Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5072
Revised: March 9, 2012
Accepted: March 20, 2012
Published online: September 28, 2012
AIM: To assess the prognostic value of preoperative 18 fluorodeoxyglucose positron emission tomography (FDG-PET)/computed tomography (CT) in patients with resectable colorectal cancer.
METHODS: One hundred sixty-three patients with resectable colorectal cancer who underwent FDG-PET/CT before surgery were included. Patient data including pathologic stage at presentation, histology, treatment, disease-free survival and the maximum standardized uptake value (SUVmax) of the primary tumor on FDG-PET/CT were retrospectively analyzed. Median follow up duration was 756 (range, 419-1355). The primary end point was disease-free survival.
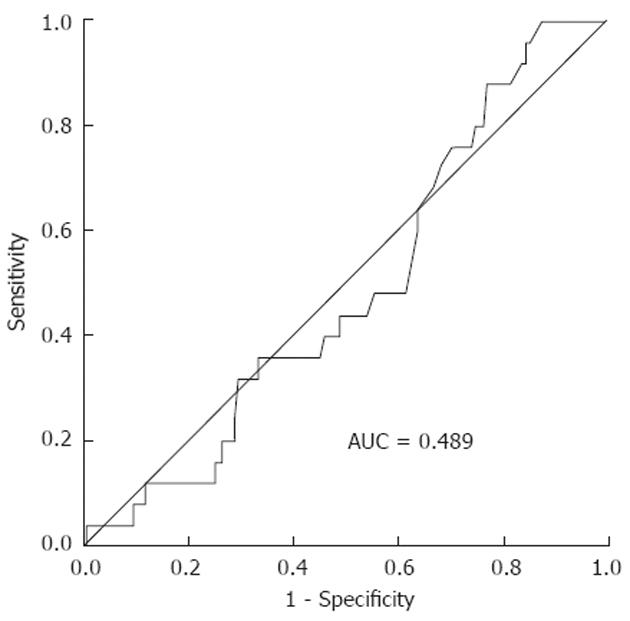
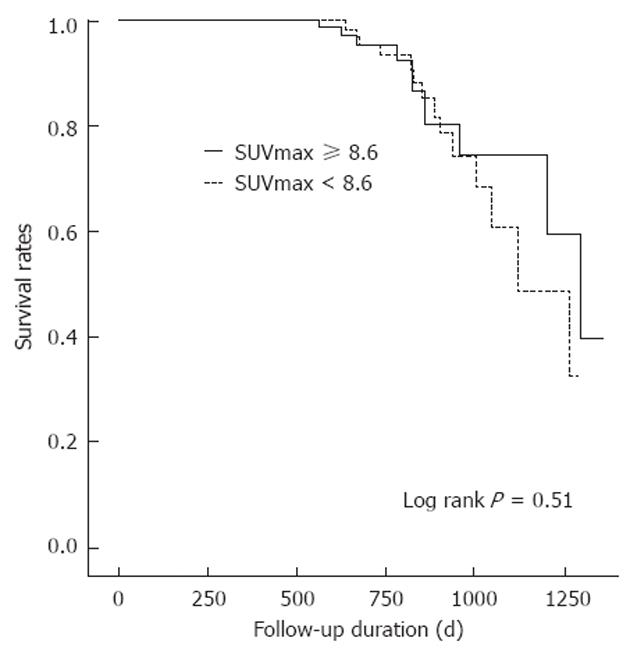
RESULTS: Twenty-five of 163 patients (15.3%) had recurrences. The median SUVmax values of the recurrence and no-recurrence groups were 8.9 (range, 5-24) and 8.2 (range, 0-23, P = 0.998). Receiver operating characteristic (ROC) curve analysis showed no significant association between SUVmax and recurrence (area under the curve = 0.5, P = 0.998, 95% CI: 0.389-0.611). Because a statistically significant value was not found, SUVmax was dichotomized at its median of 8.6. The disease-free survival curve was analyzed using the median SUVmax (8.6) as the cut off. Univariate and multivariate analysis did not provide evidence that disease-free survival rates for the subgroups defined by the median SUVmax were significantly different (P = 0.52, P = 0.25).
CONCLUSION: Our study suggests that the high FDG uptake of primary mass in resectable colorectal cancer doesn’t have a significant relationship with tumor recurrence and disease-free survival.
- Citation: Lee JE, Kim SW, Kim JS, Choi KY, Kang WK, Oh ST, Yoo IR, Kim SH. Prognostic value of 18-fluorodeoxyglucose positron emission tomography-computed tomography in resectable colorectal cancer. World J Gastroenterol 2012; 18(36): 5072-5077
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5072.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5072
Colorectal cancer is the third most common malignancy and the second most frequent cause of cancer-related deaths in the United States, with approximately 51 370 cancer-related deaths in 2010[1]. Despite improvement in diagnosis and treatment modalities, mortality from colon cancer has decreased slightly over the past 30 years. Therefore, methods for identifying patients at high risk of recurrence could influence posttreatment surveillance with the ultimate goal of improving survival.
Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) is a functional imaging method for measurement of tumor glucose use. FDG-PET combined with computed tomography (FDG-PET/CT) was recently introduced and is expected to provide more precise anatomical data along with metabolic information. FDG-PET/CT imaging may be useful for diagnosis of patients with malignant disease, assessment of the extension of disease, detection of tumor recurrence and for monitoring responses to therapy[2-5].
In addition, some researchers have suggested that metabolic activity on FDG-PET may be a predictive marker for some types of malignancies[6-9]. Recent studies have demonstrated that there is a correlation between the maximum standardized uptake value (SUVmax) and survival in patients with gastrointestinal cancers such as esophageal and stomach cancer[10-12]. However, the prognostic value of FDG-PET/CT for colorectal cancer is less well studied. Several studies have found that metabolic activity on FDG-PET/CT is correlated with tumor biology and survival in colon cancer[13-15]. However, these studies were limited by experimental studies, small numbers of patients, and one report focused on stage IV colorectal cancer[14]. Therefore, in this study, we evaluated FDG-PET/CT imaging of patients with resectable colorectal cancer and determined whether metabolic activity is correlated with recurrence and disease-free survival.
We retrospectively reviewed the tumor registry at our institution and identified all patients who were diagnosed with colorectal cancer and underwent curative resection between January 2006 and June 2008. Patients were required to have undergone the FDG-PET/CT study before surgery and to have received no treatment before the FDG-PET/CT. Primary colorectal cancer was confirmed by endoscopic biopsy. Patients who received neoadjuvant treatment or had a previous history of another malignancy were excluded. Patients with colorectal perforation or less than 1 year of follow-up were also excluded. Patients who did not receive adjuvant therapy despite lymph node-positive cancer were excluded from the analysis.
All patients underwent full imaging of the colon by colonoscopy unless an obstructing lesion was encountered. In this instance, colonoscopy was performed after recovery from surgical treatment. All surgeries were performed by two experienced colorectal surgeons.
Data were collected on demographic details, tumor node metastasis (TNM) and Duke staging at presentation, recurrence, histology, survival, disease-free survival, and SUVmax of the primary tumor. Tumor stage was classified according to the sixth edition of the TNM classification of the Union for International Cancer Control[16].
A total of 163 patients (65 women and 98 men) were eligible for this study. The median age of the patients was 60 years (range, 30 to 81 years).
Patient anonymity was preserved and the Institutional Review Board of Seoul St. Mary’s Hospital approved the study. The study protocol was in complete compliance with the Declaration of Helsinki, as revised in Edinburgh in 2000.
All patients were fasted for at least 6 h before FDG injection; their blood glucose levels were determined from capillary blood samples collected before intravenous FDG injection. In our institution, the cut-off blood glucose level that contraindicates FDG injection is 8 mmol/L.
PET images were acquired 1 h after injection of 370-570 MBq of 18F-FDG. The patients were scanned generally from the base of the skull to the upper thighs with their arms raised above their heads.
FDG/PET-CT images were reviewed using fusion software (Syngo, Siemens; Knoxville, TN). An experienced nuclear medicine physician reviewed the PET/CT images. PET, CT and fused whole-body images displayed in axial, coronal, and sagittal planes were available for review. The PET data were also displayed as a rotating maximum-intensity projection. Abnormal FDG uptake, SUVmax of the primary tumor and distant metastases were evaluated.
All patients who were included in this study underwent curative resection based on a standardized technique and principle, which were not changed during the period of this study. Curative resection was defined as achieving complete microscopic/macroscopic clearance with no evidence of metastatic spread (except resectable liver or lung metastasis) at the time of surgical resection. The surgical margins were reported by experienced pathologists and all the margins were clear.
After curative resection of the tumor, adjuvant chemotherapy or chemoradiotherapy were indicated for all patients with lymph node-positive colorectal cancer. Patients were scheduled for follow-up visits every 3-6 mo for the first 2 years, every 6-12 mo for up to 4 years, and annually thereafter. Follow-up evaluations included physical examinations at each visit, analysis of carcinoembryonic antigen (CEA) levels, abdominal and pelvic CT scans, and chest X-rays. A colonoscopy was performed 1 year after resection and then as indicated clinically. Abnormal physical findings or laboratory results mandated further screening with ultrasonography, CT, magnetic resonance imaging, or FDG-PET/CT, as indicated according to the clinician’s decision.
In the previous study, the 2-year survival rates for an SUVmax of less than 10 and an SUVmax of 10 or more were 90% and 65%, respectively[14]. Based on the findings of our previous report, we hypothesized that the high SUVmax group would have a higher recurrence rate (the assumed odds ratio was 3 or more). The assumed odds ratio was decided based on the previous study for other malignancies, such as lung cancer and stomach cancer. We calculated that 163 patients would be required to detect these differences with an alpha level of 0.05 and a power of 0.80.
The predictive value of SUVmax for tumor recurrence was determined by analysis of the area under the receiver operating characteristic (ROC) curve. Because a statistically significant value was not found, SUVmax was dichotomized at its median of 8.6. The significance of the predictive value was analyzed by log-rank testing in univariate analysis and by Cox proportional hazards regression testing in multivariate analysis.
For statistical analysis, group means were compared using the Mann-Whitney or χ2 tests, and disease-free survival was evaluated using a Kaplan-Meier survival analysis. For all tests, a P value of < 0.05 was considered statistically significant. All statistical analysis were performed using the Statistical Package for Social Sciences (SPSS), version 16 (SPSS, Chicago, IL).
The characteristics of the 163 patients enrolled in this study are shown in Table 1. The pathologic tumor stages were stageIin 23 patients, stage II in 67 patients, stage III in 64 patients, and stage IV in nine patients. Nine patients with stage IV tumors had metastases confined to the liver. Metastatic nodules on the liver numbered 1 or 2. The median size of the metastatic nodules was 1.2 cm (range, 0.5-2.5 cm). During the follow-up, recurrence was observed in 25 of 163 patients (15.3%). Three patients died with a median follow-up of 25.2 mo.
| Characteristic | Value |
| Age (yr), median (range) | 60 (30-81) |
| Male/female, n | 98/65 |
| Stage, n | |
| I | 23 |
| II | 67 |
| III | 64 |
| IV | 9 |
| Follow-up duration (d), median (range) | 756 (419-1355) |
| Recurrence, n | 25 |
| Death, n | 3 |
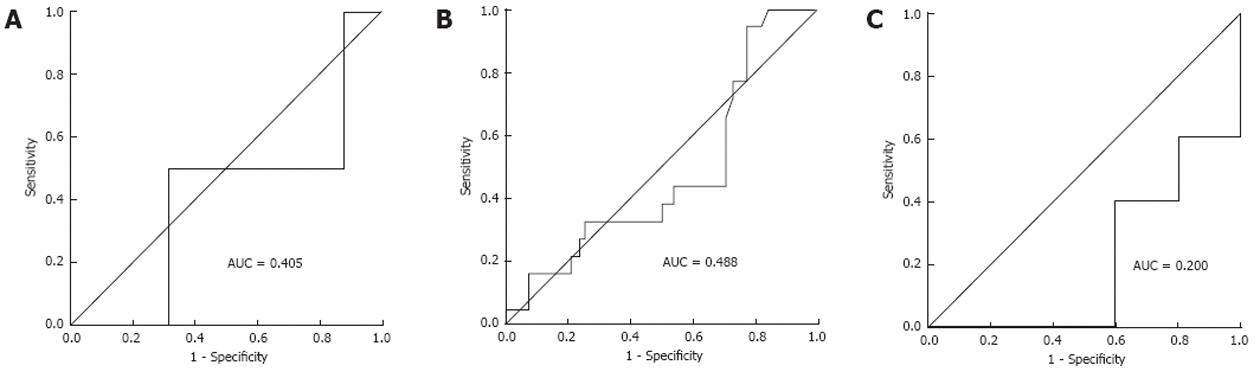
The median of SUVmax of the primary tumor was 8.6. ROC curve analysis showed no significant association between the SUVmax and recurrence (area under the curve = 0.5, P = 0.998, 95% CI: 0.389-0.611; Figure 1). As survival in patients with colorectal cancer is stage related, ROC curve analysis was conducted according to tumor stage. Patients with stageItumors were excluded because they had no recurrence. Three ROC curves (stage II and III). None showed a statistically significant result. ROC curves according to stage showed that SUVmax was not a valid marker for predicting recurrence. Because a statistically significant value was not found, SUVmax was dichotomized at its median of 8.6. The disease-free survival curve was analyzed using the median SUVmax (8.6) as the cut off (Figure 2).
In a comparison between two groups differing in median SUVmax, tumor size (P = 0.02) and T stage (P = 0.018) showed significant differences. However, tumor recurrence, N stage, M stage, and histologic grade showed no significant differences (Table 2).
| SUVmax < 8.6 (n = 80) | SUVmax≥8.6 (n = 83) | P value | |
| Age (yr), median (range) | 61.0 (37-81) | 60.0 (30-81) | 0.799 |
| Sex (male), n | 47 | 51 | 0.872 |
| Stage, n | 0.346 | ||
| I | 15 | 8 | |
| II | 33 | 34 | |
| III | 28 | 36 | |
| IV | 4 | 5 | |
| Tumor size (cm), median (range) | 4.0 (2-13.5) | 4.5 (2.4-9.5) | 0.02 |
| T stage, n | 0.01 | ||
| 1 | 5 | 3 | |
| 2 | 12 | 8 | |
| 3 | 59 | 57 | |
| 4 | 4 | 15 | |
| N stage, n | 0.955 | ||
| 0 | 47 | 44 | |
| 1 | 17 | 24 | |
| 2 | 16 | 15 | |
| M stage, n | 0.562 | ||
| 0 | 76 | 78 | |
| 1 | 4 | 5 | |
| Histology | 0.54 | ||
| WD | 6 | 7 | |
| MD | 71 | 67 | |
| PD | 4 | ||
| Mucinous | 3 | 5 | |
| Median follow-up (d) | 776 | 756 | 0.285 |
| Recurrence, n | 14 | 11 | 0.509 |
| Death, n | 2 | 1 | 0.547 |
A disease-free survival curve calculated according to the Kaplan-Meier method for SUVmax of the primary tumor is shown in Figure 3 and Table 3. The difference in disease-free survival of patients categorized by median SUVmax was not statistically significant (P = 0.53).
| Variable | No. of patients | Two-year DFS (%) | Log-rank P |
| Age (yr) | 0.29 | ||
| ≥ 60 | 81 | 90.1 | |
| < 60 | 82 | 85.4 | |
| Sex | 0.08 | ||
| Male | 98 | 83.7 | |
| Female | 65 | 93.8 | |
| TNM stage | < 0.01 | ||
| I | 23 | 100 | |
| II | 67 | 98.5 | |
| III | 64 | 78.1 | |
| IV | 9 | 44.4 | |
| SUVmax | 0.53 | ||
| ≥ 8.6 | 83 | 86.7 | |
| < 8.6 | 80 | 88.8 | |
| CEA (ng/mL) | < 0.01 | ||
| > 5 | 36 | 72.2 | |
| ≤ 5 | 123 | 93.5 |
After multivariate survival analysis, N stage and elevated CEA were independent prognostic predictors for disease-free survival (Table 4).
| Variable | Relative risk | 95% CI | P value |
| SUVmax ≥ 8.6 | 0.8 | 0.35-2.16 | 0.76 |
| T stage (T3, T4) | 3.1 | 0-6.6 | 0.94 |
| N stage (N2) | 9.78 | 2.01-49.5 | 0.01 |
| M stage (M1) | 2.51 | 0.78-7.98 | 0.12 |
| Age ≥ 60 yr | 0.6 | 0.35-2.16 | 0.35 |
| Male | 1.9 | 0.71-5.15 | 0.20 |
| CEA > 5 ng/mL | 3.2 | 1.26-8.42 | 0.02 |
FDG-PET/CT has been proposed for use in the diagnosis and staging of several malignancies[4]. Furthermore, recent studies have demonstrated that there is a correlation between SUVmax and survival in patients with gastrointestinal cancers such as esophageal and stomach cancer[10-12].
This study focused on the role of FDG-PET/CT in providing prognostic information on patients with resectable colorectal cancer. We analyzed whether the SUVmax in the primary mass was associated with recurrence and disease-free survival. Our study demonstrates that the SUVmax of the primary mass in patients with resectable colorectal cancer provides no prognostic information.
FDG-PET/CT is a molecular imaging tool that monitors tissue glucose metabolism[17]. The biologic correlations of FDG accumulation are less well studied for colorectal cancer than for other cancers. That cellular glucose metabolism may be correlated with tumor growth and aggressiveness has been suggested in some studies[17-21]. Previous in vitro and clinical studies indicated an association between elevated FDG uptake and colon tumor glucose metabolism, such as the activity of GLUT1, Ki-67, and p53[13,22-25]. A study by Gu et al[22] indicated that tumor size and depth of invasion were associated with higher SUVs in both primary colon tumors and hepatic metastatic foci. However, the study was limited by a small sample size (20 patients) and survival analysis was not performed. The other report demonstrated that a high SUVmax is correlated with poor prognosis and predicts mortality for patients with colorectal cancer after liver metastasectomy[14]. However, in this study, the highest SUVmax value was selected regardless of whether the lesion was primary or metastatic. In addition, the authors did not state whether complete resection was achieved and multivariate analysis of prognostic factors was not performed. Past studies that showed a correlation between survival and SUV were focused on stage IV colorectal cancer. In this study, we included only resectable colorectal cancers, in which clinicians are most interested. The main prognostic factors for disease-free survival were tumor stage, especially nodal stage, and elevated CEA. The SUVmax of the primary tumor was not a significant prognostic factor.
There are several limitations to this study. First, this study was a retrospective review with variation between treatment protocols. However, to reduce the heterogeneity of the study population, we excluded patients who did not receive adjuvant therapy, despite a positive lymph node status. Most adjuvant regimens consisted of FOLFOX (infusion of 5-fluorouracil, leucovorin, and oxaliplatin). Second, the duration of follow-up was relatively short. However, a recent analysis showed that most relapses occur within 2 years of surgery. This study suggested that disease-free survival after 2 and 3 years of follow-up is an appropriate end point for clinical trials in the adjuvant setting[26]. Third, immunohistochemical information on glucose metabolism in colorectal cancer was unavailable. Therefore, a biological explanation for the correlation between FDG accumulation and the behavior of the tumor was not suggested.
In conclusion, our study suggests that high FDG uptake of the primary mass in resectable colorectal cancer is not associated significantly with tumor recurrence or disease-free survival.
Despite improvement in diagnosis and treatment modalities, mortality from colon cancer has decreased slightly over the past 30 years. Therefore, methods for identifying patients at high risk of recurrence could influence posttreatment surveillance with the ultimate goal of improving survival. Fluorine-18 fluorodeoxyglucose positron emission tomography (FDG-PET) is a functional imaging method for measurement of tumor glucose use. Some researchers have suggested that metabolic activity on FDG-PET may be a predictive marker for some types of malignancies. However, the prognostic value of FDG-PET/CT for colorectal cancer is less well studied.
FDG-PET/CT is a molecular imaging tool that monitors tissue glucose metabolism. That cellular glucose metabolism may be correlated with tumor growth and aggressiveness has been suggested in some studies. The biologic correlations of FDG accumulation are less well studied for colorectal cancer than for other cancers. Therefore, in this study, authors evaluated FDG-PET/CT imaging of patients with resectable colorectal cancer and determined whether metabolic activity is correlated with recurrence and disease-free survival.
Several studies have found that metabolic activity on FDG-PET/CT is correlated with tumor biology and survival in colon cancer. However, these studies were limited by experimental studies, small numbers of patients, and one report focused on stage IV colorectal cancer. This study focused on the role of FDG-PET/CT in providing prognostic information on patients with resectable colorectal cancer. Authors included only resectable colorectal cancers, in which clinicians are most interested. Univariate and multivariate analysis did not provide evidence that disease-free survival rates for the subgroups defined by the median SUVmax were significantly different.
High FDG uptake of primary mass in resectable colorectal cancer doesn’t have a significant relationship with tumor recurrence and disease-free survival.
This manuscript evaluate the prognostic value of preoperative FDG-PET/CT in patients with resectable colorectal cancer. This study suggests that the high FDG uptake of primary mass in resectable colorectal cancer doesn’t have a significant relationship with tumor recurrence and disease-free survival. The results demonstrate evidence can be considerate by clinicians.
Peer reviewers: Shu Zheng, Professor, Cancer Institute, Zhejiang University, No.88 Jiefang road, Hangzhou 310009, Zhejiang Province, China; Dr. Freddy Penninckx, Professor, Department of Abdominal Surgery, University Clinic Gasthuisberg, Herestraat 49, 3000 Leuven, Belgium
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10002] [Cited by in RCA: 10453] [Article Influence: 696.9] [Reference Citation Analysis (0)] |
| 2. | de Geus-Oei LF, Vriens D, van Laarhoven HW, van der Graaf WT, Oyen WJ. Monitoring and predicting response to therapy with 18F-FDG PET in colorectal cancer: a systematic review. J Nucl Med. 2009;50 Suppl 1:43S-54S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Davey K, Heriot AG, Mackay J, Drummond E, Hogg A, Ngan S, Milner AD, Hicks RJ. The impact of 18-fluorodeoxyglucose positron emission tomography-computed tomography on the staging and management of primary rectal cancer. Dis Colon Rectum. 2008;51:997-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Lin M, Wong K, Ng WL, Shon IH, Morgan M. Positron emission tomography and colorectal cancer. Crit Rev Oncol Hematol. 2011;77:30-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Flamen P. Positron emission tomography in colorectal cancer. Best Pract Res Clin Gastroenterol. 2002;16:237-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, Rusch V. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value predicts survival after lung cancer resection. J Clin Oncol. 2004;22:3255-3260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 265] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Sanghera B, Wong WL, Lodge MA, Hain S, Stott D, Lowe J, Lemon C, Goodchild K, Saunders M. Potential novel application of dual time point SUV measurements as a predictor of survival in head and neck cancer. Nucl Med Commun. 2005;26:861-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, Fujii H, Shimahara Y. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Pleiss C, Risse JH, Biersack HJ, Bender H. Role of FDG-PET in the assessment of survival prognosis in melanoma. Cancer Biother Radiopharm. 2007;22:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Cerfolio RJ, Bryant AS. Maximum standardized uptake values on positron emission tomography of esophageal cancer predicts stage, tumor biology, and survival. Ann Thorac Surg. 2006;82:391-394; discussion 394-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Kato H, Nakajima M, Sohda M, Tanaka N, Inose T, Miyazaki T, Fukuchi M, Oriuchi N, Endo K, Kuwano H. The clinical application of (18)F-fluorodeoxyglucose positron emission tomography to predict survival in patients with operable esophageal cancer. Cancer. 2009;115:3196-3203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Chung HW, Lee EJ, Cho YH, Yoon SY, So Y, Kim SY, Lee MH, Kim JH, Lee SY, Sung IK. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol. 2010;136:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Burt BM, Humm JL, Kooby DA, Squire OD, Mastorides S, Larson SM, Fong Y. Using positron emission tomography with [(18)F]FDG to predict tumor behavior in experimental colorectal cancer. Neoplasia. 2001;3:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Riedl CC, Akhurst T, Larson S, Stanziale SF, Tuorto S, Bhargava A, Hricak H, Klimstra D, Fong Y. 18F-FDG PET scanning correlates with tissue markers of poor prognosis and predicts mortality for patients after liver resection for colorectal metastases. J Nucl Med. 2007;48:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Wang H, Zhang J, Tian J, Qu B, Li T, Chen Y, Liu J, Wang S. Using dual-tracer PET to predict the biologic behavior of human colorectal cancer. J Nucl Med. 2009;50:1857-1864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. 6th ed. New York: Wiely-Liss 2002; . [DOI] [Full Text] |
| 17. | Lin M. Molecular imaging using positron emission tomography in colorectal cancer. Discov Med. 2011;11:435-447. [PubMed] |
| 18. | Kurokawa T, Yoshida Y, Kawahara K, Tsuchida T, Okazawa H, Fujibayashi Y, Yonekura Y, Kotsuji F. Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer. 2004;109:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Chung JK, Lee YJ, Kim SK, Jeong JM, Lee DS, Lee MC. Comparison of [18F]fluorodeoxyglucose uptake with glucose transporter-1 expression and proliferation rate in human glioma and non-small-cell lung cancer. Nucl Med Commun. 2004;25:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, van Diest PJ, Comans EF, Joshi U, Semenza GL, Hoekstra OS, Lammertsma AA. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 21. | Knox WE, Jamdar SC, Davis PA. Hexokinase, differentiation and growth rates of transplanted rat tumors. Cancer Res. 1970;30:2240-2244. [PubMed] |
| 22. | Gu J, Yamamoto H, Fukunaga H, Danno K, Takemasa I, Ikeda M, Tatsumi M, Sekimoto M, Hatazawa J, Nishimura T. Correlation of GLUT-1 overexpression, tumor size, and depth of invasion with 18F-2-fluoro-2-deoxy-D-glucose uptake by positron emission tomography in colorectal cancer. Dig Dis Sci. 2006;51:2198-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Haber RS, Rathan A, Weiser KR, Pritsker A, Itzkowitz SH, Bodian C, Slater G, Weiss A, Burstein DE. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Jadvar H, Alavi A, Gambhir SS. 18F-FDG uptake in lung, breast, and colon cancers: molecular biology correlates and disease characterization. J Nucl Med. 2009;50:1820-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 25. | Sakashita M, Aoyama N, Minami R, Maekawa S, Kuroda K, Shirasaka D, Ichihara T, Kuroda Y, Maeda S, Kasuga M. Glut1 expression in T1 and T2 stage colorectal carcinomas: its relationship to clinicopathological features. Eur J Cancer. 2001;37:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O'Callaghan C. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 509] [Article Influence: 31.8] [Reference Citation Analysis (0)] |