Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4787
Revised: May 31, 2012
Accepted: June 15, 2012
Published online: September 14, 2012
A 46-year-old female patient with terminal ileum Crohn’s disease and ankylosing spondylitis presented with recurrent angioedema and urticaria. Investigations ruled out hereditary angioedema, and environmental or food allergen triggers. She was diagnosed with chronic idiopathic urticaria with angioedema, and was treated with a trial of intravenous immunoglobulin immunotherapy, danazol, prednisone and hydroxyzine. Due to ongoing bowel and arthritic complaints, she was started on infliximab infusions and within 2 treatments, she had complete resolution of the angioedema and urticaria, as well as of the bowel and arthritic symptoms. Unfortunately she developed allergic reactions to the infliximab and was switched to another anti-tumor necrosis factor (TNF)-α agent, adalimumab. Since then, she has had no further angioedema or urticaria, and her Crohn’s disease has been quiescent. This is the first known case report of chronic idiopathic urticaria with angioedema coexistent with Crohn’s disease that was successfully treated with anti-TNF-α agents.
- Citation: Habal F, Huang V. Angioedema associated with Crohn's disease: Response to biologics. World J Gastroenterol 2012; 18(34): 4787-4790
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4787.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4787
Studies have shown that there is a strong autoimmune involvement in the pathophysiology of urticaria and angioedema. There are increases in pro-inflammatory cytokines, such as interleukin (IL)-1 β, IL-12p70, tumor necrosis factor (TNF)-α, IL-6, IL-10 and IL-17, in chronic idiopathic urticaria. Crohn’s disease also has autoimmune involvement, and there is evidence for an altered cytokine milieu leading to mucosal inflammation. Recent studies have shown that T-cell production of certain cytokines play a strong role. One common thread in the pathophysiology of chronic idiopathic urticaria and Crohn’s disease is the derangement in cytokine levels, in particular, IL-17 and TNF-α. The IL-17 cytokines are T-cell derived cytokines that stimulate various cells to secrete cytokines and chemokines, and therefore play an important role in many autoimmune diseases. It has been shown that IL-17A positive cells are increased in the inflamed mucosa of inflammatory bowel disease (IBD) patients, and IL-17F mRNA expression is elevated in the mucosa of Crohn’s disease patients. Infliximab and adalimumab are anti-TNF-α agents that block the inflammatory cascade. Given the similarity in cytokine derangements found in chronic idiopathic urticaria and in Crohn’s disease, medications that target these cytokines, such as anti-TNF-α agents should be effective in both conditions.
This case brings to attention the common pathophysiology between autoimmune diseases, and the need for further research looking into the changes in the cytokine milieu as potential targets for treatment.
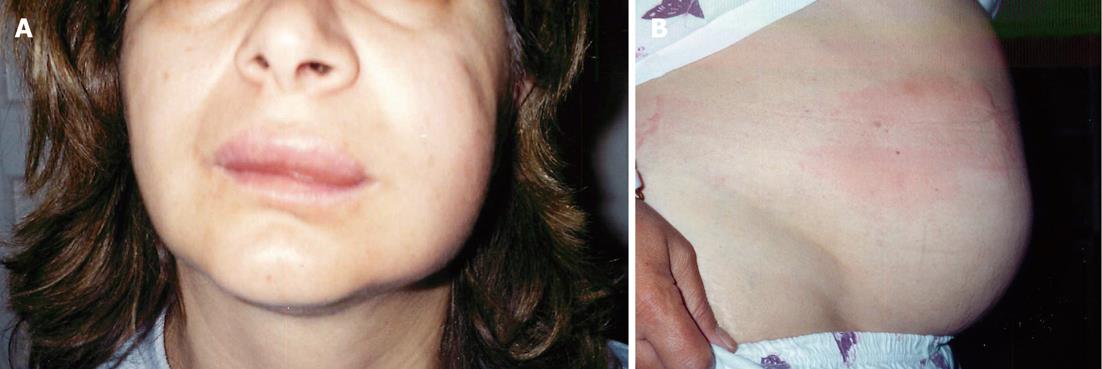
A 46-year-old female with terminal ileum Crohn’s disease and ankylosing spondylitis presented to our Gastroenterology clinic with recurrent angioedema and urticaria. Her Crohn’s disease was diagnosed in 1993 when she began having abdominal pain and diarrhea. A small bowel follow through revealed terminal ileum involvement, and she was started on Pentasa at that time. She remained well on Pentasa and for unclear reasons discontinued the medication in 2001. She was diagnosed with ankylosing spondylitis in 2004 just prior to presentation. On examination, she had angioedema and urticaria involving the tongue, lips, eyes, face, feet, hands and genital areas (Figure 1). Laboratory investigations revealed white blood cell 1.0 (normal, range: 4.0 × 109 - 11.0 × 109/L), neutrophils 10.0 (normal, range: 2.0 × 109 - 7.50 × 109/L), erythrocyte sedimentation rate 68 (normal, range: 0-20 mm/h). She was restarted on an aminosalicylate, Pentasa, for treatment of active Crohn’s disease with the concern that the angioedema may be related to the activity of the Crohn’s disease. A subsequent colonoscopy revealed aphthous ulceration in the terminal ileum. Biopsies of the ileocecal valve and terminal ileum showed mild acute on chronic inflammation with no granulomas and no dysplasia.
After restarting the Pentasa, her urticaria and angioedema subsided for 3 mo. Unfortunately her symptoms then recurred. The episodes became more frequent, and she required Prednisone and Atarax to control her symptoms. An allergist performed skin testing which revealed environmental allergies but no significant food allergies. Further investigations revealed an elevated IgM (3.02, range: 0.2-2.2), elevated IgE (1120) without eosinophilia, normal C3 (1.19), and normal C4. There was a question of hereditary angioedema, however she had normal C1 esterase inhibitor levels (313). Based on these results, she was diagnosed with chronic idiopathic recurrent urticaria with angioedema.
She was treated with a trial of intravenous immunoglobulin immunotherapy, but developed aseptic meningitis, and was instead treated with repeated courses of prednisone. A trial of Danazol did not alleviate her symptoms and she continued to be dependent on Prednisone and Atarax.
In 2007, because of her ongoing bowel, arthritis, and urticaria/angioedema symptoms, she was started on intravenous infliximab infusions (5 mg/kg) and within two treatments (weeks 0 and 2); she improved with resolution of the angioedema and urticaria, as well as of the bowel and arthritic symptoms. Unfortunately, while receiving her thirteenth infusion, she developed allergic reactions to the Infliximab. She was switched to another anti-TNF-α agent, adalimumab (160 mg subcut week 0, 80 mg week 2, 40 mg week 4 then 40 mg 2 wk). We have continued to follow her and since then, she has had no further angioedema or urticaria, and her Crohn’s disease has been quiescent. This is the first known case report of chronic idiopathic urticaria with angioedema coexistent with Crohn’s disease that was successfully treated with anti-TNF-α agents.
Urticaria refers to swelling of the superficial skin layer, and can be acute or chronic (present for at least 6 wk). When no external cause can be found for chronic urticaria, the patient is referred to as having “chronic idiopathic urticaria”[1]. Urticaria can occur alone (approximately 40% of patients), or in combination with angioedema (approximately 50% of patients)[1]. Angioedema refers to non-pruritic, non-pitting areas of swelling or cutaneous and mucosal tissues, affecting the deep dermal and subcutaneous/submucosal skin layers[1,2]. Angioedema can be further categorized into hereditary, acquired, associated with allergic reactions, and idiopathic. Degranulation and histamine release by mast cells and basophils leads to the erythema and edema typical of urticaria and angioedema.
Studies have shown that there is a strong autoimmune involvement in the pathophysiology of urticaria and angioedema. These conditions have been associated with other autoimmune diseases such as autoimmune thyroid disease and celiac disease[1,2]. Chronic urticaria and angioedema have been attributed to reactions to food, drugs, environmental antigens, and Helicobacter pylori infection, but the etiology for a majority of patients remains unknown. However, even for these “idiopathic” chronic urticaria patients, it is believed that the underlying mechanism is an autoimmune phenomenon[1,3,4].
Up to 30%-50% of patients with chronic urticaria have autoantibodies to the α-chain of the high affinity receptor for IgE (FceRIa); it is thought that these autoantibodies cross-link the IgE receptors, therefore activating the infiltrating basophils and skin mast cells, leading to histamine release[1,3,4].
In addition, other circulating mediators may play a role in activation and histamine release studies have shown increases in pro-inflammatory cytokines, such as IL-1β, IL-12p70, TNF-α, IL-6, IL-10 and IL-17, in chronic idiopathic urticaria[5,6].
Crohn’s disease is also a disease with autoimmune involvement, and there is evidence for an altered cytokine milieu leading to mucosal inflammation. Although the exact mechanism of Crohn’s disease has not been determined, recent studies have shown that T-cell production of certain cytokines play a strong role in the pathophysiology of Crohn’s disease[7-11].
A thorough literature review has revealed very few case reports of urticaria or angioedema associated with IBDs. These include cases of Hereditary angioedema associated with Crohn’s disease[12,13], angioedema of the small intestine masquerading as Crohn’s disease[14,15], and a single case of chronic urticaria without angioedema in a patient who was subsequently diagnosed with Crohn’s disease[16]. There has also been a case report of chronic urticaria and ulcerative colitis[17].
One possible common thread in the pathophysiology of chronic idiopathic urticaria and Crohn’s disease is the derangement in cytokine levels, in particular, IL-17 and TNF-α. The IL-17 cytokines are T-cell derived cytokines that stimulate various cells to secrete cytokines and chemokines, and therefore play an important role in many autoimmune diseases[7], The Th17 CD4+ T cells produce a distinct set of cytokines (IL-17A, IL-17F, IL-6, IL-22 and IL-26) which enhance immune and host defenses. IL-17A plays a role in the expansion and recruitment of innate immune cells (neutrophils), and interacts with toll-like receptor ligands, IL-1 β, and TNF-α to enhance inflammatory reactions. IL-17F induces the secretion of other inflammatory cytokines such as IL6, IL-8 and LIF. It has been shown that Il-17A positive cells are increased in the inflamed mucosa of IBD patients[9], and IL-17F mRNA expression is elevated in the mucosa of Crohn’s disease patients[8].
Infliximab and adalimumab are anti-TNF-α agents that block the inflammatory cascade. Both of these agents have been found to be effective in the treatment of Crohn’s disease[18,19]. Given the similarity in cytokine derangements found in chronic idiopathic urticaria and in Crohn’s disease, anti-inflammatory medications that target these cytokines should be effective in both conditions. Anti-TNF-α agents are still experimental for the treatment of urticaria, and have been tried in patients with various forms of urticaria, with a few case reports that have indicated successful treatment[20].
In summary, this is the first known case report of chronic idiopathic urticaria with angioedema coexistent with Crohn’s disease that was successfully treated with anti-TNF-α agent. We hypothesize that the derangement in cytokines, especially IL-17 and TNF-α, may be the reason the anti-TNF-α agents were successful, and that there may be a common pathophysiology between autoimmune diseases. Patients with IBD and concurrent angioedema or urticaria could have their cytokine levels checked and compared to see if there is any trend. These levels could be checked before and after treatment with biologics to confirm the biologic effect on the cytokine milieu in these two diseases. This case brings to attention the need for further research looking into the changes in the cytokine milieu as potential targets for treatment.
Peer reviewer: Bruno Bonaz, MD, PhD, Professor, Department of Gastroenterology, Grenoble University, 38000 Grenoble, France
S- Editor Gou SX L- Editor A E- Editor Xiong L
.
| 1. | Najib U, Sheikh J. The spectrum of chronic urticaria. Allergy Asthma Proc. 2009;30:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Banerji A, Sheffer AL. The spectrum of chronic angioedema. Allergy Asthma Proc. 2009;30:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Philpott H, Kette F, Hissaria P, Gillis D, Smith W. Chronic urticaria: the autoimmune paradigm. Intern Med J. 2008;38:852-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Kaplan AP, Greaves M. Pathogenesis of chronic urticaria. Clin Exp Allergy. 2009;39:777-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 194] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 5. | Dos Santos JC, Azor MH, Nojima VY, Lourenço FD, Prearo E, Maruta CW, Rivitti EA, da Silva Duarte AJ, Sato MN. Increased circulating pro-inflammatory cytokines and imbalanced regulatory T-cell cytokines production in chronic idiopathic urticaria. Int Immunopharmacol. 2008;8:1433-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Hermes B, Prochazka AK, Haas N, Jurgovsky K, Sticherling M, Henz BM. Upregulation of TNF-alpha and IL-3 expression in lesional and uninvolved skin in different types of urticaria. J Allergy Clin Immunol. 1999;103:307-314. [PubMed] |
| 7. | Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154-5161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 85] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 8. | Bene L, Falus A, Baffy N, Fulop AK. Cellular and molecular mechanisms in the two major forms of inflammatory bowel disease. Pathol Oncol Res. 2011;17:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 10. | Peyrin-Biroulet L, Desreumaux P, Sandborn WJ, Colombel JF. Crohn's disease: beyond antagonists of tumour necrosis factor. Lancet. 2008;372:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756-1767. [PubMed] [DOI] [Full Text] |
| 12. | Farkas H, Gyeney L, Nemesánszky E, Káldi G, Kukán F, Masszi I, Soós J, Bély M, Farkas E, Füst G. Coincidence of hereditary angioedema (HAE) with Crohn's disease. Immunol Invest. 1999;28:43-53. [PubMed] |
| 13. | Freeman HJ. Hereditary angioneurotic edema and familial Crohn's disease. Can J Gastroenterol. 2000;14:337-339. [PubMed] |
| 14. | Malcolm A, Prather CM. Intestinal angioedema mimicking Crohn's disease. Med J Aust. 1999;171:418-420. [PubMed] |
| 15. | Burak KW, May GR. C1 inhibitor deficiency and angioedema of the small intestine masquerading as Crohn's disease. Can J Gastroenterol. 2000;14:349-451. [PubMed] |
| 16. | Mansueto P, Carroccio A, Corsale S, Di Lorenzo G, Di Prima L, Pirrone G, Florena AM, Di Fede G. Chronic urticaria as a presenting symptom of Crohn's disease. BMJ Case Rep. 2009;2009 Sep 7; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Naimeh LG, Muller BA. Chronic urticaria in a 17-year-old patient with a past history of bowel disease. Ann Allergy Asthma Immunol. 2001;86:511-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Lee TW, Fedorak RN. Tumor necrosis factor-α monoclonal antibodies in the treatment of inflammatory bowel disease: clinical practice pharmacology. Gastroenterol Clin North Am. 2010;39:543-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:644-59, quiz 660. [PubMed] |
| 20. | Magerl M, Philipp S, Manasterski M, Friedrich M, Maurer M. Successful treatment of delayed pressure urticaria with anti-TNF-alpha. J Allergy Clin Immunol. 2007;119:752-754. [PubMed] |