Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5027
Revised: May 18, 2012
Accepted: May 26, 2012
Published online: September 28, 2012
AIM: To evaluate gastric motility using electrical bio-impedance (EBI) and gastric changes as a result of stress induced by psychological tests.
METHODS: A group of 57 healthy women, aged 40-60 years, was recruited, and a clinical history and physical examination were performed. The women were free from severe anxiety, chronic or acute stress, severe depression, mental diseases and conditions that affect gastric activity. The women were evaluated under fasting conditions, and using a four-electrode configuration, the gastric signals were obtained through a BIOPAC MP-150 system. The volunteers were evaluated using the following paradigm: basal state, recording during the Stroop Test, intermediate resting period, recording during the Raven Test, and a final resting period. We analyzed the relative areas of the frequency spectrum: A1 (1-2 cpm), A2 (2-4 cpm), A3 (4-8 cpm), and A4 (8-12 cpm), as well as the median of area A2 + A3. The data were analyzed by an autoregressive method using a Butterworth filter with MatLab and Origin. Analysis of variance (ANOVA) and Friedman ANOVA (for nonparametric variables) were performed; in addition, pairs of groups were compared using the T dependent and Wilcoxon T tests.
RESULTS: The results of the main values of area A2 were not significantly different comparing the five steps of the experimental paradigm. Nevertheless, there was a tendency of this A2 region to decrease during the stress tests, with recuperation at the final resting step. When an extended gastric region was considered (1-4 cpm), significant differences with the psychological stress tests were present (F = 3.85, P = 0.005). The A3 region also showed significant changes when the stress psychological tests were administered (F = 7.25, P < 0.001). These differences were influenced by the changes in the adjacent gastric region of A2. The parameter that we proposed in previous studies for the evaluation of gastric motility by electrical bio-impedance (EBI) was the median of the area under the region from 2 to 8 cpm (A2 + A3). The mean values of these frequencies (median of the A2 + A3 area) with the stress test showed significant changes (F = 5.5, P < 0.001). The results of the Wilcoxon T test for the A4 area parameter, which is influenced by the breathing response, changed significantly during the Raven stress test (P < 0.05).
CONCLUSION: We confirm that the gastric response to acute psychological stress can be evaluated by short-term EBI.
- Citation: Huerta-Franco MR, Vargas-Luna M, Montes-Frausto JB, Morales-Mata I, Ramirez-Padilla L. Effect of psychological stress on gastric motility assessed by electrical bio-impedance. World J Gastroenterol 2012; 18(36): 5027-5033
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5027.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5027
The relationship between emotions and gastric motility has been documented since the beginning of the last century by Cabanis and Beaumont and thereafter by Pavlov and Canon, who were the pioneers in determining the gastric response after an emotional stimulus in animal models[1-3]. In 1934, Hall described the autonomic response of rats exposed to unfamiliar events[1,4]. The effects of stress on the gastrointestinal (GI) tract have been reported since the first studies on the general adaptation syndrome by Selye[5] in 1936, who confirmed the physical, physiological and psychological responses of a living organism due to stress. In healthy humans, anger, fear, labyrinthine stimulation, painful stimuli, preoperative anxiety and intense exercise are some of the causes of slow gastric emptying[1,6,7]. The inhibition of gastric emptying and stimulation of colonic motor function are the most commonly encountered patterns induced by various stressors[1].
In the study of psychological stress as a risk factor for human health, there are few scientific reports in relation to the GI system, and most of the studies in humans have focused on the cardiovascular system because there is a clinical association of psychological and physical stress with a high risk for stroke and cardiovascular events, as well as with increased morbidity and mortality of the population. However, the evaluation of gastric function, particularly gastric motility and emptying, during psychological stress has not been given sufficient scientific attention despite the high prevalence of gastric problems due to psychological stress, such as gastritis, abdominal pain and gastroenteritis[8]. The alterations in the GI motility pattern by various stressors have been documented, although scarcely, with the development of quantitative techniques to monitor GI motility and transit in experimental animals and humans[9,10]. The gold standard technique accepted for gastric activity evaluation is scintigraphy, although this technique measures gastric emptying as the main consequence of gastric motility[11]; however, this technique is invasive. For the direct assessment of gastric motility, there are other invasive methods, such as manometry, which uses a probe placed at a specific point in the GI tract to record directly the frequency and amplitude of the GI movements[12].
Electrogastrography (EGG) is considered an invasive procedure when placing the electrodes in the GI cavity to record the myoelectrical activity of a specific inner surface of the tract[13,14]. However, EGG is a noninvasive technique in which the electrodes are placed on the skin. Another similar technique is the use of electrical bio-impedance (EBI), which also uses cutaneous electrodes in the gastric region[15-17]. In these last two techniques, the main challenge is the interpretation of the signal due to the overlapping information from different regions in the gastric system, particularly with respect to lung region movements in the case of EBI; in both cases, different motility frequencies are involved[16,18]. In general terms, gastric clinical research requires a strong link between the accuracy and reliability of the method and the comfort of the patient[14]. Thus, from this point of view, noninvasive EGG and EBI assessments have advantages over other techniques[19]. Therefore, the use of short-term EBI can be a good tool to evaluate the gastric response to psychological stress.
Furthermore, the possibility of permanent monitoring at a low cost is the preamble for a feedback program to be used in stress treatment and control. Gastric signals are not frequently used for this purpose, although this physiological function is definitively affected by stress. Gastric waves occur at a rate of 3 cpm on average and up to 12 cpm in the intestinal region, therefore, a long recording period is likely needed to assess gastric motility accurately, which contrasts with the need for short, acute stress tests and hence the need for short-term gastric motility recordings. Our hypothesis is that by observing the relative changes of the entire GI activity in the window of frequencies from 1 to 8 cpm, gastric motility can be used to monitor the responses to acute stress that results from the application of psychological tests that last < 10 min, such as the Stroop[20,21] and Raven tests[22].
A group of 57 healthy women, aged 40-60 years (mean ± SD: 48.19 ± 5.98 years), was recruited after the protocol was approved by the Division of Health Sciences Campus Leon at the University of Guanajuato, Mexico. Written informed consent was obtained from all participants, and the study was conducted according to the guidelines outlined in the Declaration of Helsinki (World Health Organization, 1996)[23].
Before starting the experiment, clinical assessments were conducted for all participants. These assessments included the following: (1) general information, including name, age, sex, and occupation; (2) clinical history, with a clinical evaluation of GI-related diseases; (3) physiological data, anthropometric measurements, and body mass index; (4) lifestyle information, including exercise, sleep habits, and substance abuse (tobacco, alcohol, medication); and (5) additional information obtained by a physical examination. For the purpose of this study, this information was used to assure the internal validity of the study (homogeneity of the study group and the absence of health problems related to the GI tract). To participate in the study, the women had to have met the following criteria: 40-60 years of age, no prior GI disease, and no prior disease that may affect the GI system (diabetes, Parkinson’s disease, amyloidosis, myotonic dystrophy, polymyositis, human immunodeficiency virus infection or cytomegalovirus infection). The women were required not to have been taking any treatment that could interfere with gastric activity, have any other endocrine disorder, or have had significant weight loss within 3 mo prior to the study. In addition to these physical criteria, the women had to be free from psychological problems, such as severe anxiety, chronic or acute stress, severe depression and mental disease, because these conditions could potentially affect gastric activity though endocrine and central nervous system modulation[1].
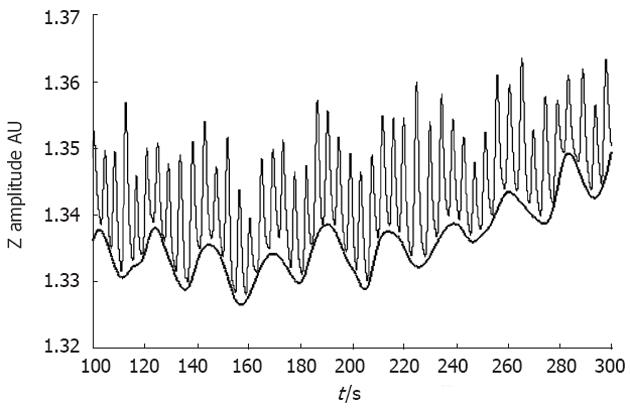
Prior to the evaluation, each woman was asked to arrive under fasting conditions (more than 8 h). In addition, the women refrained from smoking, alcohol consumption, strenuous exercise and caffeine for 24 h before the evaluation. The women were evaluated while in the semi-Fowler supine position to enable them to observe a screen on the wall with the projection of the psychological test (Stroop and Raven tests). For this experiment, we used the four-electrode configuration. This configuration consisted of two electrodes placed on the abdomen and two on the back. One pair (one electrode placed on the back and the other placed on the anterior part) was used for the current injection, and the other pair was used for voltage recording. The first electrode was placed near the rib cage using the midline of the abdomen as a reference. The second electrode was placed approximately 5 cm from the first, 45 degrees down and left of the umbilicus. The back electrodes were placed at 2.5 cm off the spine at the level of both electrodes on the abdomen. The electrode configuration used in this study was similar to that used in previous studies, which has shown good results[24]. The gastric signals were obtained through a BIOPAC MP-150 system and the corresponding EBI module[24]. The rough data mainly show oscillations due to breathing and a slower oscillation due to gastric movements (Figure 1).
The gastric function for all volunteers was evaluated before, during and after being subjected to two psychological tests that have been shown to produce psychological stress: the Stroop and Raven tests[20-22]. The Stroop Test consisted of a 3-min session during which words in color were projected upon a screen at the rate of one word per second. The participant was asked to say the color of the word. During the first 40 s, words that name several objects were projected on a screen, followed by 1 min of words that name colors. The written color sometimes corresponded to the color of the word and sometimes did not[20]. Finally, words that named several objects but not colors were shown[21]. The Raven Test is a psychological test to determine the intelligence quotient (IQ), which consists of solving sequences and completing figures or truncated shapes. This test was performed without a time limit because the purpose was not the evaluation of IQ but simply to induce a state of psychological stress in the participant[22].
The following paradigm was used in this study: (1) 10 min of basal gastric motility (BGM) recording by EBI (before the test); (2) EBI recording during the Stroop Test, lasting 3 min; (3) an intermediate resting period (IRP) of 3 min; (4) EBI recording during the Raven Test, lasting approximately 4 min; and (5) the final resting period (FRP), lasting 3 min.
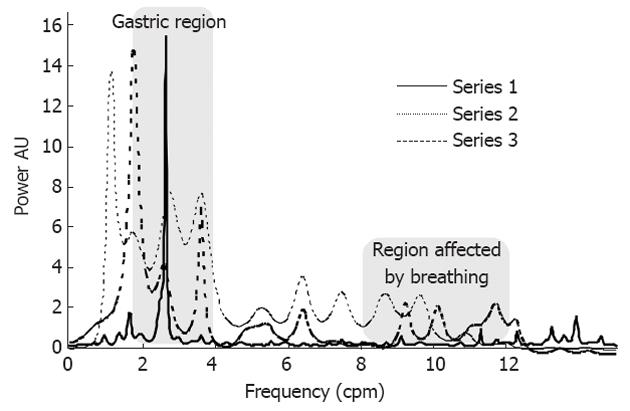
In general, the parameter used to evaluate gastric motility is the position of the main peak in the region from 2 to 4 cpm of the frequency spectrum of gastric activity. In this study, we proposed alternative parameters when EBI was used[16] to examine the gastric motility changes due to psychological stress. We proposed the analysis of the relative area under the frequency domain curve in different regions of the spectrum as a relative measure of the gastric global activity in each frequency region. The frequency range was divided into four regions: the first (A1) included frequencies from 1 to 2 cpm and defined the low frequencies; the second (A2) included the frequencies from 2 to 4 cpm and defined the main frequency range of the gastric region activity according to the literature[1,7,9] (although some authors define a more restricted region for gastric motility as 2.5 to 3.75 cpm); the third (A3) included the frequencies from 4 to 8 cpm and contained the frequencies for the regions where bowel movements were important, which was mainly from the ileum (7-8 cpm)[1] or when tachygastria was present; and the fourth (A4) included the frequencies from 8 to 12 cpm and accounted for duodenum motility, breath frequency and tachygastria. The latter region, due to the strong contribution of the respiratory system, was not considered useful for gastric activity analysis because stress causes the breath frequency to change to higher values, diminishing the contribution of this area. A typical frequency spectrum and gastric changes occurring during the stress tests is shown in Figure 2.
We recorded the areas relative to the total area AT from 1 to 12 cpm. In addition, the median of the area from 2 to 8 cpm (frequency for which the area under the spectrum was divided into two equal regions) was considered for analysis. This median changed when the relative areas of 2-4 cpm and/or 4-8 cpm changed, providing a good indication of the importance of the gastric motility compared with the adjacent frequency region. The advantage of these parameters was that small changes in the frequency spectrum, due to small variations in the data or data analysis, did not significantly affect these global parameters.
The data were analyzed by an autoregressive method using a Butterworth filter in the frequency range from 1 to 12/min (or cpm), i.e., 0.017-0.2 Hz. Particular attention was given to the region from 2 to 4/min (0.033-0.066 Hz) and from 4 to 8/min (0.066-0.132 Hz). In addition to this range, we analyzed the adjacent region, from 1 to 2 cpm, presented as the integrated region from 1 to 4 cpm, which is viewed as an extended gastric region. These analyses were performed using MatLab 6.5 and Origin 6.0 according to previous studies[16,24].
The comparison analysis within and between the experimental paradigm steps (BGM, Stroop Test, IRP, Raven Test, FRP) was performed using analysis of variance (ANOVA) and post-hoc analysis for variables with a normal distribution (A2, A1 + A2, A3, and the median under A2 + A3) and using the Friedman ANOVA for variables with a non-normal distribution (A4). Considering that the same women were evaluated before and after the psychological stress tests, we compared the dependent variables in the different experimental paradigm steps (BGM, Stroop Test, IRP, Raven Test, FRP) using the T dependent test on the normally distributed data (A1, A2, and A3 regions and the median of the area under the A2 + A3 region), and the T of the Wilcoxon test when non-normal distribution was found (A4 region). The statistical analyses were performed using STATISTICA 7 (Stat-Soft, Inc. Tulsa, OK, United States). We considered a P value ≤ 0.05 to be significant.
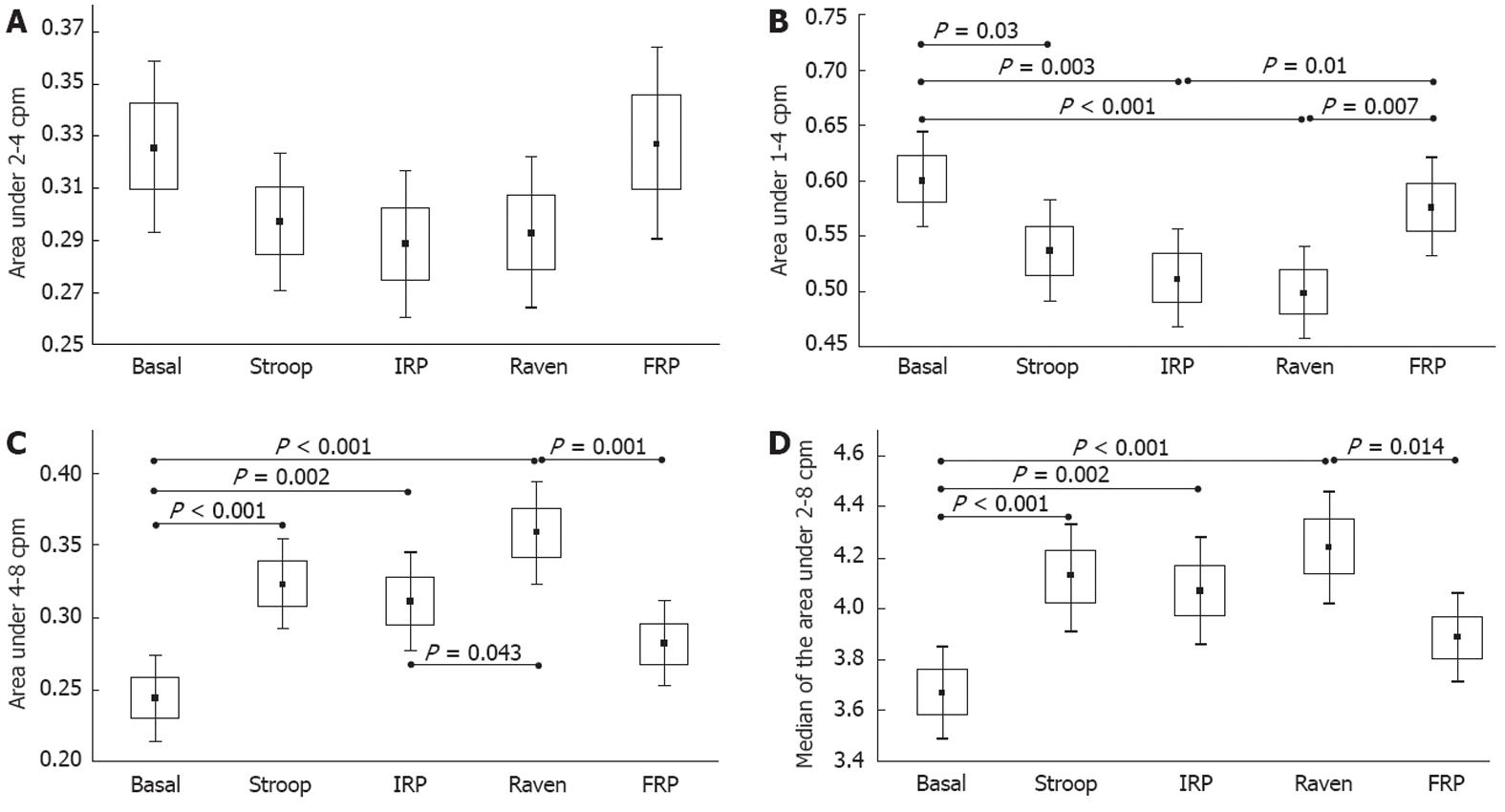
The results of the gastric activity A2 area, indicated by the main range from 2 to 4 cpm, are presented in Figure 3A. We observed that none of the mean values of this A2 region changed significantly by ANOVA (F = 1.46, P = 0.22), even when we compare pairs of steps from the experimental paradigm (BGM, Stroop Test, IRP, Raven Test, FRP). However, a tendency towards a decrease in this A2 region during the stress tests was observed with a remarkable recovery during the FRP.
The results of the ANOVA for A1 + A2, A3 and the median under A2 + A3 had significant differences among the steps of the experimental paradigm (F = 3.85, P = 0.005, F = 7.25, P < 0.001, and F = 5.5, P < 0.001, respectively). Pairs for the group data analysis are presented in Figure 3B, which shows the results for the region from 1 to 4 cpm (referred to as the extended gastric region). We observed significant changes from the BGM step to the Stroop Test (P = 0.02), from the BGM step to the IRP (P = 0.003), and from the BGM step to the Raven Test (P < 0.001). In addition, we found significant changes from the IRP and Raven Test to the FRP, with P = 0.01 and P = 0.007, respectively. Similar results to the A1 + A2 region were observed in the A3 region (4-8 cpm), which showed significant changes when the stress psychological tests were administrated from BGM to Stroop Test, IRP, and Raven Test (P < 0.001, P = 0.002, and P < 0.001, respectively) (Figure 3C). There were also significant changes from the IRP to the Raven Test (P = 0.043) and from the Raven Test to the FRP (P = 0.001) (Figure 3C). These differences were influenced by changes in the adjacent gastric region, A2 (2-4 cpm), and the higher frequency region, A4 (8-12 cpm), but not as much by the decreased ileum activity. As we described in the methodology, one of the parameters that we have proposed in previous studies[16,24] as a useful parameter for EBI gastric studies is the median of the area under the region from 2 to 8 cpm (A2 + A3); namely, the frequency that divides this area into two equal parts. The mean values of these parameters (median of the A2 + A3 area) are presented in Figure 3D, which also shows the statistical changes when the stress psychological tests were administered, with significant variations from the BGM step to the Stroop Test, IRP and Raven Test (P < 0.001, P = 0.002, and P < 0.001, respectively). We also observed significant changes from the Raven Test to the FRP (P = 0.014).
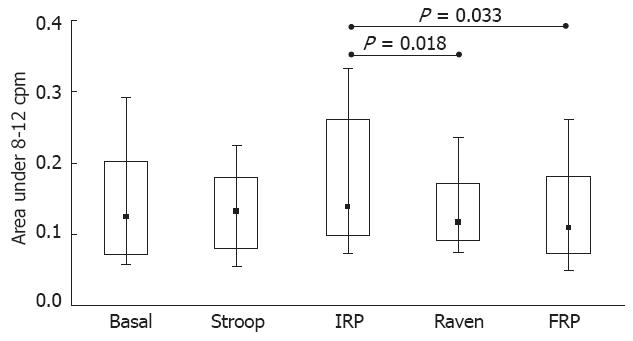
The results of the A4 (8-12 cpm) Friedman ANOVA did not show a significant difference (χ2 = 8.44, P = 0.077). The comparisons among the groups of variables are presented in Figure 4, which gives the results of the Wilcoxon T test for the A4 area. This region, which was influenced by the breathing response, changed significantly during the Raven Test, with significant changes from the IRP step to the Raven Test and from the IRP to the FRP (P = 0.018 and P = 0.033, respectively).
We used the short-term EBI technique to evaluate the physiological changes in gastric activity due to psychological stress caused by the Stroop[20,21] and Raven[22] Tests in healthy women. Several studies have shown that gastric motility alterations are good clinical indicators of diseases[18,25-28]. Using EGG and EBI as noninvasive techniques for assessing gastric motility, we can ensure that the accuracy and reliability of the test and the comfort of the patient are the prevailing conditions under which each patient is assessed[29-32].
As described above, the most widely used parameter for the assessment of gastric motility is the highest peak of activity in the region from 2 to 4 cpm of the frequency spectrum. This parameter could not be trusted in our case because the EBI recorded information from other parts of the GI tract and other systems (breathing)[33-35] strongly interfered with the desired signal. In addition, the short-term EBI recording prevented us from relying on a parameter as being punctual in the low frequency region (2-4 cpm). The area under a specific frequency region is expected to give the relative level of activity in that frequency region without needing the details of the spectrum. An alternative to the EBI gastric motility measurement of two or more areas is the recording of the median of the entire area. In this study, the changes found in gastric motility, as measured by the relative areas, were considered to have statistical significance when P was ≤ 0.05, and in other cases, we included the graph only to present the tendencies that were physiologically compatible with what we expected as a response to gastric stress[24].
Taking the basal gastric measurement as a reference, we observed that the Stroop Test induced changes in the A1 + A2, A3 and the median of the A2 + A3 parameters (Figure 3B-D), in agreement with the inhibition of gastric activity during psychological stress. We hypothesized that the decrease in the activity in the gastric region produced the increase in the A3 region (Figure 3C). The region from 8 to 12 cpm did not show significant changes during the Stroop Test.
In the intermediate resting period, we observed a slight recovery in the A3 and A4 regions and in the median of the A2 + A3 region; the A1 + A2 region showed a continuing effect of the Stroop Test. None of these changes were statistically significant. During the Raven Test, the A3 region changed significantly by a strengthened effect of the stress caused by the Stroop Test. This effect was also observed in the A4 region. The final resting period change was highly significant in the regions A1 + A2 and A3 and in the median of A2 + A3.
Some general observations should be addressed at this point. All of the parameters consider relative areas under the whole region from 1 to 12 cpm or the median frequency of a relative area; thus, a change in any of these parameters is not only due to the local change, but is also influenced by the changes in the other parameters. The apparent stability of the relative area of A4, which represents approximately 15% of the total area and which includes the breathing frequency, is the result of the breath going beyond the higher frequency limit (12 cpm) and the real decrease in the GI activity (A1 + A2). We believe that the continuing effects of stress after completing the Stroop Test were due in part to the knowledge of the participants that they would receive a second test, which predisposed them towards having a continuous stress response (Figures 3B-D and 4). The immediate recovery of the participants at the end of the Stoop and Raven Tests, as shown by the evaluated parameters reaching the basal values, was likely affected by the knowledge of the participants that the test was complete (Figure 3B-D).
Changes due to stress vary between participants. Typically, the stress starts at the beginning of the psychological test, with a variable reaction time, duration and intensity[24]. Gastric motility changes, with an acceptable physiological explanation, give a clear indication that short-term gastric EBI recording could be useful in routine studies of the response to psychological stress. Further research should address alternative signal analysis approaches, validation, movement discrimination, limitations, variability factors, the normal range of significant parameters, and assessment protocols.
Psychological stressors may have different patterns, accompanied by increases in the sympathetic nervous system activity and total blood vessel peripheral resistance[36]. Therefore, more cardiovascular activity is involved in a specific task that requires active action (as in the Stroop Test) than in those that are considered passive, maintaining a close similarity to the response to a real situation. After using the Stroop Test as a stressor, there was an increase in anxiety, heart rate, respiration, electrodermal activity, muscle tension and the levels of catecholamines, among other psychophysiological variables. The Stroop Test includes complex neuropsychological processes related to the ability to classify information, act selectively, and block non-requested information linked to frontal lobe functions. It is based on the observation that naming colors occurs noticeably slower than reading words. This test assesses some cognitive functions, such as attention, mental flexibility, and the inhibition of automatic responses, related to executive functions associated with the frontal lobes. The other test that has been certified widely in the literature for its usefulness is the Raven Test; however, it is limited by the verbal and cultural influences, level of schooling, economic level, and age. Taken together, the results presented here indicate that short-term gastric EBI has great potential to become a user-friendly methodology to assess gastric motility, particularly with respect to the evaluation of the acute stress response.
In conclusion, the results from this study, which was performed in healthy women, clearly demonstrated that using different parameters, such as the relative areas under the frequency spectrum and the analysis of the median of those areas, in short-term recordings of gastric EBI may be useful for the evaluation of the sympathetic nervous system response to acute psychological stress. The relatively simple signal analysis required for the gastric EBI could make this technique a good candidate for a basic clinical evaluation and even an ambulatory method to assess gastric motility. One of the main advantages of this technique is that it can be implemented using small and inexpensive devices once the frequency and amplitude of the stimulation are known.
The authors thank all the volunteers for their enthusiastic participation. In addition, Huerta-Franco MR and Vargas-Luna M thank Research and Graduate Department and the University of Guanajuato for financial support.
The relationship between emotions and gastric motility has been documented since the last century. Likewise, psychological stress produces alterations in gastrointestinal tract response in humans. There are several methods for measuring gastric response to stress, but many of these methods are invasive. In this study, authors proposed a novel technique, which was inexpensive and easy to manage, to measure the gastric response to psychological stress by electrical bio-impedance (EBI).
In healthy humans, anger, fear, painful stimuli, preoperative anxiety and intense exercise are some of the causes of slow gastric emptying. The principal noninvasive techniques to evaluate the gastric motility response during psychological stress are electrogastrography (EGG) and the EBI. In these two techniques, the main challenge is the interpretation of the signal due to the overlapping information from different regions in the gastric system, particularly with respect to lung region movements in the case of EBI; in both cases, different motility frequencies are involved.
The authors confirm that the gastric response to acute psychological stress can be evaluated by short-term EBI.
The short-term technical EBI is useful for measuring the gastric response to psychological stress. Besides, it may be useful for frequent monitoring of health status of patients with gastric disorders; being an easy-to-use method of clinical monitoring and diagnosis.
EGG is a technique to record the myoelectrical activity of a specific inner surface of the gastrointestinal tract. EGG is a noninvasive technique, in which the electrodes are placed on the skin. EBI records the motility of the stomach using cutaneous electrodes placed in the gastric region. Stroop and Raven Tests are valid and reliable tests to produce psychological stress.
This study clearly demonstrated that short-term EBI may be useful for evaluating the gastric activity response to acute psychological stress.
Peer reviewers: Ming Li, Associate Professor, Tulane University Health Sciences Center, 1430 Tulane Ave Sl-83, New Orleans, LA 70112, United States; Guang-Yin Xu, MD, PhD, Assistant Professor, Division of Gastroenterology, Department of Internal Medicine, University of Texas Medical Branch, Galveston, TX 77555-0655, United States; Cesare Tosetti, MD, Department of Primary Care, Health Care Agency of Bologna, Via Rosselli 21, 40046 Porretta Terme (BO), Italy
S- Editor Lv S L- Editor Kerr C E- Editor Li JY
| 1. | Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173-G177. [PubMed] |
| 3. | Schäfer PK, Sauerbruch T. [Rudolf Schindler (1888--1968)--"father" of gastroscopy]. Z Gastroenterol. 2004;42:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Holt S, McDicken WN, Anderson T, Stewart IC, Heading RC. Dynamic imaging of the stomach by real-time ultrasound--a method for the study of gastric motility. Gut. 1980;21:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2007] [Cited by in RCA: 1447] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 6. | Rao SS, Hatfield RA, Suls JM, Chamberlain MJ. Psychological and physical stress induce differential effects on human colonic motility. Am J Gastroenterol. 1998;93:985-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Tache Y. Stress-induced alterations of gastric emptying. Stress an Digestive Motility. Montrouge, France: John Libbey Eurotext 1989; 123-132. |
| 8. | Li Z, Ren C. Gastric motility measurement and evaluation of functional dyspepsia by a bio-impedance method. Physiol Meas. 2008;29:S373-S382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Mariani G, Boni G, Barreca M, Bellini M, Fattori B, AlSharif A, Grosso M, Stasi C, Costa F, Anselmino M. Radionuclide gastroesophageal motor studies. J Nucl Med. 2004;45:1004-1028. [PubMed] |
| 10. | Ajaj W, Lauenstein T, Papanikolaou N, Holtmann G, Goehde SC, Ruehm SG, Debatin JF. Real-time high-resolution MRI for the assessment of gastric motility: pre- and postpharmacological stimuli. J Magn Reson Imaging. 2004;19:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Vantrappen G. Methods to study gastric emptying. Dig Dis Sci. 1994;39:91S-94S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Stanghellini V, Ghidini C, Tosetti C, Franceschini A, Ricci Maccarini M, Corinaldesi R, Barbara L. [Comparison of methods: gastro-duodenal manometry and study of gastric emptying]. Minerva Chir. 1991;46:125-130. [PubMed] |
| 13. | Chen JD, Schirmer BD, McCallum RW. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am J Physiol. 1994;266:G90-G98. [PubMed] |
| 14. | Jonderko K, Kasicka-Jonderko A, Błońska-Fajfrowska B. Does body posture affect the parameters of a cutaneous electrogastrogram? J Smooth Muscle Res. 2005;41:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | McClelland GR, Sutton JA. Epigastric impedance: a non-invasive method for the assessment of gastric emptying and motility. Gut. 1985;26:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Huerta-Franco R, Vargas-Luna M, Hernandez E, Capaccione K, Cordova T. Use of short-term bio-impedance for gastric motility assessment. Med Eng Phys. 2009;31:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Giouvanoudi A, Amaee WB, Sutton JA, Horton P, Morton R, Hall W, Morgan L, Freedman MR, Spyrou NM. Physiological interpretation of electrical impedance epigastrography measurements. Physiol Meas. 2003;24:45-55. [PubMed] |
| 18. | Amaris MA, Sanmiguel CP, Sadowski DC, Bowes KL, Mintchev MP. Electrical activity from colon overlaps with normal gastric electrical activity in cutaneous recordings. Dig Dis Sci. 2002;47:2480-2485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Smout AJ, Jebbink HJ, Akkermans LM, Bruijs PP. Role of electrogastrography and gastric impedance measurements in evaluation of gastric emptying and motility. Dig Dis Sci. 1994;39:110S-113S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Delaney JP, Brodie DA. Effects of short-term psychological stress on the time and frequency domains of heart-rate variability. Percept Mot Skills. 2000;91:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: general emotional or disorder specificity? J Anxiety Disord. 2001;15:147-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Andersson S, Finset A. Heart rate and skin conductance reactivity to brief psychological stress in brain-injured patients. J Psychosom Res. 1998;44:645-656. [PubMed] |
| 23. | Rickham PP. Human experimentation. Code of ethics of the world medical association. declaration of helsinki. Br Med J. 1964;2:177. [PubMed] |
| 24. | Huerta-Franco MR, Vargas-Luna M, Capaccione KM, Yañez-Roldán E, Hernández-Ledezma U, Morales-Mata I, Córdova-Fraga T. Effects of metoclopramide on gastric motility measured by short-term bio-impedance. World J Gastroenterol. 2009;15:4763-4769. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Gilger MA, Boyle JT, Sondheimer JM, Colletti RB. A medical position statement of the North American Society for Pediatric Gastroenterology and Nutrition. Indications for pediatric esophageal manometry. J Pediatr Gastroenterol Nutr. 1997;24:616-618. [PubMed] |
| 26. | Gilger MA. Gastroenterologic endoscopy in children: past, present, and future. Curr Opin Pediatr. 2001;13:429-434. |
| 27. | Daghastanli NA, Braga FJ, Oliveira RB, Baffa O. Oesophageal transit time evaluated by a biomagnetic method. Physiol Meas. 1998;19:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Tack J, Coulie B, Verbeke K, Janssens J. Influence of delaying gastric emptying on meal-related symptoms in healthy subjects. Aliment Pharmacol Ther. 2006;24:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Ferdinandis TG, Dissanayake AS, De Silva HJ. Effects of carbohydrate meals of varying consistency on gastric myoelectrical activity. Singapore Med J. 2002;43:579-582. [PubMed] |
| 30. | Koyama S, Hosoda S. [Evaluation of gastric motility using vector analysis of electrogastrography]. J Smooth Muscle Res. 1994;30:21-34. [PubMed] |
| 31. | Podczeck F, Mitchell CL, Newton JM, Evans D, Short MB. The gastric emptying of food as measured by gamma-scintigraphy and electrical impedance tomography (EIT) and its influence on the gastric emptying of tablets of different dimensions. J Pharm Pharmacol. 2007;59:1527-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 32. | Huerta-Franco MR, Vargas-Luna M, Montes-Frausto JB, Flores-Hernandez C, Morales-Mata I. Electrical Bioimpedance and other techniques for gastric emptying and motility evaluation. World J Gastrointest Pathophysiol. 2012;3:10-18. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Doglietto F, Prevedello DM, Jane JA, Han J, Laws ER. Brief history of endoscopic transsphenoidal surgery--from Philipp Bozzini to the First World Congress of Endoscopic Skull Base Surgery. Neurosurg Focus. 2005;19:E3. [PubMed] |
| 34. | Janssen P, Vanden Berghe P, Verschueren S, Lehmann A, Depoortere I, Tack J. Review article: the role of gastric motility in the control of food intake. Aliment Pharmacol Ther. 2011;33:880-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 35. | Quigley EM. Review article: gastric emptying in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2004;20 Suppl 7:56-60. [PubMed] |
| 36. | Perna FM, Schneiderman N, LaPerriere A. Psychological stress, exercise and immunity. Int J Sports Med. 1997;18 Suppl 1:S78-S83. [PubMed] |