Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5065
Revised: April 26, 2012
Accepted: May 13, 2012
Published online: September 28, 2012
AIM: To assess clinical and endoscopic response to propionyl-L-carnitine hydrochloride (PLC) in colonic inflammatory bowel disease.
METHODS: Patients suffering from mild to moderate ulcerative colitis (UC) or Crohn’s disease (CD) colitis, with disease activity index (DAI) between 3 and 10 and under stable therapy with oral aminosalicylates, mercaptopurine or azathioprine, for at least 8 wk prior to baseline assessments, were considered suitable for enrollment. Fourteen patients were enrolled to assume PLC 2 g/d (two active tablets twice daily) orally. Clinical-endoscopic and histological activity were assessed by DAI and histological index (HI), respectively, following a colonoscopy performed immediately before and after 4 wk treatment. Clinical response was defined as a lowering of at least 3 points in DAI and clinical remission as a DAI score ≤ 2. Histological response was defined as an improvement of HI of at least 1 point. We used median values for the analysis. Differences pre- and post-treatment were analyzed by Wilcoxon signed rank test.
RESULTS: All patients enrolled completed the study. One patient, despite medical advice, took deflazacort 5 d before follow-up colonoscopy examination. No side effects were reported by patients during the trial. After treatment, 71% (SE 12%) of patients achieved clinical response, while 64% (SE 13%) obtained remission. Separating UC from CD patients, we observed a clinical response in 60% (SE 16%) and 100%, respectively. Furthermore 60% (SE 16%) of UC patients and 75% (SE 25%) of CD patients were in clinical remission after therapy. The median DAI was 7 [interquartile range (IQR): 4-8] before treatment and decreased to 2 (IQR: 1-3) (P < 0.01) after treatment. Only patients with UC showed a significant reduction of DAI, from a median 6.5 (IQR: 4-9) before treatment to 2 (IQR: 1-3) after treatment (P < 0.01). Conversely, in CD patients, although displaying a clear reduction of DAI from 7 (IQR: 5.5-7.5) before therapy to 1.5 (IQR: 0.5-2.5) after therapy, differences observed were not significant (P = 0.06). Seventy-nine percent (SE 11%) of patients showed improvement of HI of at least 1 point, while only one CD and two UC patients showed HI stability; none showed HI worsening. Median HI decreased from 1 (IQR: 1-2), to 0.5 (IQR: 0-1) at the endoscopic control in the whole population (P < 0.01), while it changed from 1 (IQR: 1-2) to 0.5 (IQR: 0-1) in UC patients (P < 0.01) and from 1.5 (IQR: 1-2) to 0.5 (IQR: 0-1) in CD patients (P = not significant). The two sample tests of proportions showed no significant differences in clinical and histological response or in clinical remission between UC and CD patients. No side effects were reported during treatment or at 4 wk follow-up visit.
CONCLUSION: PLC improves endoscopic and histological activity of mild to moderate UC. Further studies are required to evaluate PLC efficacy in colonic CD patients.
- Citation: Merra G, Gasbarrini G, Laterza L, Pizzoferrato M, Poscia A, Scaldaferri F, Arena V, Fiore F, Cittadini A, Sgambato A, Franceschi F, Gasbarrini A. Propionyl-L-carnitine hydrochloride for treatment of mild to moderate colonic inflammatory bowel diseases. World J Gastroenterol 2012; 18(36): 5065-5071
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5065.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5065
Inflammatory bowel diseases (IBDs) are idiopathic, immune-mediated and multifactorial diseases, including Crohn’s disease (CD) and ulcerative colitis (UC)[1]. IBD pathogenesis has not been definitely clarified and UC and CD are considered complex diseases of unknown etiology, in which genetic and environmental factors force an abnormal intestinal mucosa immune reaction towards food and bacterial antigens[2-4]. Regardless of the main and unknown cause, several mechanisms sustain chronic inflammation and the vicious circle in IBD. Among those factors, energy deficiency[5-7] and peroxidative damage[8,9] of colonocytes play a pivotal role in IBD pathogenesis.
Propionyl-L-carnitine hydrochloride (PLC) is the (R)-3-(1oxo-propoxy)-4-(N,N,N-trimethyl amonium chloride) butanoic acid. It is a natural derivative of L-carnitine, produced in the body by the carnitine-acetyltransferase-mediated reaction between propionyl-CoA and L-carnitine. Physiologically, L-carnitine has an important role in energy regulation, taking part in fatty acid β-oxidation and glucose metabolism, and it has been used as a therapeutic option in conditions characterized by decreased ATP levels, such as heart failure and ischemia[10]. Moreover, PLC acts as an antioxidant molecule, reducing the lipid peroxidation in coronary endothelial cells[11-15]. Furthermore, PLC is a carrier of propionate moiety, which enters the Kreb’s cycle, once transformed in succinate, contributing to energetic sources of the cell[16]. Finally, PLC has been used for the therapy of cutaneous trophic ulcers[17].
Based on these results, a similar effect of PLC in colonic IBD has been supposed; a condition characterized by peroxidative damage, energy impairment, ulcers and abnormal epithelial function of colonocytes. Beyond intriguing pathogenic considerations, the effect of PLC on IBD and in particular on UC patients, has been recently addressed by a positive phase II trial[18] on mild to moderate UC patients.
Carnitine transport deficiency could also play a role in CD pathogenesis, as suggested by genetic studies, showing mutations in genes coding for carnitine transporters, OCTN1 (SLC22A4) and OCTN2 (SLC22A5), in CD patients[19]. Furthermore, in a murine model with loss-of-function mutation in the OCTN2 gene, the defect in carnitine transporter function in intestinal epithelial cells leads to atrophy of the small intestine and colon, and subsequent onset of inflammation[20] .
Based on these considerations, in this proof-of-concept pilot study, we investigated the efficacy of PLC not only in UC, but also in colonic CD patients.
This was a prospective, single center, open label, proof-of-concept pilot study on 14 patients, to investigate the efficacy of PLC (ST 261) colon release tablets at the dosage of 2 g/d in patients affected by mild to moderate colonic IBD, with stable oral treatment.
The protocol was approved by the Ethics Committee of “A. Gemelli” Hospital, Rome, Italy. The trial was conducted according to the Helsinki Declaration and the Good Clinical Practice Guidelines adopted by the European Medicines Agency in 1996. Every patient signed a written informed consent.
Primary endpoint of the study was to evaluate the percentage of patients displaying clinical/endoscopic response following 4 wk of stable therapy, defined as a lowering of the disease activity index (DAI score, Table 1) of at least 3 points upon the baseline. Percentage of patients reaching clinical remission, defined by a DAI score ≤ 2 with no individual subscore > 1, was also evaluated. As secondary endpoint, histological response to the treatment was assessed, defined as an improvement of the histological index (HI) of at least 1 point at the end of the study compared to baseline.
| Stool frequency | Mucosal appearance | Rectal bleeding | Physician’s overall assessment of disease severity | |
| 0 | Normal number of stools for the patient | Normal | None | Normal |
| 1 | 1-2 stools/d more than normal | Mild friability | Streaks of blood | Mild |
| 2 | 3-4 stools/d more than normal | Moderate friability | Obvious blood | Moderate |
| 3 | 5 or more stools/d more than normal | Exudation, spontaneous bleeding | Mostly blood | Severe |
Patients suitable for the enrollment met all the following inclusion criteria: age between 16 and 80 years; if female, not pregnant or nursing; for women of childbearing potential, willingness to avoid a pregnancy during the treatment period and for at least 1 mo from the last dose of drug; availability of pancolonoscopy and histology both confirming the diagnosis of active inflammatory bowel disease; DAI between 3 and 10 (mild to moderate disease); under stable therapy with oral aminosalicylates (mesalazine, balsalazide, olsalazine or sulfasalazine for ≥ 8 wk prior to baseline assessments), mercaptopurine or azathioprine for ≥ 8 wk prior to baseline assessments.
The exclusion criteria were: first diagnosis of IBD; known CD of the small intestine or of other sites rather than the colon; current or previous (in the past 30 d before the screening) use of systemic corticosteroids; use of antibiotics in the past 7 d before the screening; use of NSAIDs in the past 7 d preceding screening; positive stool culture (when performed, according to investigator’s judgment, to assess possible parasitological infection); significantly impaired liver, renal, pulmonary or cardiovascular function; history of total colon resection; active or chronic infection.
During a screening phase of 2 wk, each patient was clinically evaluated and underwent colonoscopy with biopsy sampling for histology, to assess HI and DAI score. Clinical/endoscopic evaluation was performed using DAI, a validated index[18] for UC patients, whose criteria are reported on Table 1. In this trial, we also used DAI to assess Crohn’s colitis. In agreement with its use in other clinical trials, a DAI score of 3-10 was considered evidence of a mild (3-6) to moderate (7-10) disease[18].
After the enrollment, patients were administered with two PLC colon release 500 mg tablets twice daily (1 h before breakfast and 1 h before dinner) for a total daily dose of 2 g, for 4 wk. After 4 wk of treatment, at a control visit, each patient underwent colonoscopy with biopsy sampling for histology and clinical evaluation with assessment of HI and DAI score. After 4 wk since the last visit, a follow-up visit was performed to assess safety of the treatment.
To assess safety and tolerability, vital signs, physical examinations, electrocardiograms and laboratory tests were registered at each visit (at the enrollment and after 4 and 8 wk).
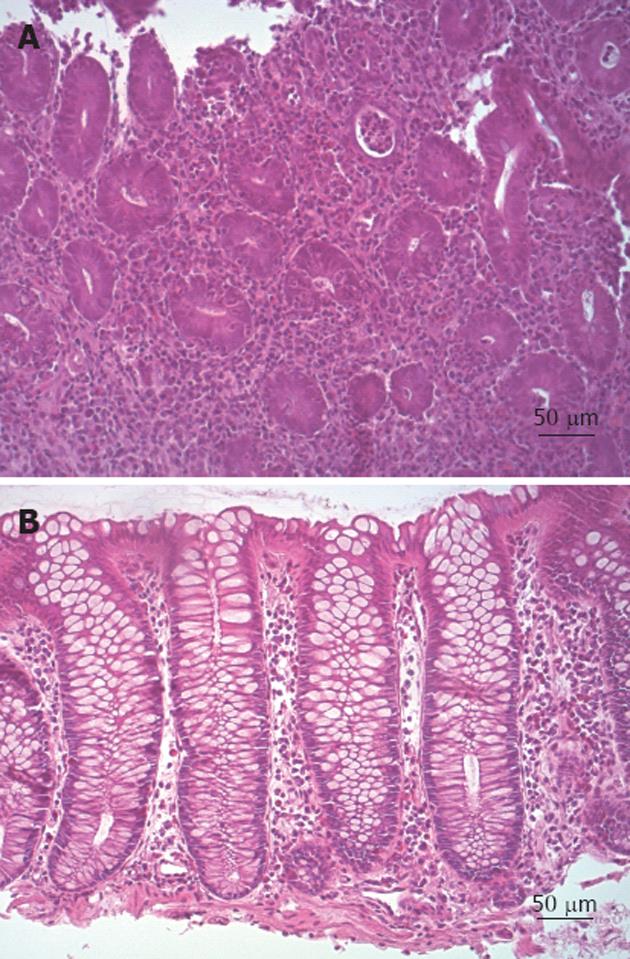
All histological scores were calculated by a single expert pathologist, following histological criteria described in Mikhailova et al[18] and modified from Truelove et al[21]. Briefly, inflammation was classified as grade 0: if indistinguishable from normal intestinal mucosa or, where structural features of UC were present, there were no polymorphs in the crypt epithelium; grade 1: if there was an increased number of leukocytes in the lamina propria and initial infiltration of crypt epithelium, but no abscesses or epithelial erosions were present; grade 2: if crypt abscesses (mandatory) and erosive foci, but no ulcerative lesions, were detected; and grade 3: if ulcerative lesions were clearly evident. The same score was also used to classify colonic CD. Lowering of HI by at least 1 point was considered as histological response.
Statistical analysis was performed by using IC STATA Release 12.0 for MAC. Data were reported as absolute numbers, percentage, median and interquartile ranges (IQRs), as specified. Mann-Whitney test was used to compare UC and CD patients. Differences were analyzed by Wilcoxon signed rank test, while the proportion of binary variables (clinical or histological response/clinical remission) between UC and CD patients was evaluated by a two-sample test of proportions. P < 0.05 was considered statistically significant. We used median values for the analysis, because of the small number of patients enrolled, and we preferred nonparametric tests.
Fourteen patients were enrolled. The mean age, female/male ratio and demographics are indicated in Table 2. With regard to therapy, six patients were treated with mesalamine 2.4 g/d; three with azathioprine at 2 mg/kg; and one with 7.5 mg/d deflazacort, starting 5 d before follow-up colonoscopy, despite medical advice to the contrary; the remaining four patients did not receive any treatment. CD patients were overall younger than UC patients and they were all male, but they were comparable to UC patients in term of DAI and HI at the time of enrollment. However, no significant differences were detected between UC and CD patients in term of demographic data.
| Patients features | All | UC | CD |
| No. of patients | 14 | 10 | 4 |
| Sex (F/M) | 5/9 | 5/5 | 0/4 |
| Age (yr), mean ± SD | 49.4 ± 17.7 | 54.9 ± 17.5 | 34.7 ± 7.6 |
| Disease duration at enrollment (yr), mean ± SD | 4.8 ± 3.1 | 4.9 ± 3.1 | 4.5 ± 3.7 |
| Diagnosis | |||
| Pancolitis | 8 | - | - |
| Distal colitis | 2 | - | - |
| Colonic CD | 4 | - | - |
| DAI at time of enrollment, median ± SE | 7 ± 4 | 6.5 ± 5 | 7 ± 2 |
| DAI after the treatment, median ± SE | 2 ± 2 | 2 ± 2 | 1.5 ± 2 |
| HI at time of enrollment, median ± SE | 1 ± 1 | 1 ± 1 | 1.5 ± 1 |
| HI after the treatment, median ± SE | 0.5 ± 1 | 0.5 ± 1 | 0.5 ± 1 |
| DAI at time of enrollment, mean ± SD | 6.6 ± 2.4 | 6.6 ± 2.7 | 6.5 ± 1.7 |
| DAI after treatment, mean ± SD | 1.9 ± 1.5 | 2.0 ± 1.6 | 1.5 ± 1.3 |
| HI at time of enrollment, mean ± SD | 1.4 ± 0.5 | 1.4 ± 0.5 | 1.5 ± 0.6 |
| HI after treatment, mean ± SD | 0.5 ± 0.5 | 0.5 ± 0.5 | 0.5 ± 0.6 |
| Time between 1st and 2nd colonoscopy (wk), mean ± SD | 4.7 ± 4 | 5.1 ± 4.6 | 3 ± 2.8 |
| Other therapy (n) | |||
| Mesalazine | 6 | 5 | 1 |
| AZA | 3 | 1 | 2 |
| Deflazacort | 1 | 1 | 0 |
| No other therapy | 4 | 3 | 1 |
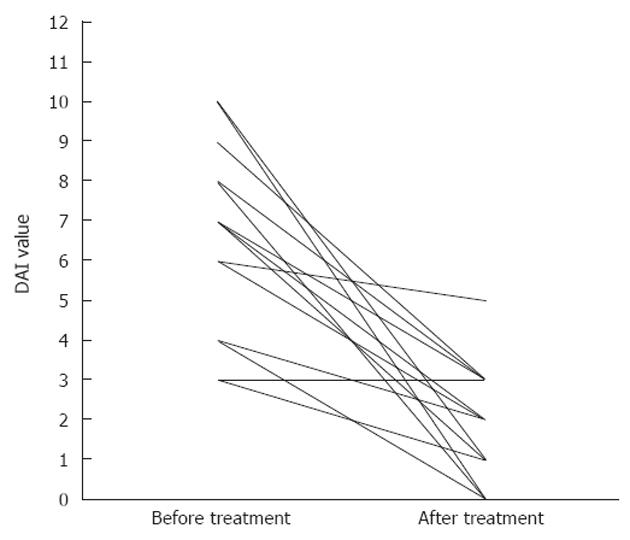
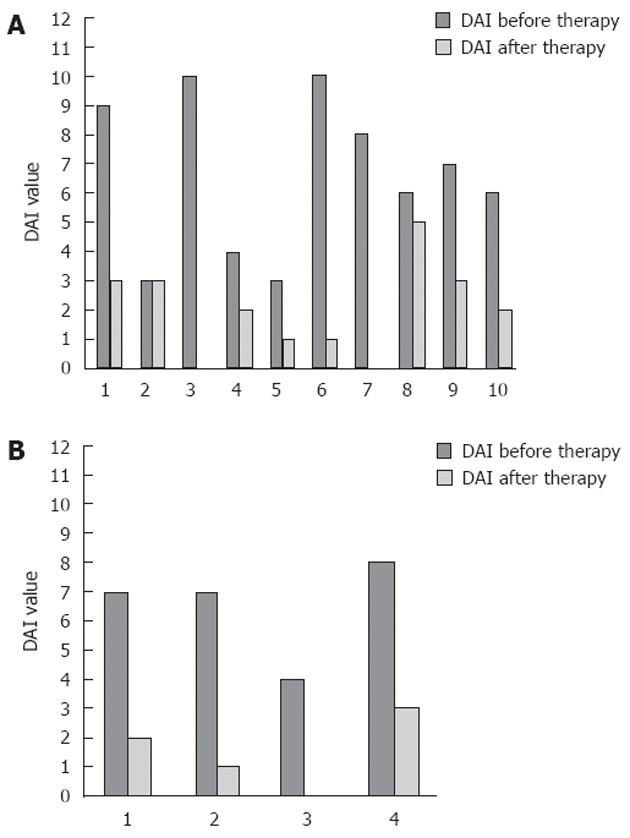
After treatment, 71% (SE 12%) and 64% (SE 13%) of patients achieved a clinical response, or obtained remission, respectively. Separating UC patients from CD patients, we observed a clinical response after therapy in 60% (SE 16%) of UC patients and in 100% of CD patients; furthermore 60% (SE 16%) of UC patients and 75% (SE 25%) of CD patients were in clinical remission after therapy. The median DAI was 7 (IQR: 4-8) before treatment and decreased to 2 (IQR: 1-3) (P < 0.01) after treatment (Table 2 for mean values and Figure 1 for details). Stratifying by disease, only patients with UC showed a significant reduction of DAI, from a median value of 6.5 (IQR: 4-9) before treatment to a median value of 2 (IQR: 1-3) (P < 0.01) after treatment (Figure 2A). Conversely, in CD patients, although displaying a clear reduction of DAI from 7 (IQR: 5.57-5) before therapy to 1.5 (IQR: 0.5-2.5) after therapy, differences observed were not significant (P = 0.06) (Figure 2B).
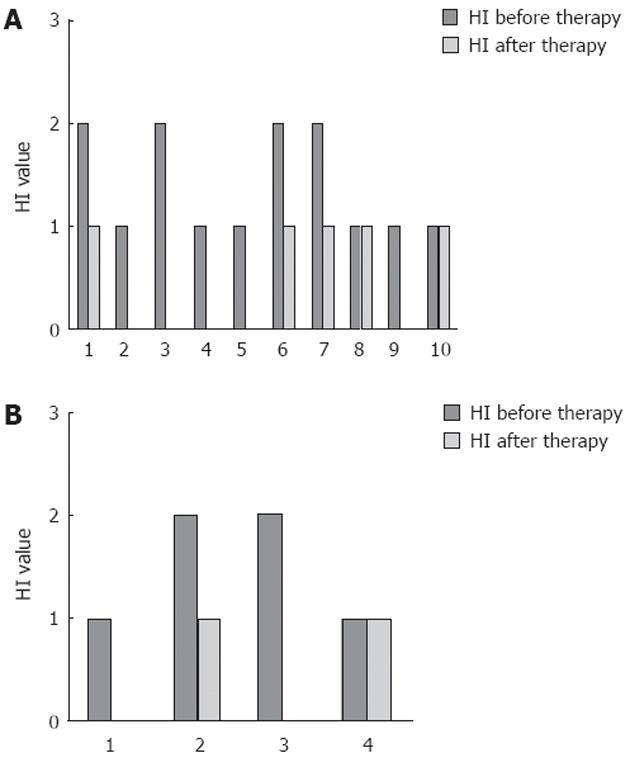
Overall, 79% (SE 11%) of patients showed an improvement in HI of at least 1 point, while only one CD and two UC patients showed HI stability; none showed HI worsening (Figure 3). Median HI decreased from 1 (IQR: 1-2), to 0.5 (IQR: 0-1) at the endoscopic control in the whole population (P < 0.01), while it changed from 1 (IQR: 1-2) to 0.5 (IQR: 0-1) in UC patients (P < 0.01) (Figure 4A) and from 1.5 (IQR: 1-2) to 0.5 (IQR: 0-1) in CD patients (P = not significant) (Figure 4B). The two-sample tests of proportions showed no significant differences in clinical/histological responses or clinical remissions between UC and CD patients.
No side effects were reported during treatment or at 4 wk follow-up visit.
We enrolled 14 patients who were under stable oral therapy, without a complete control of symptoms and with evidence of endoscopic/histological disease. Therapeutic options sustained by strong clinical data for these patients are nowadays particularly limited.
Each patient underwent colonoscopy with biopsy sampling for histology and clinical evaluation with calculation of DAI score before starting treatment and repeated the same evaluation after 4 wk of therapy with PLC (ST 261) colon release tablets at a dose of 2 g/d. Following active treatment, the majority of them displayed a clear clinical improvement (70% of patients achieved clinical response) and 79% of patients presented with histological improvement. Our study confirmed the good clinical efficacy of PLC, in contrast to other studies using only SCFA or butyrate enemas in patients with more severe forms of UC[22-25]. Our results confirmed preclinical data showing the efficacy of PLC in reducing the damaged colon area in acute and chronic models of murine colitis. In humans, PLC given intrarectally shows good results in improving clinical and histological activity in patients suffering from UC[26]. Oral formulation of PLC is more agreeable for patients, and probably improves patient compliance with therapy.
Our single center study confirms the results from a multicentre phase II, double-blind, parallel-group trial by Mikhailova et al[18] in patients suffering from UC. That trial showed a clinical/endoscopic response rate of 75% in patients exposed to PLC 1 g/d and a response rate of 69% in patients exposed to PLC 2 g⁄d, with remission rates of 55%, 49% and 35% respectively for PLC 1 g, 2 g and placebo groups. Histological responses rates were 40%, 33% and 35%, respectively. Histological assessment, however, was not significantly different among the groups. We showed a similar clinical/endoscopic remission rate and, moreover, a higher histological response rate after therapy. Furthermore, we also investigated the efficacy of PLC in CD patients, who showed a significant clinical/endoscopic response.
Nevertheless, our study had several limitations. This was an open label study, without a placebo group. Furthermore, one patient, considered in the final analysis, took 7.5 mg/d deflazacort for 5 d, despite medical advice, just before the follow-up endoscopy evaluation. This particular patient had UC, but was still considered for the final analysis because we did not consider this duration and dose of deflazacort to affect clinical and histological outcome significantly, for the purpose of our study. Furthermore, our patients, as happens frequently in clinical practice, were receiving concomitant therapy with mesalamine and/or azathioprine. The limited number of patients did not permit a stratification based on use of concomitant medication. However, we can exclude that the clinical response and remission in these patients were dependent on other therapy, which remained unchanged for at least 2 mo before enrollment and throughout the study. Patients enrolled in this study were a mixed population of UC and colonic CD patients. One can argue that these different diseases would lead to nonhomogenous results among subgroups and potentially different responses, but the number of patients enrolled was too small to allow subgroup analysis. Therefore, with our data, we cannot make conclusions about different responses to treatment in UC and colonic CD.
However, based on the supposed mechanisms of action of this drug, we expected a response in both UC and CD. In fact colonocytes from UC and colonic CD patients, showing defect in carnitine functions, would probably both benefit from PLC therapy.
Compared with the study of Sandborn et al[27], the present trial suggests a possible role of this drug in colonic CD patients, even if our results in these patients did not reach statistical significance; probably because of the small number of patients. Nevertheless, the clinical response in CD patients was good and, despite the limitation of the absence of a placebo arm, it can be argued that it was higher than expected from the response to a placebo arm, as based on the literature. For this reason, this proof-of-concept pilot study lays the foundations for future studies on the use of PLC in colonic CD patients.
In conclusion, PLC (ST 261) colon release tablets at a dose of 2 g/d were effective in inducing clinical and endoscopic/histological improvement in mild to moderate colonic IBD. Other studies are required to define the real efficacy and the correct therapeutic dosage of this promising drug for both colonic forms of IBD, and specially to evaluate its efficacy in CD patients.
In memory of Dr. Claudio Cavazza, for his love for research and for having believed in this project.
There are few options in inflammatory bowel disease (IBD) therapy, mainly because pathogenetic mechanisms of these diseases are still unclear. Several components could play a role in IBD pathogenesis, including peroxidative damage, energy impairment and abnormal epithelial function on colonocytes. Propionyl-L-carnitine hydrochloride (PLC) modulation of these impaired functions could be useful in IBD therapy.
PLC has demonstrated efficacy in reducing clinical and endoscopic disease activity in patients suffering from ulcerative colitis (UC), but only a few studies have investigated its use. Based on its mechanism of action, this drug could be effective also in patients affected by Crohn’s disease (CD), but no clinical trials have assessed the efficacy of this drug in CD.
This is one of the first studies to investigate the use of PLC in patients suffering from UC and CD. The data suggest that this drug demonstrates efficacy and safety in both conditions.
With regard to UC, these data are in accordance with previous findings emerging from a multicenter, double-blind, placebo-controlled clinical trial. Furthermore, they suggest a possible use of this drug also in CD colitis and, even if few patients have been treated, this proof-of-concept study lays the foundations for future wider clinical trials.
PLC has an important role in energy regulation, taking part in fatty acid β-oxidation and glucose metabolism. Moreover, PLC acts as an antioxidant molecule and it is a carrier of propionate moiety, which enters the Kreb’s cycle, once transformed in succinate, contributing to energy sources in the cell. Finally, PLC has shown efficacy in treatment of cutaneous trophic ulcers.
This is an interesting and important study that provides clinical evidence of the benefit of this novel therapy in patients with colitis. The topic is hot and of great clinical importance and these promising data would support further clinical assessment of this agent.
Peer reviewers: Andrew S Day, Professor, University of Otago, Christchurch Hospital, Christchurch 8140, New Zealand; Dr. Ferenc Sipos, 2nd Department of Internal Medicine, Semmelweis University, Szentkir¨¢lyi u. 46., 1088 Budapest, Hungary
S- Editor Gou SX L- Editor Kerr C E- Editor Li JY
| 1. | Hanauer SB. Inflammatory bowel disease. N Engl J Med. 1996;334:841-848. [PubMed] |
| 2. | Rotter JI, Yang H. Delineating the major aetiological risk factors for IBD: the genetic susceptibilities. Inflammatory Bowel Diseases. Pathophysiology as Basis of Treatment. Dordrecht: Kluwer Academic Publishers 1993; 9-18. |
| 3. | MacDonald TT, Monteleone G, Pender SL. Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol. 2000;51:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Blumberg RS, Strober W. Prospects for research in inflammatory bowel disease. JAMA. 2001;285:643-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Roediger WE. The colonic epithelium in ulcerative colitis: an energy-deficiency disease? Lancet. 1980;2:712-715. [PubMed] |
| 6. | Chapman MA, Grahn MF, Boyle MA, Hutton M, Rogers J, Williams NS. Butyrate oxidation is impaired in the colonic mucosa of sufferers of quiescent ulcerative colitis. Gut. 1994;35:73-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 169] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Ahmad MS, Krishnan S, Ramakrishna BS, Mathan M, Pulimood AB, Murthy SN. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 2000;46:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 138] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Bauer V, Bauer F. Reactive oxygen species as mediators of tissue protection and injury. Gen Physiol Biophys. 1999;18 Spec No:7-14. [PubMed] |
| 9. | McCafferty DM. Peroxynitrite and inflammatory bowel disease. Gut. 2000;46:436-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Mingorance C, Rodríguez-Rodríguez R, Justo ML, Alvarez de Sotomayor M, Herrera MD. Critical update for the clinical use of L-carnitine analogs in cardiometabolic disorders. Vasc Health Risk Manag. 2011;7:169-176. [PubMed] |
| 11. | Arduini A, Rossi M, Mancinelli G, Belfiglio M, Scurti R, Radatti G, Shohet SB. Effect of L-carnitine and acetyl-L-carnitine on the human erythrocyte membrane stability and deformability. Life Sci. 1990;47:2395-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Li P, Park C, Micheletti R, Li B, Cheng W, Sonnenblick EH, Anversa P, Bianchi G. Myocyte performance during evolution of myocardial infarction in rats: effects of propionyl-L-carnitine. Am J Physiol. 1995;268:H1702-H1713. [PubMed] |
| 13. | Torielli L, Conti F, Cinato E, Ceppi E, Anversa P, Bianchi G, Ferrari P. Alterations in energy metabolism of hypertrophied rat cardiomyocytes: influence of propionyl-L-carnitine. J Cardiovasc Pharmacol. 1995;26:372-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Paulson DJ, Traxler J, Schmidt M, Noonan J, Shug AL. Protection of the ischaemic myocardium by L-propionylcarnitine: effects on the recovery of cardiac output after ischaemia and reperfusion, carnitine transport, and fatty acid oxidation. Cardiovasc Res. 1986;20:536-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 101] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Vanella A, Russo A, Acquaviva R, Campisi A, Di Giacomo C, Sorrenti V, Barcellona ML. L -propionyl-carnitine as superoxide scavenger, antioxidant, and DNA cleavage protector. Cell Biol Toxicol. 2000;16:99-104. [PubMed] |
| 16. | Brevetti G, Fanin M, De Amicis V, Carrozzo R, Di Lello F, Martone VD, Angelini C. Changes in skeletal muscle histology and metabolism in patients undergoing exercise deconditioning: effect of propionyl-L-carnitine. Muscle Nerve. 1997;20:1115-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Pola P, Flore R, Serricchio M, Tondi P. New carnitine derivatives for the therapy of cutaneous ulcers in vasculopathics. Drugs Exp Clin Res. 1991;17:277-282. [PubMed] |
| 18. | Mikhailova TL, Sishkova E, Poniewierka E, Zhidkov KP, Bakulin IG, Kupcinskas L, Lesniakowski K, Grinevich VB, Malecka-Panas E, Ardizzone S. Randomised clinical trial: the efficacy and safety of propionyl-L-carnitine therapy in patients with ulcerative colitis receiving stable oral treatment. Aliment Pharmacol Ther. 2011;34:1088-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Peltekova VD, Wintle RF, Rubin LA, Amos CI, Huang Q, Gu X, Newman B, Van Oene M, Cescon D, Greenberg G. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat Genet. 2004;36:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 561] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 20. | Shekhawat PS, Srinivas SR, Matern D, Bennett MJ, Boriack R, George V, Xu H, Prasad PD, Roon P, Ganapathy V. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(-/-)) mice. Mol Genet Metab. 2007;92:315-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Truelove SC, Richards WC. Biopsy studies in ulcerative colitis. Br Med J. 1956;1:1315-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 271] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Scheppach W, Müller JG, Boxberger F, Dusel G, Richter F, Bartram HP, Christl SU, Dempfle CE, Kasper H. Histological changes in the colonic mucosa following irrigation with short-chain fatty acids. Eur J Gastroenterol Hepatol. 1997;9:163-168. [PubMed] |
| 23. | Scheppach W, Sommer H, Kirchner T, Paganelli GM, Bartram P, Christl S, Richter F, Dusel G, Kasper H. Effect of butyrate enemas on the colonic mucosa in distal ulcerative colitis. Gastroenterology. 1992;103:51-56. [PubMed] |
| 24. | Steinhart AH, Hiruki T, Brzezinski A, Baker JP. Treatment of left-sided ulcerative colitis with butyrate enemas: a controlled trial. Aliment Pharmacol Ther. 1996;10:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Vernia P, Marcheggiano A, Caprilli R, Frieri G, Corrao G, Valpiani D, Di Paolo MC, Paoluzi P, Torsoli A. Short-chain fatty acid topical treatment in distal ulcerative colitis. Aliment Pharmacol Ther. 1995;9:309-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 159] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Gasbarrini G, Mingrone G, Giancaterini A, De Gaetano A, Scarfone A, Capristo E, Calvani M, Caso V, Greco AV. Effects of propionyl-L-carnitine topical irrigation in distal ulcerative colitis: a preliminary report. Hepatogastroenterology. 2003;50:1385-1389. [PubMed] |
| 27. | Sandborn WJ, Schreiber S, Feagan BG, Rutgeerts P, Younes ZH, Bloomfield R, Coteur G, Guzman JP, D'Haens GR. Certolizumab pegol for active Crohn's disease: a placebo-controlled, randomized trial. Clin Gastroenterol Hepatol. 2011;9:670-678.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |