Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5034
Revised: July 3, 2012
Accepted: July 9, 2012
Published online: September 28, 2012
AIM: To elucidate the role of neuropilin-1 (Nrp-1) and semaphorin 3A (Sema3A) in sinusoidal remodeling during liver regeneration in rats.
METHODS: Male Wistar/ST rats at 7 wk of age, weighing about 200 g, were used for all animal experiments. In vivo, at 24, 48, 72, 96, 144 and 192 h after two-thirds partial hepatectomy (PHx), the remnant livers were removed. Liver tissues were immunohistochemically stained for Nrp-1, Sema3A and SE-1, a liver sinusoidal endothelial cell (SEC) marker. Total RNA of the liver tissue was extracted and reversely transcribed into cDNA. The mRNA expression of Sema3A was analyzed by quantitative real-time polymerase chain reaction and normalized to that of ribosomal protein S18. In vitro, SECs were isolated from rat liver and cultured in endothelial growth medium containing 20 ng/mL vascular endothelial cell growth factor. Migration of SECs in primary culture was assessed by cell transwell assay with or without recombinant Sema3A. Apoptotic cells were determined by a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling method.
RESULTS: In vitro, immunohistochemistry study revealed that Sema3A and Nrp-1 were constitutively expressed in hepatocytes and SECs, respectively, in normal rat liver tissues. Nrp-1 expression in SECs was quantified by the percentage of immunostained area with anti-Nrp-1 antibody in relation to the area stained with SE-1. Between 24 h and 96 h following resection of liver, Nrp-1 expression in SECs was transiently increased. Compared with the baseline (5.2% ± 0.1%), Nrp-1 expression in SECs significantly increased at 24 h (17.3% ± 0.7%, P < 0.05), 48 h (39.1% ± 0.6%, P < 0.01), 72 h (46.9% ± 4.5%, P < 0.01) and 96 h (29.9% ± 3.8%, P < 0.01) after PHx, then returned to the basal level at termination of liver regeneration. Interestingly, the expression of Sema3A was inversely associated with that of Nrp-1 in liver after PHx. Sema3A mRNA expression was significantly reduced by about 75% over the period 24-144 h after PHx (P < 0.05), and returned to basal levels at 192 h after PHx. In vitro, SECs isolated from rats after PHx (PHx-SECs) were observed to migrate to the lower chamber of the cell transwell system after incubation for 24 h, but not cells from normal rats (CONT-SECs), indicating that mobility of PHx-SECs increases as compared with that of CONT-SECs. Moreover, recombinant Sema3A significantly attenuated migration in PHx-SECs in primary culture (vehicle-treated 100% ± 7.9% vs Sema3A-treated 42.6% ± 5.4%, P < 0.01), but not in CONT-SECs. Compared with CONT-SECs, the apoptotic rate of PHx-SECs decreased by 78.3% (P < 0.05). There was no difference in apoptosis between CONT-SECs that were treated with vehicle and Sema3A. However, in PHx-SECs, apoptosis was induced by the presence of 5 nmol Sema3A for 24 h (vehicle-treated 21.7% ± 7.6% vs Sema3A-treated 104.3% ± 8.9%, P < 0.05). In addition, immunohistochemistry confirmed the increased expression of Nrp-1 in PHx-SECs, while it was noted to a lesser extent in CONT-SECs.
CONCLUSION: The interplay of Nrp-1 and Sema3A shown in our results may lead to a better understanding of interaction between sinusoidal remodeling and SECs during liver regeneration.
- Citation: Fu L, Kitamura T, Iwabuchi K, Ichinose S, Yanagida M, Ogawa H, Watanabe S, Maruyama T, Suyama M, Takamori K. Interplay of neuropilin-1 and semaphorin 3A after partial hepatectomy in rats. World J Gastroenterol 2012; 18(36): 5034-5041
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5034.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5034
In liver, adult hepatocytes are long-lived and normally do not undergo cell division, but they maintain the ability to proliferate in response to toxic injury or partial resection of liver[1,2]. The process of liver regeneration (LR) is complex and involves a finely orchestrated balance of cell proliferation to reconstruct the normal liver architecture. Although hepatocyte proliferation is a major feature of the regenerating liver, it involves the interactions of other hepatic cell types (non-parenchymal cells), including Kupffer cells, stellate cells, sinusoidal endothelial cells (SECs) and biliary epithelial cells[3-5].
In the rat liver after two-thirds hepatectomy, the rate of DNA synthesis in hepatocytes begins to increase after about 12 h and peaks around 24 h. However, the induction of DNA synthesis occurs later in the non-parenchymal cells (at approximately 48 h for Kupffer and biliary epithelial cells, and at approximately 96 h for SECs), and subsequently, LR terminates at approximately 192 h[6,7]. In the late stage of this process, angiogenesis and/or vasculogenesis is essential to supply blood flow to newly replicating avascular islands of hepatocytes, in which SECs migrate and proliferate, leading to the formation of new functional sinusoids[8,9].
Indeed, many of the growth factors have been reported to be upregulated in a regenerating liver. Vascular endothelial growth factor (VEGF) is a major proangiogenic factor and is thought to improve sinusoid reconstruction during the LR process[10,11]. Hepatocyte growth factor (HGF) levels are dramatically increased after partial hepatectomy, but HGF is also a potent proangiogenic factor in vivo and stimulates endothelial cell protease production, motility, proliferation and differentiation in vitro.
Recently, the importance of neuropilins (Nrps) as proangiogenic factors has been highlighted[12]. Nrps are transmembrane glycoproteins with large extracellular domains that interact with both class 3 semaphorins and VEGF, and they are involved in the regulation of many physiological pathways including angiogenesis[13,14]. The semaphorins were initially described as axon guidance factors that affect the development of the central nervous system[15]. However, semaphorin receptors were subsequently found to be expressed by multiple types of cells including endothelial cells and cancer cells. These observations were followed by studies that indicate that the semaphorins can modulate the behavior of cancer cells and endothelial cells[16,17].
In this study, we report the inverse expression of Nrp-1 and semaphorin 3A (Sema3A) in liver tissues during liver regeneration in rats. We further show that Sema3A significantly attenuated migration and induced apoptosis in SECs isolated from rats after partial hepatectomy, but not in cells from normal rats.
Male Wistar/ST rats, 7 wk of age, weighing about 200 g (Sankyo lab, Tokyo, Japan), were used for all animal experiments. Two-thirds partial hepatectomy (PHx) was performed under sevoflurane inhalant anesthesia according to the method of Higgins et al[18]. The left lateral and median lobes of the liver were excised. At 24, 48, 72, 96, 144 and 192 h after PHx, the rats were anesthetized and the remnant livers were removed. Control livers were obtained from sevoflurane-anesthetized non-manipulated animals.
Liver tissues were obtained from the right lateral lobe, embedded in Tissue Tek OCT Compound (Electron Microscopy Sciences, Hatfield, PA), frozen and stored at -80 °C until use. All procedures involving animals were approved by the Animal Care and Use Committee of Juntendo University, which conforms to the National Institutes of Health Guideline.
Double staining of Nrp-1 and SE-1 was performed as follows: tissue fixed with 4% paraformaldehyde for 10 min at room temperature, incubated with goat polyclonal anti-Nrp-1 antibody (R and D Systems, Minneapolis, MN) (diluted 1:50) and mouse monoclonal SE-1 (Immuno-Biological Laboratories, Gunma, Japan) (diluted 1:20) at 4 °C overnight prior to incubation with Alexa-488 rabbit anti-goat (diluted 1:300) and Alexa-594 donkey anti-mouse antibody (diluted 1:300) (Invitrogen, Carlsbad, CA) for 1 h, then mounted in Vectashield mounting medium with 4,6-diamidino-2-phenylindole dihydrochloride (Vector Laboratories, Burlingame, CA). The immunostained samples were observed and analyzed with a BIOREVO BZ-9000 microscope system (KEYENCE, Osaka, Japan).
For Sema3A staining, frozen sections of liver tissues were fixed with -20 °C acetone for 5 min, incubated with rabbit polyclonal anti-Sema3A antibody (diluted 1:200) (Abcam, Cambridge, United Kingdom) at 4 °C overnight prior to incubation with Alexa-488 goat anti-rabbit antibody (diluted 1:300) for 1 h.
Total RNA of the liver tissue was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA), and reversely transcribed into cDNA using the RT reagent kit (TaKaRa Bio Inc., Shiga, Japan). Quantitative real-time polymerase chain reaction (PCR) was performed using ABI PRISM 7900HT Sequence Detector System (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. The following primers were used: Sema3A (accession number: NM_017310.1) CTG CTC CGA CTT GCA GCA TC (sense) and CGC CTC TGA AAT TGC CAA TAT ACC (antisense); ribosomal protein S18 (RPS18) (accession number: NM_213557.1) AAG TTT CAG CAC ATC CTG CGA GTA (sense) and TTG GTG AGG TCA ATG TCT GCT TTC (antisense). The Sema3A mRNA expression was normalized to that of RPS18.
SECs were isolated from rat liver based on a modification of the method of Braet et al[19], as we previously reported[20]. Briefly, liver was perfused with 0.05% collagenase A and 0.001% DNase. SECs were then purified by isopycnic sedimentation in a two-step Percoll gradient (25% and 50%). SECs were cultured in EBM-2 medium containing 20 ng/mL VEGF. These cells displayed a number of fenestrae in sieve plates, the characteristic of SECs in vivo. In separate experiments, SECs were isolated from rats 4 d after PHx (PHx-SECs).
SEC transwell migration assay was performed using 24-well culture inserts (BD Biosciences, San Jose, CA). Polyethylene terephthalate membranes with a pore size of 8 μm were coated with fibronectin. SECs were loaded to the upper chamber. Recombinant Sema3A (R and D Systems, Minneapolis, MN) or vehicle was added to the lower chamber. After incubation for 24 h, non-migrated cells on the upper surface of the filter were removed with a cotton swab and the cells which traversed and spread on the lower surface of the membrane were fixed with 2.5% glutaraldehyde and stained with hematoxylin. The average number of the cells per field was counted ×10 objective magnification from five microscopic fields. Migration index represents the number of migratory cells/number of migratory cells in vehicle-treated control.
After incubation with 5 nmol recombinant Sema3A for 24 h, SECs were fixed with 4% paraformaldehyde for 10 min at room temperature. Apoptotic cells were determined by a terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling (TUNEL) method with a fluorescent apoptosis detection system kit (Promega, Madison, WI).
Results are expressed as the mean ± SE. Statistical analysis were performed using GraphPad Prism v5.00 for Windows (GraphPad Software, San Diego, CA). Statistical significance was determined by one-way analysis of variance.
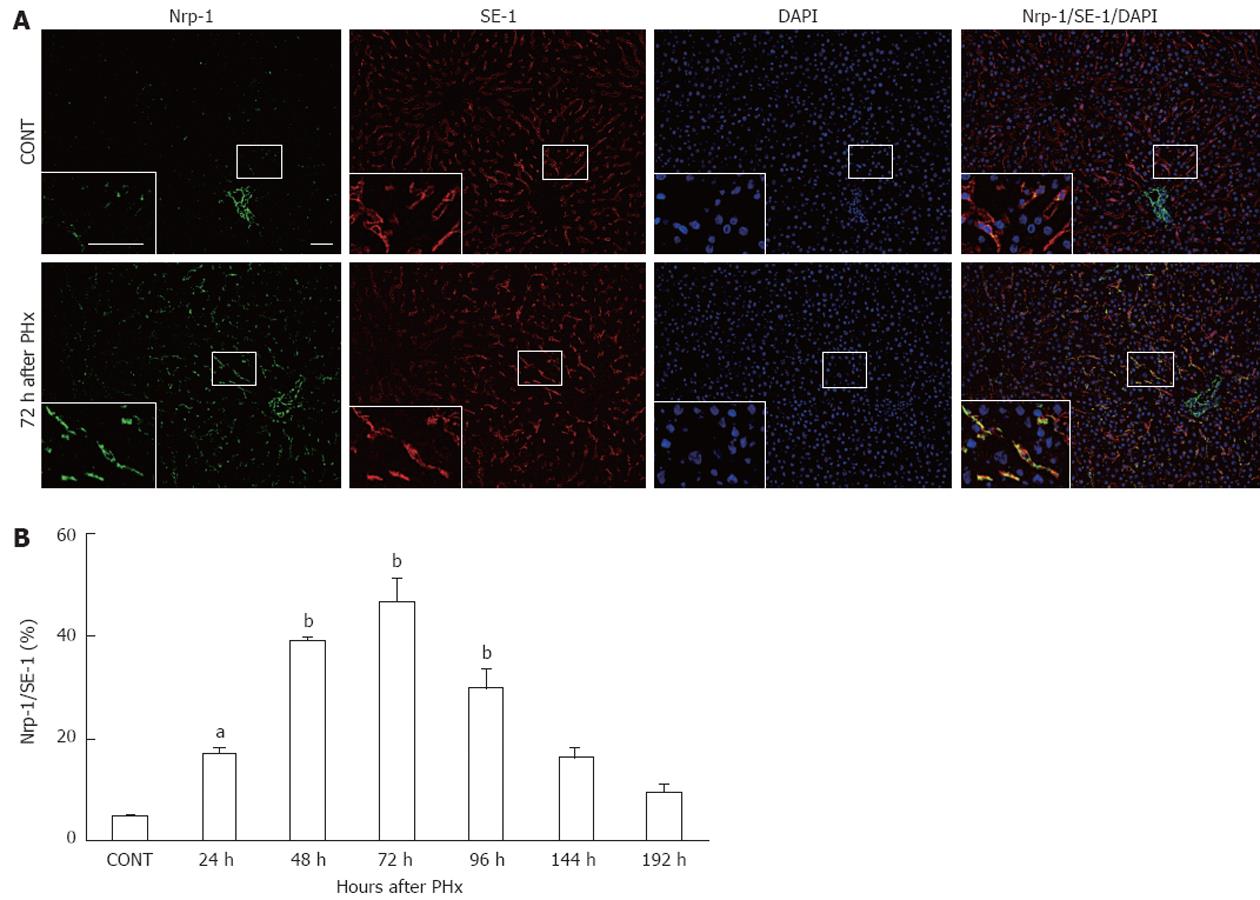
To investigate Nrp-1 expression in SECs during LR, liver tissues after PHx were analyzed by immunofluorescent staining with anti-Nrp-1 antibody and SE-1, a monoclonal antibody that specifically reacts with rat liver SECs[21,22]. As shown in Figure 1A, SECs stained with SE-1 were positive for Nrp-1 in control liver, and Nrp-1 expression in SECs was enhanced in regenerating liver at 72 h after PHx.
Nrp-1 expression in SECs was quantified by the percentage of immunostained area with anti-Nrp-1 antibody in relation to the area stained with SE-1. Nrp-1 expression in SECs significantly increased at 24 h (P < 0.05), peaked at 72 h (P < 0.01), and recovered to the basal level at 144 h after PHx (Figure 1B).
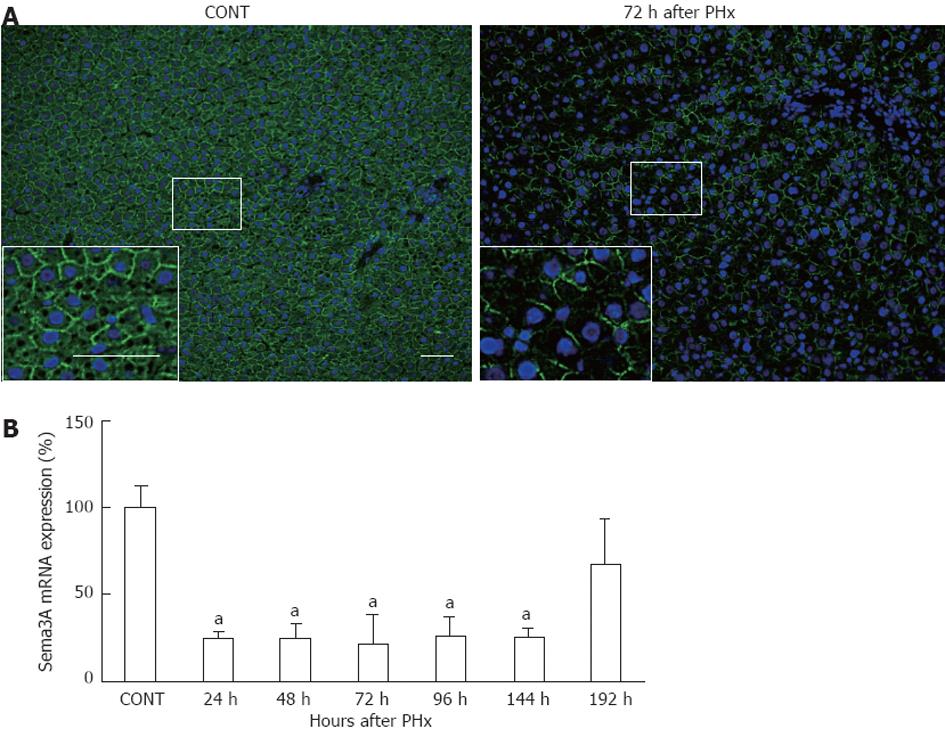
To test the expression of Sema3A during LR, liver tissues were examined by immunofluorescence with anti-Sema3A antibody in control rats and rats at 72 h after PHx. Sema3A was strongly stained in hepatocytes, which display round and big nuclei, in control liver. However, Sema3A expression was attenuated at 72 h after PHx (Figure 2A). Furthermore, real-time PCR analysis confirmed the decline of Sema3A expression during the process of LR. As shown in Figure 2B, Sema3A mRNA expression was significantly reduced by about 75% over the period 24-144 h after PHx (P < 0.05), and returned to baseline at 192 h after PHx.
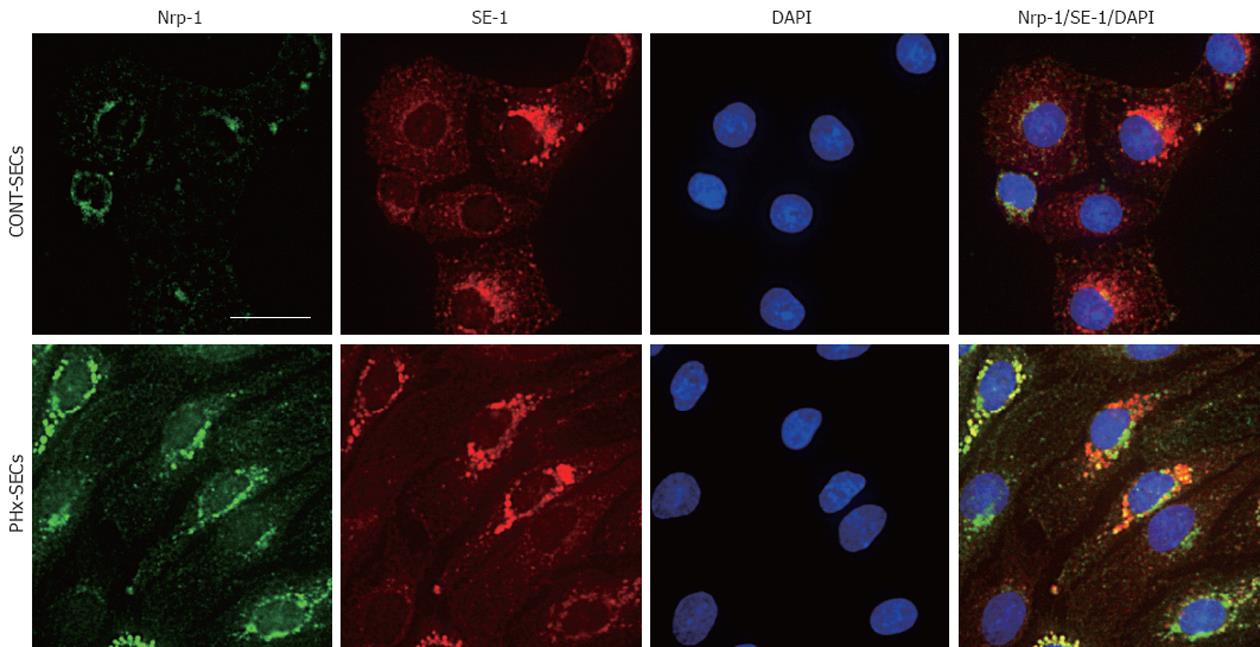
To confirm that Nrp-1 is expressed in SECs, immunofluorescent analysis was performed in SECs isolated from rat liver. Both CONT-SECs and PHx-SECs at 24 h in culture were stained positive for Nrp-1 and SE-1, and Nrp-1 expression was increased in PHx-SECs as compared with CONT-SECs (Figure 3A and B). The upregulated Nrp-1 expression in PHx-SECs was consistent with in vivo data shown in Figure 1. Although fluorescence is observed in the perinuclear region as well as the plasma membrane, this may suggest that Nrp-1 presents intracellular trafficking between plasma membrane and intracellular compartments, such as endoplasmic reticulum, Golgi, endosomes and lysosomes.
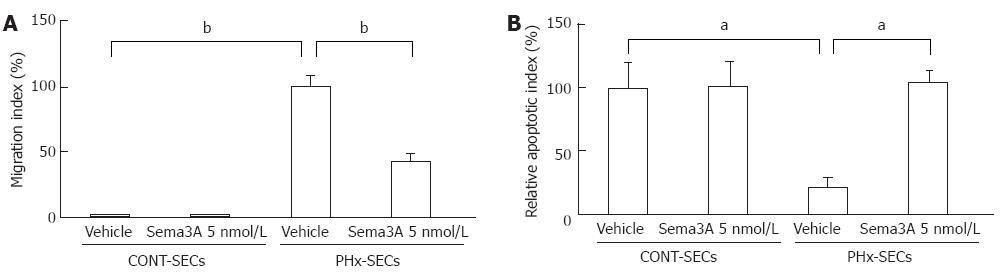
To investigate the effect of Sema3A on SEC mobility, migration of cells was quantified by cell transwell assay in SECs isolated from rats after PHx (PHx-SECs) and normal rats (CONT-SECs). As a result, PHx-SECs were observed to migrate to the lower chamber after incubation for 24 h, but not CONT-SECs, indicating that mobility of PHx-SECs increases as compared with that of CONT-SECs. Moreover, 5 nmol Sema3A significantly attenuated migration of PHx-SECs by 57% (P < 0.01, Figure 4A), while it did not affect proliferation of PHx-SECs (data not shown).
Based on previous reports showing that Sema3A induces apoptosis[23-25], we explored the effect of Sema3A on apoptotic rate in PHx-SECs and CONT-SECs with TUNEL assay. Compared with CONT-SECs, apoptotic rate in PHx-SECs decreased by 78% (P < 0.05, Figure 4B). There was no difference in apoptosis between CONT-SECs that were treated with vehicle and Sema3A. However, in PHx-SECs, apoptosis was induced by the presence of 5 nmol Sema3A for 24 h (P < 0.05, Figure 4B).
The adult hepatocyte is normally a quiescent, highly differentiated cell, but almost immediately it can begin the process of replication in response to conditions that induce cell loss by physical, infectious, or toxic injury. The events involved in the regenerative response are precise, carefully orchestrated, and highly regulated. Indeed, the hepatocyte is a key player in the process of LR, but other cell types, including non-parenchymal cells, also play important roles in reformation of the normal liver architecture.
Among non-parenchymal cells, SECs line the intrahepatic circulatory vessels, named sinusoids, and provide a large surface area for nutrient absorption. Thus, in regenerating liver, SECs play a central role in reconstruction of sinusoids through which blood flow is supplied into the hepatocytes[8,9,26]. Previous reports using electron microscopy have shown that SECs proliferate and migrate into initially formed avascular clusters of proliferating hepatocytes, leading to the formation of new functional sinusoids[9].
In the process of sinusoidal remodeling, various proangiogenic factors are produced in response to upregulation of hypoxia-inducible factor, which is generated within the avascular clusters of hepatocytes. Among these, vascular endothelial growth factor (VEGF) has been shown to be a key regulator of hepatic neovascularization, and is involved in three tyrosine kinase receptors (VEGFR1, R2, and R3), and two non-tyrosine kinase receptors (Nrp-1 and Nrp-2) in inducing signal transduction[27].
Neuropilins were first identified as receptors for class 3 semaphorins, a family of soluble molecules with neuronal guidance functions[28,29]. During development, Nrp-1 is preferentially expressed in arteries, and Nrp-2 is expressed in veins and lymphatics[30]. There is a degree of specificity in semaphorin/neuropilin binding and activity, with semaphorin 3A (Sema3A) binding to Nrp-1, Sema3F binding to Nrp-2 and Sema3B binding to both receptors on endothelial cells[13]. Recently it has been suggested that these semaphorins may interact with the vasculature in an inhibitory manner. Indeed, in vitro studies suggest that class 3 semaphorins compete with VEGF for Nrp-1/Nrp-2 binding, thereby inhibiting mitogenic effects of VEGF in endothelial cells, and leading to apoptosis and inhibition of migration in breast cancer cell lines[31,32].
In this study, to investigate interplay of Nrp-1 and Sema3A in sinusoidal remodeling during LR, we examined the expression of Nrp-1 in liver tissues after PHx in rats, and found that Nrp-1 is constitutively expressed in SECs in normal liver, but its expression is increased following resection of liver, returning to the basal level at termination of liver regeneration. This is consistent with well-documented findings that, following hepatocyte proliferation, DNA synthesis of SECs begins in the late stage of LR[2,7]. Increased expression of Nrp-1 was further confirmed by in vitro study with SECs isolated from rats after PHx, while it was noted to a lesser extent in cells from normal liver.
Interestingly, the inverse expression of Sema3A and Nrp-1 was observed in liver during the process of LR. Immunohistochemistry also indicated the expression of Sema3A in hepatocytes, suggesting the possibility that Sema3A may interact with Nrp-1 that is expressed in SECs. Indeed, our in vitro results show that Sema3A significantly attenuated migration and induced apoptosis in SECs isolated from rats after PHx, but not in cells from normal rats.
Recently, many studies have illustrated the crucial role of Nrp-1 in cell proliferation in development and cancer. The absence of a functional Nrp-1 receptor results in embryonic lethality as a result of impaired heart and blood vessel development, indicating that this receptor plays a central regulatory role during developmental angiogenesis[33]. Furthermore, in endothelial-specific Nrp-1 knockouts, certain arterial markers are missing from arterioles and arteries[34,35]. Alternatively, a selective Sema3A inhibitor has been shown to enhance regenerative responses and functional recovery of the injured spinal cord[36].
Although further studies are required to reveal the underlying mechanism, our results showing inverse expression of Nrp-1 and Sema3A in liver after PHx may provide a better understanding of sinusoidal remodeling in LR.
We thank Dr. Mitsutoshi Tominaga for providing anti-Sema3A antibody and for useful suggestions.
The process of liver regeneration (LR) is complex and involves a finely orchestrated balance of cell proliferation to reconstruct the normal liver architecture. Although hepatocyte proliferation is a major feature of the regenerating liver, it involves the interactions of other hepatic cell types (non-parenchymal cells), including Kupffer cells, stellate cells, sinusoidal endothelial cells and biliary epithelial cells.
Many of the growth factors have been reported to be upregulated in a regenerating liver. Vascular endothelial growth factor (VEGF) is a major proangiogenic factor and is thought to improve sinusoid reconstruction during the LR process.
Recently, the importance of neuropilins (Nrps) as proangiogenic factors has been highlighted. Nrps are transmembrane glycoproteins with large extracellular domains that interact with both class 3 semaphorins and VEGF, and are involved in the regulation of many physiological pathways including angiogenesis. Semaphorin receptors were subsequently found to be expressed by multiple types of cells including endothelial cells.
The findings of this study may offer new insights into the role of Nrp-1 and semaphorin 3A (Sema3A) as a target for novel therapeutic strategies in impaired liver regeneration.
Nrp-1 was originally characterized as a Sema3A receptor regulating axon guidance, and it was later demonstrated that Nrp-1 is also important for vascular morphogenesis. Nrp-1 acts as receptor for VEGF, whereby it serves to promote VEGF-induced angiogenesis. Because Sema3A inhibits angiogenesis through Nrp-1, it has been suggested that it acts by merely competing with VEGF and thus inhibiting its proangiogenic effects.
The authors present interesting results on the interaction of neuropilin and semaphorin in control of angiogenesis during liver regeneration. Overall, the results presented support the conclusions.
Peer reviewer: George K Michalopoulos, MD, PhD, Department of Pathology, School of Medicine, University of Pittsburgh, S-410 Biomedical Science Tower, Pittsburgh, PA 15260, United States
S- Editor Gou SX L- Editor Logan S E- Editor Li JY
| 1. | Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1204] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 3. | Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1246] [Cited by in RCA: 1158] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 4. | Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1091] [Cited by in RCA: 1175] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 5. | Qiao JG, Wu L, Lei DX, Wang L. Insulin promotes sinusoidal endothelial cell proliferation mediated by upregulation of vascular endothelial growth factor in regenerating rat liver after partial hepatectomy. World J Gastroenterol. 2005;11:5978-5983. [PubMed] |
| 6. | Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842-849. [PubMed] |
| 7. | Widmann JJ, Fahimi HD. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. A light and electron microscopic cytochemical study. Am J Pathol. 1975;80:349-366. [PubMed] |
| 8. | Martinez-Hernandez A, Amenta PS. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401-1410. [PubMed] |
| 9. | Wack KE, Ross MA, Zegarra V, Sysko LR, Watkins SC, Stolz DB. Sinusoidal ultrastructure evaluated during the revascularization of regenerating rat liver. Hepatology. 2001;33:363-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene. 1994;9:2683-2690. [PubMed] |
| 11. | Shimizu H, Mitsuhashi N, Ohtsuka M, Ito H, Kimura F, Ambiru S, Togawa A, Yoshidome H, Kato A, Miyazaki M. Vascular endothelial growth factor and angiopoietins regulate sinusoidal regeneration and remodeling after partial hepatectomy in rats. World J Gastroenterol. 2005;11:7254-7260. [PubMed] |
| 12. | Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009;104:428-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 203] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 13. | Staton CA, Kumar I, Reed MW, Brown NJ. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212:237-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 14. | Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411:211-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217-227. [PubMed] |
| 16. | Maione F, Molla F, Meda C, Latini R, Zentilin L, Giacca M, Seano G, Serini G, Bussolino F, Giraudo E. Semaphorin 3A is an endogenous angiogenesis inhibitor that blocks tumor growth and normalizes tumor vasculature in transgenic mouse models. J Clin Invest. 2009;119:3356-3372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Derijck AA, Van Erp S, Pasterkamp RJ. Semaphorin signaling: molecular switches at the midline. Trends Cell Biol. 2010;20:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Higgins GM, Anderson RM. Experimental pathology of the liver. Archives of pathology. 1931;12:186-202. |
| 19. | Braet F, De Zanger R, Sasaoki T, Baekeland M, Janssens P, Smedsrød B, Wisse E. Assessment of a method of isolation, purification, and cultivation of rat liver sinusoidal endothelial cells. Lab Invest. 1994;70:944-952. [PubMed] |
| 20. | Zheng DM, Kitamura T, Ikejima K, Enomoto N, Yamashina S, Suzuki S, Takei Y, Sato N. Sphingosine 1-phosphate protects rat liver sinusoidal endothelial cells from ethanol-induced apoptosis: Role of intracellular calcium and nitric oxide. Hepatology. 2006;44:1278-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Ohmura T, Enomoto K, Satoh H, Sawada N, Mori M. Establishment of a novel monoclonal antibody, SE-1, which specifically reacts with rat hepatic sinusoidal endothelial cells. J Histochem Cytochem. 1993;41:1253-1257. [PubMed] |
| 22. | March S, Hui EE, Underhill GH, Khetani S, Bhatia SN. Microenvironmental regulation of the sinusoidal endothelial cell phenotype in vitro. Hepatology. 2009;50:920-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Shirvan A, Ziv I, Fleminger G, Shina R, He Z, Brudo I, Melamed E, Barzilai A. Semaphorins as mediators of neuronal apoptosis. J Neurochem. 1999;73:961-971. [PubMed] |
| 24. | Bagnard D, Sainturet N, Meyronet D, Perraut M, Miehe M, Roussel G, Aunis D, Belin MF, Thomasset N. Differential MAP kinases activation during semaphorin3A-induced repulsion or apoptosis of neural progenitor cells. Mol Cell Neurosci. 2004;25:722-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282:26294-26305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 174] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Krause P, Rave-Fränk M, Wolff HA, Becker H, Christiansen H, Koenig S. Liver sinusoidal endothelial and biliary cell repopulation following irradiation and partial hepatectomy. World J Gastroenterol. 2010;16:3928-3935. [PubMed] |
| 27. | Herzog B, Pellet-Many C, Britton G, Hartzoulakis B, Zachary IC. VEGF binding to NRP1 is essential for VEGF stimulation of endothelial cell migration, complex formation between NRP1 and VEGFR2, and signaling via FAK Tyr407 phosphorylation. Mol Biol Cell. 2011;22:2766-2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739-751. [PubMed] |
| 29. | Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell. 1997;90:753-762. [PubMed] |
| 30. | Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109:115-119. [PubMed] |
| 31. | Bachelder RE, Lipscomb EA, Lin X, Wendt MA, Chadborn NH, Eickholt BJ, Mercurio AM. Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Res. 2003;63:5230-5233. [PubMed] |
| 32. | Kessler O, Shraga-Heled N, Lange T, Gutmann-Raviv N, Sabo E, Baruch L, Machluf M, Neufeld G. Semaphorin-3F is an inhibitor of tumor angiogenesis. Cancer Res. 2004;64:1008-1015. [PubMed] |
| 33. | Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895-4902. [PubMed] |
| 34. | Gu C, Rodriguez ER, Reimert DV, Shu T, Fritzsch B, Richards LJ, Kolodkin AL, Ginty DD. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev Cell. 2003;5:45-57. [PubMed] |
| 35. | Mukouyama YS, Gerber HP, Ferrara N, Gu C, Anderson DJ. Peripheral nerve-derived VEGF promotes arterial differentiation via neuropilin 1-mediated positive feedback. Development. 2005;132:941-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, Okano HJ, Ikegami T, Moriya A, Konishi O. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |