peripheral blood cells among HCV-infected patients and normal controls. The authors also use a simplified method to determine the extent of mitochondrial oxidative injury (measured as mtDNA∆CT) among these two groups of patients. In addition, the authors further study the association of mitochondrial damage with different clinical parameters among HCV-infected patients.
This study aimed to detect and compare differences in mtDNA copy number and mtDNA∆CT between a normal population and HCV-infected patients. Oxidative stress and mitochondrial injury have been postulated as mechanisms in the pathogenesis of HCV-related liver injury and subsequent development of cirrhosis or hepatocellular carcinoma. Direct measurement of such changes in the liver tissue is difficult and invasive. In this study, the authors found suggestions that measurement of the peripheral blood leukocyte mtDNA copy number and oxidative stress can be used as a noninvasive marker of mitochondrial damage in HCV-infected patients.
The study results suggest that measurement of peripheral blood leukocytes for changes in mitochondrial damage provides an alternative approach to assessing hepatitis C related liver injury.
The authors examined the mtDNA copy number and oxidative damage index of mtDNA in the peripheral blood leukocytes of HCV-infected patients in comparison with normal controls. This is an interesting study utilizing unique techniques to determine the copy number of mtDNA and its oxidative damage index. The results are interesting and suggest that oxidative stress and mtDNA damage are detectable in peripheral leukocytes of HCV-infected patients. These findings may lead people to an alternative approach to treatment of HCV infection.
Peer reviewers: Fumio Imazeki, MD, Department of Medicine and Clinical Oncology, Chiba University, 1-8-1 Inohana, Chuo-ku, Chiba 260-8670, Japan; Akihito Tsubota, Assistant Professor, Institute of Clinical Medicine and Research , Jikei University School of Medicine, 163-1 Kashiwa-shita, Kashiwa, Chiba 277-8567, Japan; Hisato Nakajima, MD, Department of Gastroenterology and Hepatology, The Jikei University School of Medicine, 3-25-8, Nishi-Shinbashi, Minato-ku, Tokyo 105-8461, Japan
S- Editor Lv S L- Editor O’Neill M E- Editor Li JY
| 1. | Clay Montier LL, Deng JJ, Bai Y. Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics. 2009;36:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 405] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Piccoli C, Quarato G, Ripoli M, D'Aprile A, Scrima R, Cela O, Boffoli D, Moradpour D, Capitanio N. HCV infection induces mitochondrial bioenergetic unbalance: causes and effects. Biochim Biophys Acta. 2009;1787:539-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Yamada S, Nomoto S, Fujii T, Kaneko T, Takeda S, Inoue S, Kanazumi N, Nakao A. Correlation between copy number of mitochondrial DNA and clinico-pathologic parameters of hepatocellular carcinoma. Eur J Surg Oncol. 2006;32:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 4. | Okochi O, Hibi K, Uemura T, Inoue S, Takeda S, Kaneko T, Nakao A. Detection of mitochondrial DNA alterations in the serum of hepatocellular carcinoma patients. Clin Cancer Res. 2002;8:2875-2878. [PubMed] |
| 5. | Liu CS, Tsai CS, Kuo CL, Chen HW, Lii CK, Ma YS, Wei YH. Oxidative stress-related alteration of the copy number of mitochondrial DNA in human leukocytes. Free Radic Res. 2003;37:1307-1317. [PubMed] |
| 6. | Kim JY, Hwang JM, Ko HS, Seong MW, Park BJ, Park SS. Mitochondrial DNA content is decreased in autosomal dominant optic atrophy. Neurology. 2005;64:966-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | de Mendoza C, Soriano V. The role of hepatitis C virus (HCV) in mitochondrial DNA damage in HIV/HCV-coinfected individuals. Antivir Ther. 2005;10 Suppl 2:M109-M115. [PubMed] |
| 8. | Levent G, Ali A, Ahmet A, Polat EC, Aytaç C, Ayşe E, Ahmet S. Oxidative stress and antioxidant defense in patients with chronic hepatitis C patients before and after pegylated interferon alfa-2b plus ribavirin therapy. J Transl Med. 2006;4:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Barbaro G, Di Lorenzo G, Asti A, Ribersani M, Belloni G, Grisorio B, Filice G, Barbarini G. Hepatocellular mitochondrial alterations in patients with chronic hepatitis C: ultrastructural and biochemical findings. Am J Gastroenterol. 1999;94:2198-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Verucchi G, Calza L, Biagetti C, Attard L, Costigliola P, Manfredi R, Pasquinelli G, Chiodo F. Ultrastructural liver mitochondrial abnormalities in HIV/HCV-coinfected patients receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2004;35:326-328. [PubMed] |
| 11. | Bäuerle J, Laguno M, Mauss S, Mallolas J, Murillas J, Miquel R, Schmutz G, Setzer B, Gatell JM, Walker UA. Mitochondrial DNA depletion in liver tissue of patients infected with hepatitis C virus: contributing effect of HIV infection? HIV Med. 2005;6:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | de Mendoza C, Martin-Carbonero L, Barreiro P, de Baar M, Zahonero N, Rodriguez-Novoa S, Benito JM, González-Lahoz J, Soriano V. Mitochondrial DNA depletion in HIV-infected patients with chronic hepatitis C and effect of pegylated interferon plus ribavirin therapy. AIDS. 2007;21:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Cardin R, Saccoccio G, Masutti F, Bellentani S, Farinati F, Tiribelli C. DNA oxidative damage in leukocytes correlates with the severity of HCV-related liver disease: validation in an open population study. J Hepatol. 2001;34:587-592. [PubMed] |
| 14. | The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15-20. [PubMed] |
| 15. | Lin CS, Wang LS, Tsai CM, Wei YH. Low copy number and low oxidative damage of mitochondrial DNA are associated with tumor progression in lung cancer tissues after neoadjuvant chemotherapy. Interact Cardiovasc Thorac Surg. 2008;7:954-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Chang ML, Chen JC, Chang MY, Yeh CT, Lin WP, Liang CK, Huang SF, Dang KN, Chiu CT, Lin DY. Acute expression of hepatitis C core protein in adult mouse liver: Mitochondrial stress and apoptosis. Scand J Gastroenterol. 2008;43:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Piccoli C, Scrima R, D'Aprile A, Ripoli M, Lecce L, Boffoli D, Capitanio N. Mitochondrial dysfunction in hepatitis C virus infection. Biochim Biophys Acta. 2006;1757:1429-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Fujita N, Sugimoto R, Ma N, Tanaka H, Iwasa M, Kobayashi Y, Kawanishi S, Watanabe S, Kaito M, Takei Y. Comparison of hepatic oxidative DNA damage in patients with chronic hepatitis B and C. J Viral Hepat. 2008;15:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Fujita N, Horiike S, Sugimoto R, Tanaka H, Iwasa M, Kobayashi Y, Hasegawa K, Ma N, Kawanishi S, Adachi Y. Hepatic oxidative DNA damage correlates with iron overload in chronic hepatitis C patients. Free Radic Biol Med. 2007;42:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Bhargava A, Raghuram GV, Pathak N, Varshney S, Jatawa SK, Jain D, Mishra PK. Occult hepatitis C virus elicits mitochondrial oxidative stress in lymphocytes and triggers PI3-kinase-mediated DNA damage response. Free Radic Biol Med. 2011;51:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Pai HH, Liu CS, Chen ER. The growth effects of gamma-ray irradiation on third-stage larvae of Angiostrongylus cantonensis in snail. Kaohsiung J Med Sci. 2001;17:120-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Shen M, Zhang L, Bonner MR, Liu CS, Li G, Vermeulen R, Dosemeci M, Yin S, Lan Q. Association between mitochondrial DNA copy number, blood cell counts, and occupational benzene exposure. Environ Mol Mutagen. 2008;49:453-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Chuma M, Hige S, Nakanishi M, Ogawa K, Natsuizaka M, Yamamoto Y, Asaka M. 8-Hydroxy-2'-deoxy-guanosine is a risk factor for development of hepatocellular carcinoma in patients with chronic hepatitis C virus infection. J Gastroenterol Hepatol. 2008;23:1431-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Haque A, Nishikawa M, Qian W, Mashimo M, Hirose M, Nishiguchi S, Inoue M. Lack of mitochondrial DNA enhances growth of hepatocellular carcinoma in vitro and in vivo. Hepatol Res. 2006;36:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Yu M, Zhou Y, Shi Y, Ning L, Yang Y, Wei X, Zhang N, Hao X, Niu R. Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life. 2007;59:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 26. | Wang Y, Liu VW, Xue WC, Cheung AN, Ngan HY. Association of decreased mitochondrial DNA content with ovarian cancer progression. Br J Cancer. 2006;95:1087-1091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Lee HC, Li SH, Lin JC, Wu CC, Yeh DC, Wei YH. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat Res. 2004;547:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 207] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 28. | Kumada H, Okanoue T, Onji M, Moriwaki H, Izumi N, Tanaka E, Chayama K, Sakisaka S, Takehara T, Oketani M. Guidelines for the treatment of chronic hepatitis and cirrhosis due to hepatitis C virus infection for the fiscal year 2008 in Japan. Hepatol Res. 2010;40:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Xu Z, Zhang M, Lv X, Xiang D, Zhang X, Chen L. The inhibitory effect of celecoxib on mouse hepatoma H22 cell line on the arachidonic acid metabolic pathway. Biochem Cell Biol. 2010;88:603-609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |