Published online Sep 28, 2012. doi: 10.3748/wjg.v18.i36.5021
Revised: July 26, 2012
Accepted: July 29, 2012
Published online: September 28, 2012
AIM: To evaluate the agreement between transrectal ultrasound (TRUS) and magnetic resonance imaging (MRI) in classification of ≥ T3 rectal tumors.
METHODS: From January 2010 to January 2012, 86 consecutive patients with ≥ T3 tumors were included in this study. The mean age of the patients was 66.4 years (range: 26-91 years). The tumors were all ≥ T3 on TRUS. The sub-classification was defined by the penetration of the rectal wall: a: 0 to 1 mm; b: 1-5 mm; c: 6-15; d: > 15 mm. Early tumors as ab (≤ 5 mm) and advanced tumors as cd (> 5 mm). All patients underwent TRUS using a 6.5 MHz transrectal transducer. The MRI was performed with a 1.5 T Philips unit. The TRUS findings were blinded to the radiologist performing the interpretation of the MRI images and measuring the depth of extramural tumor spread.
RESULTS: TRUS found 51 patients to have an early ≥ T3 tumors and 35 to have an advanced tumor, whereas MRI categorized 48 as early ≥ T3 tumors and 38 as advanced tumors. No patients with tumors classified as advanced by TRUS were found to be early on MRI. The kappa value in classifying early versus advanced T3 rectal tumors was 0.93 (95% CI: 0.85-1.00). We found a kappa value of 0.74 (95% CI: 0.63-0.86) for the total sub-classification between the two methods. The mean maximal tumor outgrowth measured by TRUS, 5.5 mm ± 5.63 mm and on MRI, 6.3 mm ± 6.18 mm, P = 0.004. In 19 of the 86 patients the following CT scan or surgery revealed distant metastases; of the 51 patients in the ultrasound ab group three (5.9%) had metastases, whereas 16 (45.7%) of 35 in the cd group harbored distant metastases, P = 0.00002. The odds ratio of having distant metastases in the ultrasound cd group compared to the ab group was 13.5 (95% CI: 3.5-51.6), P = 0.00002. The mean maximal ultrasound measured outgrowth was 4.3 mm (95% CI: 3.2-5.5 mm) in patients without distant metastases, while the mean maximal outgrowth was 9.5 mm (95% CI: 6.2-12.8 mm) in the patients with metastases, P = 0.00004. Using the MRI classification three (6.3%) of 48 in the MRI ab group had distant metastases, while 16 (42.1%) of the 38 in the MRI cd group, P = 0.00004. The MRI odds ratio was 10.9 (95% CI: 2.9-41.4), P = 0.00008. The mean maximal MRI measured outgrowth was 4.9 mm (95% CI: 3.7-6.1 mm) in patients without distant metastases, while the mean maximal outgrowth was 11.5 mm (95% CI: 7.8-15.2 mm) in the patients with metastases, P = 0.000006.
CONCLUSION: There is good agreement between TRUS and MRI in the pretreatment sub-classification of ≥ T3 tumors. Distant metastases are more frequent in the advanced group.
- Citation: Rafaelsen SR, Vagn-Hansen C, Sørensen T, Pløen J, Jakobsen A. Transrectal ultrasound and magnetic resonance imaging measurement of extramural tumor spread in rectal cancer. World J Gastroenterol 2012; 18(36): 5021-5026
- URL: https://www.wjgnet.com/1007-9327/full/v18/i36/5021.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i36.5021
Depth of tumor spread beyond the rectal wall has been validated as an important prognostic indicator[1-3]. Tumors with 5 mm or less of extramural spread regardless of node status have an 85% cancer-specific survival rate compared with poorer-prognosis tumors with more than 5 mm spread, which have only a 54% 5-year cancer-specific survival rate[4]. The MERCURY study[5] showed that tumor spread can be estimated accurately with magnetic resonance imaging (MRI) in comparison with pathologic results. Studies have shown that there was good to very good agreement on measuring the maximal extramural tumor spread using MRI compared to histopathology[6,7]. The clinical guidelines for neoadjuvant therapy vary among countries.
The tumor extending 5 mm or more into perirectal fat is considered a high-risk disease[8]. Patients with a tumor infiltration depth more than 5 mm are at some institutions offered preoperative long-course chemoirradiation therapy. However, MRI cannot be used in all patients due to contraindications, pacemaker, claustrophobia, etc.
Transrectal ultrasound (TRUS) has been shown to be accurate in staging early rectal tumors[9-12] including accurate evaluation of penetration of the rectal wall[13-17]. The ability of TRUS to discriminate between early and advanced tumors has not been well documented. The aim of the present study was to compare the intermodality agreement of TRUS with MRI in measuring the depth of extramural tumor spread in rectal tumors, and to determine the incidence of distant metastases with regard to tumor invasion measured by TRUS.
The study was approved by the local ethics committee and by the National Data Protection Agency (jr. nr. 2011-41-6988), according to national law.
From January 2010 to January 2012, 153 consecutive patients with suspected rectal carcinomas were referred to the Department of Radiology for local staging. The patients were retrieved in the Radiology Information System (Kodak Carestream RIS 2008, Eastman Kodak Company, NY, United States). Fourteen patients having neither TRUS nor MRI were excluded as well as 11 patients with other cancers. The reasons for exclusion are listed in Table 1. A total of 86 patients with sonographical ≥ uT3 tumors were included in this study, five of these were uT4. The mean age of the patients was 66.4 years (range: 26-91 years). Forty-five of the 86 patients were treated with radio-chemotherapy. The diagnosis of rectal carcinoma was confirmed in all patients by histopathological examination of the biopsy material. The sub-classification of ≥ T3 was defined as: a with a penetration of the rectal wall from 0 to 1 mm, b: 1-5 mm, c: 6-15 mm, d: > 15 mm. Early ≥ T3 tumors as ab (≤ 5 mm penetration) and advanced tumors as cd (> 5 mm penetration).
| Patients with suspected rectal cancer admitted to local staging using TRUS and MRI | n |
| Excluded: | |
| No MRI: pacemaker: 3; claustrophobia: 1 | 4 |
| No TRUS: poor cleansing: 4; pain: 2; refused: 2 | 8 |
| Other cancers excluded: anal cancer: 7; carcinoid: 2; GIST: 1; prostate cancer: 1 | 11 |
| Adenocarcinomas excluded: duplex tumour: 1; stent: 1; sigmoid tumour: 5 | 7 |
| < uT3: uT1: 9; uT2: 18 | 27 |
| uT3 with insufficient documentation of outgrowth measurement | 4 |
| uT4 with insufficient documentation of outgrowth measurement | 6 |
| Total study group | 86 |
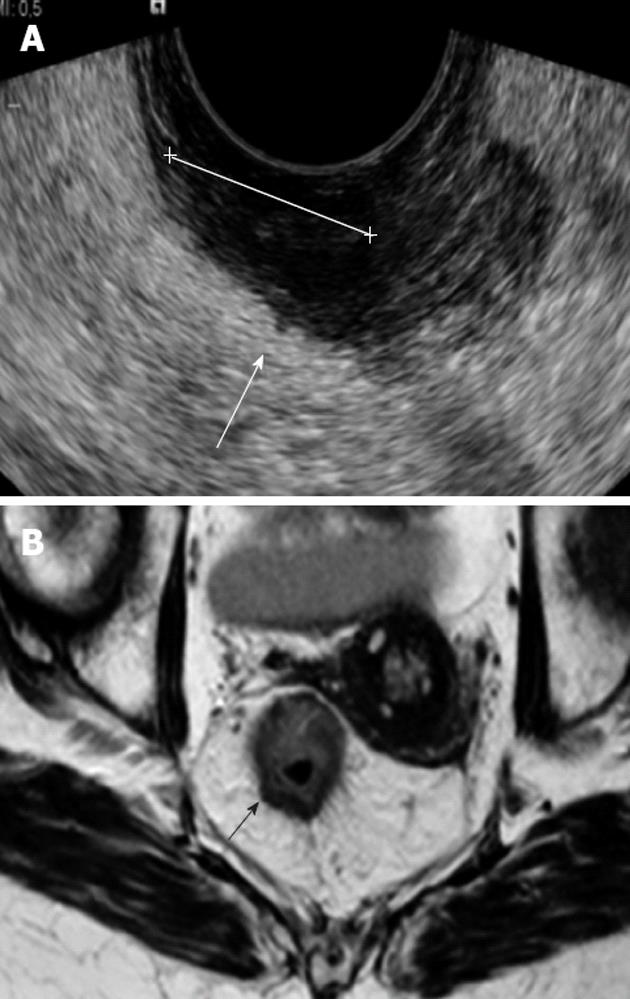
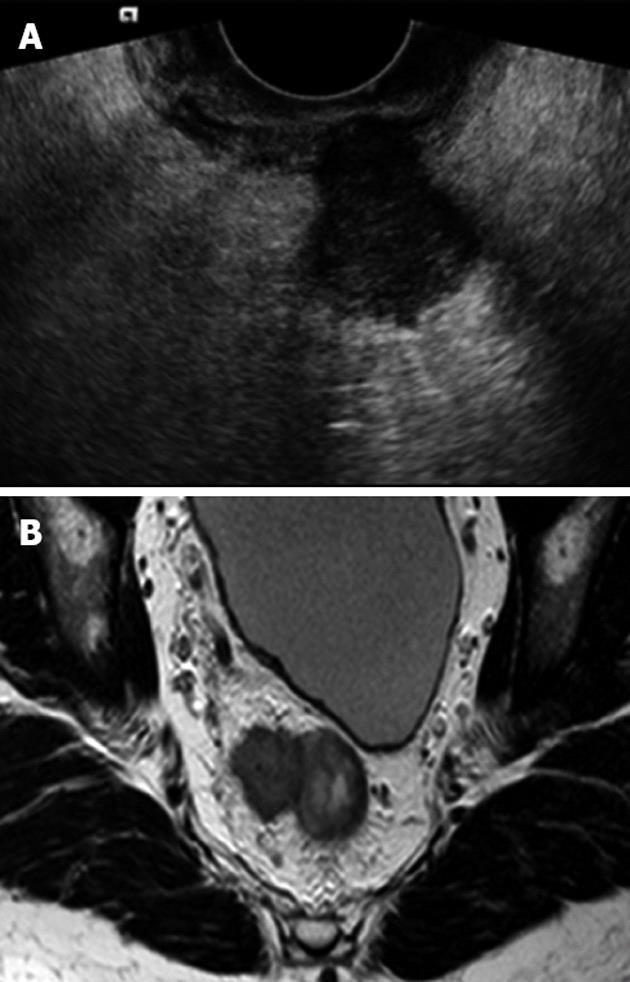
All patients underwent TRUS using a 6.5 MHz forward looking transrectal transducer (EC9-4) connected to a Siemens S 2000 ultrasound machine (Issaquah, Washington, United States). The transducer was used to facilitate visualization of stenotic tumors. Patients received a cleansing enema prior to their examination and all procedures were carried out with the patient in the left lateral position. All observers who performing and interpreting the TRUS were specialists in radiology with more than 10 years’ experience in TRUS and all had participated in a special training course. The level above the anal verge and location of the tumor were not blinded to the observers. The extent of wall invasion was assessed according to uT criteria as described by Hildebrandt et al[18]. In all ≥ T3 patients in the study population the maximal depth of extramural tumor spread was prospectively measured in mm and the ultrasound images were stored in the radiological electronic picture archive computer system (PACS).
The MRI scan was carried out using an up-graded 60 cm bore Achiva 1.5 Tesla MRI unit with a 5-element wrap-around surface coil over the pelvis (Philips, Eindhoven, The Netherlands). After localizer scans, fast T2-weighted (w) spin-echo sequences were obtained. The scan included high-resolution 3 mm axial slices at a 90- degree angle to the tumor. The axial scans were prepared by the MR radiographer with assistance from the radiologists to ensure perpendicular images. No contrast enhancement was used. Each patient underwent the TRUS and MRI examinations on the same day. The MRI images were interpreted and reported by two experienced radiologist after the TRUS examination in the daily clinical setting. The depth of extramural tumor spread was measured blindly to the TRUS findings in this study, with special focus on the axial MRI images. All images were evaluated using an Impax PACS workstation (Agfa, Mortsel, Belgium) with two Coronis monitors (1600 1200 pixels; Megapixels Diagnostic Display System; Barco, Kortijk, Belgium). Presence or absence of any distant metastasis at subsequent CT and surgery was recorded.
Statistical analysis were performed using Number Cruncher Statistical Systems (NCSS, Kaysville, Utah, United States). Intermodality agreements were assessed with Cohen’s kappa statistics. Kappa values were interpreted the following way: absence of agreement 0, slight agreement 0.20, fair agreement 0.21-0.40, moderate agreement 0.41-0.60, substantial agreement 0.61-0.80, and almost perfect agreement 0.81-1.0 as proposed by Landis et al[19]. Confidence limits were set at 95 percent. Descriptive statistics were used. Means between TRUS and MRI were analyzed using Paired t test. Comparison of proportions was performed by χ2 test or, when appropriate, Fisher’s exact test. P values less than 0.05 were considered significant.
TRUS found 51 patients to have an early ≥ T3 tumor and 35 to have an advanced tumor, whereas MRI categorized 48 as early ≥ T3 tumors and 38 as advanced tumors (Table 2). Three patients had tumors characterized as early by TRUS (uT3b), but advanced by MRI; however the extent of outgrowth was only 6 mm when measured by MRI. No patients with tumors classified as advanced by TRUS were found to be early on MRI. The unweighted and linear weighted kappa values in classifying early versus advanced ≥ T3 rectal tumors were both 0.93 (95% CI: 0.85-1.00).
| MRI-a | MRI-b | MRI-c | MRI-d | |
| TRUS-a | 10 | 6 | ||
| TRUS-b | 1 | 31 | 3 | |
| TRUS-c | 25 | 3 | ||
| TRUS-d | 2 | 5 |
The results of the subgroup classification are shown in Table 2. TRUS sub-classified 16 patients as uT3a, while MRI categorized six of these as ≥ T3b. The actual outgrowth measure by MRI was 2 mm in five and 4 mm in one of these patients. One MRI a sub-stage was b sub-stage using TRUS, the difference was 1 mm in this patient.
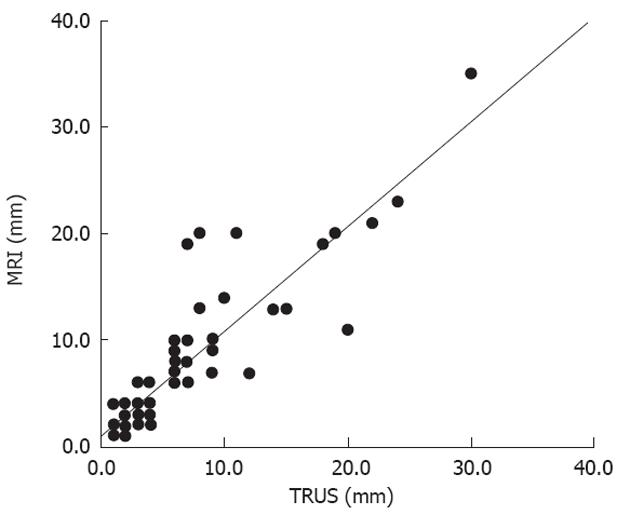
Three MRI d-group patients were only c-group on TRUS. Two patients had a difference of 12 mm and one with 9 mm (Figure 1 and Table 2). Two were in the d-group on TRUS but on MRI the same patients were in the c-group; the difference in outgrowth was 9 and 2 mm, respectively. We found a kappa value of 0.74 (95% CI: 0.63-0.86) for the total sub-classification between the two methods (Figures 2 and 3).
Figure 1 shows plots of the data from TRUS versus MRI, with respect to maximum extramural tumor spread. Multiple values occurring at a specific point were more common where the tumor outgrowth was limited.
The overall mean maximal tumor outgrowth measured on TRUS, 5.5 mm ± 5.63 mm and on MRI, 6.3 mm ± 6.18 mm, P = 0.004. The highest value in the same patient was 30 mm on TRUS and 35 mm measured by MRI.
In 19 of the 86 patients the following CT scan or surgery revealed distant metastases; of the 51 patients in the ultrasound ab group three (5.9%) had metastases, whereas 16 (45.7%) of 35 in the cd group harbored distant metastases, P = 0.00002. The odds ratio was 13.5 (95% CI: 3.5-51.6), P = 0.00002. The mean maximal ultrasound measured outgrowth was 4.3 mm (95% CI: 3.2-5.5 mm) in patients without distant metastases, while the mean maximal outgrowth was 9.5 mm (95% CI: 6.2-12.8 mm) in the patients with metastases, P = 0.00004. Using the MRI classification three (6.3%) of 48 in the MRI ab group had distant metastases, while 16 (42.1%) of the 38 in the MRI cd group, P = 0.00004. The MRI odds ratio was 10.9 (95% CI: 2.9-41.4), P = 0.00008. The mean maximal MRI measured outgrowth was 4.9 mm (95% CI: 3.7-6.1 mm) in patients without distant metastases, while the mean maximal outgrowth was 11.5 mm (95% CI: 7.8-15.2 mm) in the patients with metastases, P = 0.000006.
This first study on the correlation between TRUS and MRI in discrimination of early vs advanced rectal cancer might have clinical implications concerning patients with contraindications to MRI. The results indicate that these patients can be evaluated using TRUS to determine whether the patient has an early or advanced rectal cancer. Patients with claustrophobia, implanted cardiac prostheses, metallic cardiovascular electronic devices, intravascular stents and filters, cochlear implants, bullets, etc. are either not suitable for MRI or there is a serious risk involved in having an MRI performed[20]. Patient movements during the MRI scan are also associated with reduced image quality[21].
Patients with tumors confined to the rectal wall or early T3 tumors can be treated with surgery alone unless suspicious lymph nodes are detected, whereas tumors with advanced mesorectal involvement may benefit from preoperative treatment[22-24]. According to our results three patients were classified as T3b using TRUS, but as T3c using MRI. These three patients would not have been treated with radiochemotherapy using this setting if MRI was contraindicated. However, the invasion in the mesorectal fat was only 6 mm in all three MRI advanced patients.
The discrepancies in the sub-group staging using TRUS and MRI were only minor distances, except for a difference up to a 12 mm outgrowth in a patient with an advanced tumor on both modalities. In the patient with the most extensive outgrowth the measurement varied from 30 to 35 mm and was categorized equally as d-group with the two methods. The resolution of a 6.5 MHz transrectal tranducer is high and approximately the same or higher as the resolution for thin slice axial T2 weighted MRI, ranging about 0.3-0.4 mm. Thus, variances in resolution cannot explain this difference between large tumors. The reason could be measurements at different positions. TRUS can have difficulties in imaging the total volume of large tumors and perhaps this result in underestimation. The presence of a large tumor is significantly related to a poorer performance of TRUS[25]. This can in part explain why TRUS more often underestimated the tumors in the subclassification compared to MRI, than the vice versa (Table 2).
The anal sphincter was involved in three patients, seminal vesicles in a male patient and vagina in a female patient. A recent Japanese pathological study found a 5-year survival of 72.4% vs 30.0% in patients with early vs advanced T3/T4 tumors[26]. The depth of tumor invasion beyond the rectal wall is considered to be an important prognostic factor for patients with T3 and T4 rectal carcinoma. The patients classified as advanced had an odds ratio of 13.5 of having distant metastases in the advanced group, although both uT1 and uT2 tumors were excluded. A new MRI study reported that patients with more than 5 mm tumor outgrowth have a higher risk of harboring distant metastases with an odds ratio of six and these patients may undergo more intensive preoperative staging including FDG-PET/CT and liver MRI and be considered for chemotherapy[27]. Another study found a higher incidence of lung metastases with higher T stage[28].
The main strength of the blinded study is that we compared state of the art TRUS with MRI using experienced readers. The ultrasound measurements were performed in a prospective clinical setting but analysed retrospectively. We did not have any lack of thin-slice or high-resolution T2-weighted MRI images perpendicular to the tumor resulting in satisfactory image quality. We did not find any different correlation between TRUS and MRI regarding the TRUS observers. An inter-observer variation study of infiltration depth of ≥ T3 tumors using TRUS will require a 3-dimensional stored image block of the ultrasound data which could be blindly re-evaluated. It was not possible in our clinical setting; an inter-observer study on plane two-dimensional images would introduce an operator bias.
Only a few previous studies have focused on the aspect of tumor outgrowth using ultrasound. Harewood et al[29] used TRUS in 14 patients with minimally invasive T3 and 28 patients with advanced T3 disease. They used 2 mm invasion as a cut-off. Digital images were obtained during each TRUS procedure, at least 10 images per patient, illustrating the deepest extent of tumor involvement. These images were reviewed by two observers and a kappa value of 0.62 was reported. The mean depth of invasion beyond the rectal wall was 3.9 mm vs 5.5 mm in the present study. The mean outgrowth was 9.5 mm in the metastatic group.
A recent study by Jürgensen et al[30] found 16 patients with minimal invasive uT3 tumors and 24 with advanced uT3 cancers. The depth of infiltration beyond the muscularis propria was determined retrospectively by evaluation of paper prints and reports. Six patients could not be classified retrospectively. This discrimination between minimally invasive and advanced cancers correlated well to the pathological stage. In our study we also found a good intermodality agreement between TRUS and MRI, if the cut off between early and advanced ≥ T3 tumors was less than 5 mm.
The patient population of the present study was uniform, containing all patients with advanced rectal cancer, for determining intermodality agreement. In the study period there was no screening programme running for colorectal cancer, favouring a relatively high proportion of advanced tumors. The staging of the stenotic tumors has been accomplished by the use of a forward looking transrectal transducers[31,32] as in the present study. Side-viewing transducers including rotating transducers do not have this possibility of adequate visualising stenotic tumors[33].
The retrospective setting allowed us to evaluate a large number of MRI scans within a relatively short time period, but may invite learning bias of the MRI findings. Another limitation is the use of MRI as reference and not the histopathology of the surgical specimens. The latter was not possible since the majority of the patients underwent preoperative radiochemotherapy. This may explain that our mean invasion depth beyond the rectal wall was more pronounced than in the study by Harewood et al[29].
Another drawback of this retrospective study is the exclusion of a few patients with missing values of the precise extent of the tumor outgrowth. Four of the excluded uT3 patients were from the first part of the study period and during vacations, but there was agreement on the T-stage between TRUS and MRI. Finally, TRUS cannot detect the mesorectal fascia as with MRI[34,35], and consequently, MRI is used as a first choice staging modality.
In conclusion, there was very good intermodality agreement between TRUS and MRI in the pretreatment in classification of tumors as early and advanced ≥ T3. Patients with more than 5 mm outgrowth measured by ultrasound have an increased risk of harboring distant metastases. TRUS can be used to predict advanced rectal cancers and thus be helpful if chemoradiation is considered in patients with contraindications to MRI.
Magnetic resonance imaging (MRI) is widely used in preoperative staging of rectal cancer. Patients with claustrophobia cannot benefit from this method. The depth of tumor outgrowth is an important prognostic factor.
MRI has recently shown to be accurate in measuring tumor outgrowth and the MRI measured maximal depth of outgrowth seems to be related to presence of distant metastases.
Previously it has been shown that MRI measured extratumoral outgrowth is related to distant metastases. In the present study the authors have demonstrated an almost perfect agreement between MRI and transrectal ultrasound (TRUS) in categorizing early and advanced tumors.
TRUS can be used to discriminate between early vs advanced rectal cancer, and thus be helpful if chemoradiation is considered in patients with contraindications to MRI.
Transrectal ultrasound and MRI measurement of depth of extramural tumor spread in rectal tumors Rafaelsen. In this study the authors investigated the depth of tumor spread beyond the rectal wall using MRI and ultrasound (US) techniques in patients with suspected rectal carcinomas. They correlated the data between MRI and US and to metastasis of the cancer. Extension of the tumor beyond the rectal wall was subclassified in units of millimeters. Early tumors were considered to have ≤ 5 mm penetration while advanced tumors were defined as those with > 5 mm penetration. Three patients had tumors characterized as early by US but advanced by MRI, where in the three cases the outgrowth measured by MRI was 6 mm. No patients with tumors classified as advanced by US were classified as early type using MRI. Overall this is an interesting and well done study.
Peer reviewers: Paul E Sijens, PhD, Associate Professor, Department of Radiology, UMCG, Hanzeplein 1, 9713GZ Groningen, The Netherlands; Dr. Edward Ciaccio, Department of Medicine, Columbia University, 180 Fort Washington Avenue, NY 10032, United States
S- Editor Lv S L- Editor A E- Editor Li JY
| 1. | Miyoshi M, Ueno H, Hashiguchi Y, Mochizuki H, Talbot IC. Extent of mesorectal tumor invasion as a prognostic factor after curative surgery for T3 rectal cancer patients. Ann Surg. 2006;243:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Tokoro T, Okuno K, Hida J, Ishimaru E, Ueda K, Yoshifuji T. Depth of mesorectal invasion has prognostic significance in T3N0 low rectal cancer. Hepatogastroenterology. 2009;56:124-127. [PubMed] |
| 3. | Yoshida K, Yoshimatsu K, Otani T, Yokomizo H, Ogawa K. The depth of tumor invasion beyond the outer border of the muscularis propria as a prognostic factor for T3 rectal/rectosigmoid cancer. Anticancer Res. 2008;28:1773-1778. [PubMed] |
| 4. | Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 5. | MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 329] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Brown G, Richards CJ, Newcombe RG, Dallimore NS, Radcliffe AG, Carey DP, Bourne MW, Williams GT. Rectal carcinoma: thin-section MR imaging for staging in 28 patients. Radiology. 1999;211:215-222. [PubMed] |
| 7. | Pedersen BG, Moran B, Brown G, Blomqvist L, Fenger-Grøn M, Laurberg S. Reproducibility of depth of extramural tumor spread and distance to circumferential resection margin at rectal MRI: enhancement of clinical guidelines for neoadjuvant therapy. AJR Am J Roentgenol. 2011;197:1360-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, Tait D, Massey A, Tebbutt NC, Chau I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 9. | Rafaelsen SR, Kronborg O, Fenger C, Drue H. Comparison of two techniques of transrectal ultrasonography for the assessment of local extent of polypoid tumours of the rectum. Int J Colorectal Dis. 1996;11:183-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Kav T, Bayraktar Y. How useful is rectal endosonography in the staging of rectal cancer? World J Gastroenterol. 2010;16:691-697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Zorcolo L, Fantola G, Cabras F, Marongiu L, D'Alia G, Casula G. Preoperative staging of patients with rectal tumors suitable for transanal endoscopic microsurgery (TEM): comparison of endorectal ultrasound and histopathologic findings. Surg Endosc. 2009;23:1384-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 13. | Rafaelsen SR, Kronborg O, Fenger C. Digital rectal examination and transrectal ultrasonography in staging of rectal cancer. A prospective, blind study. Acta Radiol. 1994;35:300-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Fuchsjäger MH, Maier AG, Schima W, Zebedin E, Herbst F, Mittlböck M, Wrba F, Lechner GL. Comparison of transrectal sonography and double-contrast MR imaging when staging rectal cancer. AJR Am J Roentgenol. 2003;181:421-427. [PubMed] |
| 15. | Rafaelsen SR, Sørensen T, Jakobsen A, Bisgaard C, Lindebjerg J. Transrectal ultrasonography and magnetic resonance imaging in the staging of rectal cancer. Effect of experience. Scand J Gastroenterol. 2008;43:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Puli SR, Bechtold ML, Reddy JB, Choudhary A, Antillon MR, Brugge WR. How good is endoscopic ultrasound in differentiating various T stages of rectal cancer? Meta-analysis and systematic review. Ann Surg Oncol. 2009;16:254-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 141] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 721] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 18. | Hildebrandt U, Feifel G. Preoperative staging of rectal cancer by intrarectal ultrasound. Dis Colon Rectum. 1985;28:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 247] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43944] [Cited by in RCA: 41871] [Article Influence: 872.3] [Reference Citation Analysis (0)] |
| 20. | Baikoussis NG, Apostolakis E, Papakonstantinou NA, Sarantitis I, Dougenis D. Safety of magnetic resonance imaging in patients with implanted cardiac prostheses and metallic cardiovascular electronic devices. Ann Thorac Surg. 2011;91:2006-2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Morelli JN, Runge VM, Ai F, Attenberger U, Vu L, Schmeets SH, Nitz WR, Kirsch JE. An image-based approach to understanding the physics of MR artifacts. Radiographics. 2011;31:849-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Chang GJ, You YN, Park IJ, Kaur H, Hu CY, Rodriguez-Bigas MA, Skibber JM, Ernst RD. Pretreatment high-resolution rectal MRI and treatment response to neoadjuvant chemoradiation. Dis Colon Rectum. 2012;55:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Kojima M, Ishii G, Yamane Y, Nishizawa Y, Saito N, Ochiai A. Area of residual tumor beyond the muscular layer is a useful predictor of outcome in rectal cancer patients who receive preoperative chemoradiotherapy. Pathol Int. 2009;59:857-862. [PubMed] |
| 24. | Jakobsen A, Ploen J, Vuong T, Appelt A, Lindebjerg J, Rafaelsen SR. Dose-Effect Relationship in Chemoradiotherapy for Locally Advanced Rectal Cancer: A Randomized Trial Comparing Two Radiation Doses. Int J Radiat Oncol Biol Phys. 2012;May 12; Epub ahead of print. [PubMed] |
| 25. | Fernández-Esparrach G, Ayuso-Colella JR, Sendino O, Pagés M, Cuatrecasas M, Pellisé M, Maurel J, Ayuso-Colella C, González-Suárez B, Llach J. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Katsumata D, Fukui H, Ono Y, Ichikawa K, Tomita S, Imura J, Abe A, Fujita M, Watanabe O, Tsubaki M. Depth of tumor invasion in locally advanced rectal cancer correlates with patients' prognosis: the usefulness of elastic stain for its measurement. Surg Today. 2008;38:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Hunter CJ, Garant A, Vuong T, Artho G, Lisbona R, Tekkis P, Abulafi M, Brown G. Adverse features on rectal MRI identify a high-risk group that may benefit from more intensive preoperative staging and treatment. Ann Surg Oncol. 2012;19:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Kirke R, Rajesh A, Verma R, Bankart MJ. Rectal cancer: incidence of pulmonary metastases on thoracic CT and correlation with T staging. J Comput Assist Tomogr. 2007;31:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Harewood GC, Kumar KS, Clain JE, Levy MJ, Nelson H. Clinical implications of quantification of mesorectal tumor invasion by endoscopic ultrasound: All T3 rectal cancers are not equal. J Gastroenterol Hepatol. 2004;19:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Jürgensen C, Teubner A, Habeck JO, Diener F, Scherübl H, Stölzel U. Staging of rectal cancer by EUS: depth of infiltration in T3 cancers is important. Gastrointest Endosc. 2011;73:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Nielsen MB, Pedersen JF, Christiansen J. Rectal endosonography in the evaluation of stenotic rectal tumors. Dis Colon Rectum. 1993;36:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 32. | Akahoshi K, Kondoh A, Nagaie T, Koyanagi N, Nakanishi K, Harada N, Nawata H. Preoperative staging of rectal cancer using a 7.5 MHz front-loading US probe. Gastrointest Endosc. 2000;52:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Beer-Gabel M, Assouline Y, Zmora O, Venturero M, Bar-Meir S, Avidan B. A new rectal ultrasonographic method for the staging of rectal cancer. Dis Colon Rectum. 2009;52:1475-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Engelen SM, Beets GL, Beets-Tan RG. Role of preoperative local and distant staging in rectal cancer. Onkologie. 2007;30:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Beets GL, Beets-Tan RG. Pretherapy imaging of rectal cancers: ERUS or MRI? Surg Oncol Clin N Am. 2010;19:733-741. [PubMed] |