Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4771
Revised: June 8, 2012
Accepted: June 15, 2012
Published online: September 14, 2012
AIM: To evaluate the diagnosis of different differentiated gastric intraepithelial neoplasia (IN) by magnification endoscopy combined with narrow-band imaging (ME-NBI) and confocal laser endomicroscopy (CLE).
METHODS: Eligible patients with suspected gastric IN lesions previously diagnosed by endoscopy in secondary hospitals and scheduled for further diagnosis and treatment were recruited for this study. Excluded from the study were patients who had liver cirrhosis, impaired renal function, acute gastrointestinal (GI) bleeding, coagulopathy, esophageal varices, jaundice, and GI post-surgery. Also excluded were those who were pregnant, breastfeeding, were younger than 18 years old, or were unable to provide informed consent. All patients had all mucus and bile cleared from their stomachs. They then received upper GI endoscopy. When a mucosal lesion is found during observation with white-light imaging, the lesion is visualized using maximal magnification, employing gradual movement of the tip of the endoscope to bring the image into focus. Saved images are analyzed. Confocal images were evaluated by two endoscopists (Huang J and Li MY), who were familiar with CLE, blinded to the related information about the lesions, and asked to classify each lesion as either a low grade dysplasia (LGD) or high grade dysplasia (HGD) according to given criteria. The results were compared with the final histopathologic diagnosis. ME-NBI images were evaluated by two endoscopists (Lu ZS and Ling-Hu EQ) who were familiar with NBI, blinded to the related information about the lesions and CLE images, and were asked to classify each lesion as a LGD or HGD according to the “microvascular pattern and surface pattern” classification system. The results were compared with the final histopathologic diagnosis.
RESULTS: The study included 32 pathology-proven low grade gastric IN and 26 pathology-proven high grade gastric IN that were detected with any of the modalities. CLE and ME-NBI enabled clear visualization of the vascular microsurface patterns and microvascular structures of the gastric mucosa. The accuracy of the CLE and the ME-NBI diagnosis was 88% (95% CI: 78%-98%) and 81% (95% CI: 69%-93%), respectively. The kappa coefficient of agreement between the histopathology and the in vivo CLE imaging was 0.755; between the histopathology and the in vivo CLE imaging was 0.615. McNemar’s test (binomial distribution used) indicated that the agreement was significant (P < 0.05). When patients were diagnosed by ME-NBI with CLE, the overall accuracy of the diagnosis was 86.21% (95% CI: 73%-96%), and the kappa coefficient of agreement was 0.713, according to McNemar’s test (P < 0.05).
CONCLUSION: Higher diagnostic accuracy, sensitivity and specificity of CLE over ME-NBI indicate the feasibility of these two techniques for the efficacious diagnostic classification of gastric IN.
- Citation: Wang SF, Yang YS, Wei LX, Lu ZS, Guo MZ, Huang J, Peng LH, Sun G, Ling-Hu EQ, Meng JY. Diagnosis of gastric intraepithelial neoplasia by narrow-band imaging and confocal laser endomicroscopy. World J Gastroenterol 2012; 18(34): 4771-4780
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4771.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4771
Gastric cancer is one of the most common malignant diseases of the gastrointestinal (GI) tract. Substantial interest has arisen in recent years in the diagnosis and management of early gastric cancer because of the high cure rate achieved with treatment of these lesions. Diagnosis and localization of early gastric cancer is problematic because of the lack of any endoscopic characteristics, even with multiple random biopsies.
In recent years, endoscopic image quality has improved as the devices have advanced technologically, including chromoendoscopy, high resolution and magnification endoscopy[1]. Additionally, magnification endoscopy combined with narrow-band imaging (ME-NBI) has been used to achieve endoscopic pathology in gastric disease.
Although these techniques improve the visualization and detection of mucosal lesions, a biopsy of the targeted lesion must still be performed for a formal histological diagnosis of cellular and architectural atypia. However, there are several disadvantages that may be associated with biopsies or endoscopic resection, including bleeding and perforation. Non-representative biopsies may miss relevant portions of tissue, leading to an underestimation of the diagnosis. Random biopsy sampling or endoscopic resection for non-neoplastic lesions can also be time-consuming. It would be ideal if a definite diagnosis could be made during endoscopy without the need for a biopsy.
Recent technological advances in miniaturization have allowed for a confocal scanning microscope to be integrated into a conventional flexible endoscope, or into trans-endoscopic probes, a technique now known as confocal endomicroscopy (CEM) or confocal laser endomicroscopy (CLE)[2-4]. This newly-developed technology has enabled endoscopists to collect real-time in vivo histological images, or “virtual biopsies”, of the GI mucosa during endoscopy, and has stimulated significant interest in the application of this technique in clinical gastroenterology.
Patients with suspected gastric intraepithelial neoplasia (IN) lesions previously diagnosed by endoscopy in secondary hospitals and scheduled for further diagnosis and treatment were recruited for this study from September 2008 to July 2009 at the General Hospital of the People’s Liberation Army, Beijing, China.
Excluded from the study were patients who had liver cirrhosis, impaired renal function, acute GI bleeding, coagulopathy, esophageal varices, jaundice, and GI post-surgery. Also excluded were those who were pregnant, breastfeeding, were younger than 18 years old, were unable to provide informed consent, or had a known allergy to fluorescein sodium. The General Hospital of the People’s Liberation Army’s clinical research ethics committee approved the protocol, and written informed consent was obtained from all participants.
The magnifying video endoscope used in this study was a GIF-H260Z (Olympus Medical Systems Co, Tokyo, Japan).
The videoendoscope is mounted on the charge coupled device (CCD) positioned on the tip of the endoscope as the imaging sensing device, with a xenon lamp used as the light source. The system is based on a black and white CCD, in which color separation is achieved through the use of a red-green-blue (RGB) rotary filter wheel equipped within the light source unit. The RGB filter consists of three broadband optical filters and covers all wavelengths of the visible spectrum, ranging from approximately 400 to 800 nm.
The NBI system uses two narrow band illuminations of 415 nm and 540 nm by NBI filter. Under NBI observation, the broadband white light derived from the xenon lamp splits into two bands (wavelength of 415 nm and 540 nm) and illuminates the surface of the mucosa. In the RGB sequential system, the NBI filter is placed on the light path in front of the RGB rotary filters. When observing with the NBI filter, the light illumination passes only B and G filters to obtain 415 nm and 540 nm images. Then, 415 nm is allocated to B and G channels so that blood vessels on the mucosal surface are reproduced in a brownish color, and 540 nm is allocated to the R channel so that the vessels in the deeper layer can be indicated in a greenish-blue color.
All procedures were performed using a confocal laser endomicroscope (EC-3870K; OptiScan, Notting Hill, Australia and Pentax Corp., Tokyo, Japan)[5].
The confocal microscope integrated into the conventional endoscope collects images at a scan rate of 1.6 frames per second (1024 × 512 pixels) or 0.8 frames per second (1024 × 1024 pixels) with an adjustable scanning depth ranging from 0 to 250 μm, a field of view of 475 μm × 475 μm, a lateral resolution of 0.7 μm, and an axial resolution of 7 μm.
It is important to clear all mucus and bile from the stomach before upper GI endoscopy. Patients were given the following mixture 30 min before the endoscopic procedure: 100 mL of water with 10 000 units of pronase (Kaken Pharmaceutical, Tokyo, Japan), 1 g of sodium bicarbonate, and 10 mL of dimethylpolysiloxane (20 mg/mL, Horii Pharmaceutical Ind., Osaka, Japan).
A soft black hood (MB-162 for GIF-Q240Z, or MB-46 for GIFH260Z and GIF-Q160Z; Olympus, Tokyo) is essential for optimal magnification endoscopy. Prior to the NBI examination, the hood is mounted on the tip of the endoscope to enable the endoscopist to consistently fix the mucosa at a distance of approximately 2 mm so that maximal magnification of the endoscopic image can be obtained.
Before detailed inspection, adherent mucus is removed by water flushing. After the endoscope was inserted, the entire stomach was initially observed with conventional white light to exclude obvious lesions. At least 35 photographs were taken from the entire stomach, even if there were no obvious lesions.
Once a mucosal lesion is found during observation with white-light imaging, it is then visualized using maximal magnification, employing gradual movement of the tip of the endoscope to bring the image into focus, with the distally attached soft black hood being used to stabilize the tip of the endoscope onto the tissue. Instead of pressing the tip of the hood, gentle suction should be applied, without causing mucosal injury, and the lesion is then observed by magnification endoscopy with NBI. The images were saved to a specific folder for later analysis.
After observation via ME-NBI, fluorescent contrasts should be used for CEM[6] (such as intravenously delivered fluorescein or topically administered acriflavine) and deployed through a spraying catheter.
Intravenously delivered fluorescein distributes throughout the extracellular matrix of the surface epithelium and lamina propria, but does not stain cell nuclei. Topically administered acriflavine stains cell nuclei of the surface epithelium, but does not penetrate to deeper layers of the GI mucosa. Acriflavine is a mutagenic dye and a potential human carcinogen, which limits its clinical utility.
Fluorescein is usually administered within 5 min of imaging; however, the dose and timing of contrast administration have not been standardized. After the contrast administration, the tip of the confocal endomicroscope or miniprobe is positioned in gentle contact with the area of interest to obtain high-resolution confocal images. Accumulated images can be saved for post-procedural analysis.
The resected specimens were fixed in 4% methanal, embedded in paraffin, sectioned into 4 mm thick samples, and stained with hematoxylin-eosin for routine histopathologic examination. Slides were coded and examined blindly by two pathologists (Wang YL and Wei LX), who were blinded to the endomicroscopic result, with diagnoses being confirmed by both individuals. The histological diagnostic criteria were based on the WHO classification. Early gastric cancer is defined as a malignant tumor limited to the mucosa or submucosa irrespective of the presence of lymph node metastases[7,8]. This includes the two types of low-grade neoplasia/dysplasia (LGD) and high-grade neoplasia/dysplasia (HGD), which are categorized according to the modified Vienna classification[9]. Early gastric cancer is further classified into two major groups: differentiated early gastric cancer which has tubular or papillary structures resembling the metaplastic intestinal epithelium; and undifferentiated early gastric cancer, which includes poorly differentiated carcinoma, and is characterized by discretely proliferating tumor cells with sparse or no tubular formation[10]. In the images of magnification endoscopy with NBI, an irregular microvascular pattern (MVP) and/or an irregular microsurface pattern (MSP), together with a clear demarcation line, are the hallmarks of early gastric cancer.
All the confocal laser endoscopy images were stored in a specific folder, with the quality of each image being scored as good (no moving artifacts, gastric pit structure and vessel can be identified clearly), average (artifacts present but gastric pit and vascular architecture can be recognized), and poor (artifacts do not allow judgment of the image). The images were evaluated by two endoscopists (Huang J and Li MY) who were familiar with CLE, blinded to the related information about the lesions, and asked to classify each lesion as either a LGD or HGD according to the aforementioned criteria. The results were compared with the final histopathologic diagnosis.
All the NBI magnified images were stored in a specific folder. The images were evaluated by two endoscopists (Lu ZS and Ling-Hu EQ) who were familiar with NBI, blinded to the related information about the lesions and CLE images, and asked to classify each lesion as either a LGD or HGD according to the “microvascular pattern (V) and surface pattern (S)” classification system[11]. The results were compared with the final histopathologic diagnosis.
The statistical analysis was performed using the statistical software package SPSS 13.0 (SPSS, Chicago, IL, United States). The accuracy, sensitivity and specificity of the CLE and ME-NBI diagnosis were respectively calculated in comparison with the histopathologic diagnosis.
A total of 59 patients participated in this study. The mean (± SD) age of the participants was 63.1 years, and the study population consisted of 44 male and 15 female patients.
In CLE, the 36 patients with low grade gastric intraepithelial neoplasia (LIN) included 9 women (25%) and 27 men (85%), with an average age of 60 years (range: 35-85 years). The 23 patients with high grade gastric intraepithelial neoplasia (HIN) showed significant male predominance as well, with 17 men (74%) and 6 women (23%). The average age was 68 years (range: 45-84 years).
A total of 1912 CLE images obtained from 62 lesions were used for analysis. The mean duration of the CLE procedure for each patient was 19 min (range: 11-30 min). After CLE, all patients developed a slight yellow coloration of the skin and urine, which disappeared in all cases within 24 h. No severe side-effects were noted.
In ME-NBI, the 45 patients with LIN included 12 women (27%) and 33 men (73%), with an average age of 63 years (range: 40-84 years). The 14 patients with HIN showed significant male predominance as well, with 11 men (79%) and 3 women (21%). The average age was 65 years (range: 36-85 years). The endoscopic and pathologic features of all the lesions are summarized below.
A total of 688 ME-NBI images obtained from 62 lesions were used for analysis. The mean duration of the ME-NBI procedure for each patient was 17 min (range: 10-25 min).
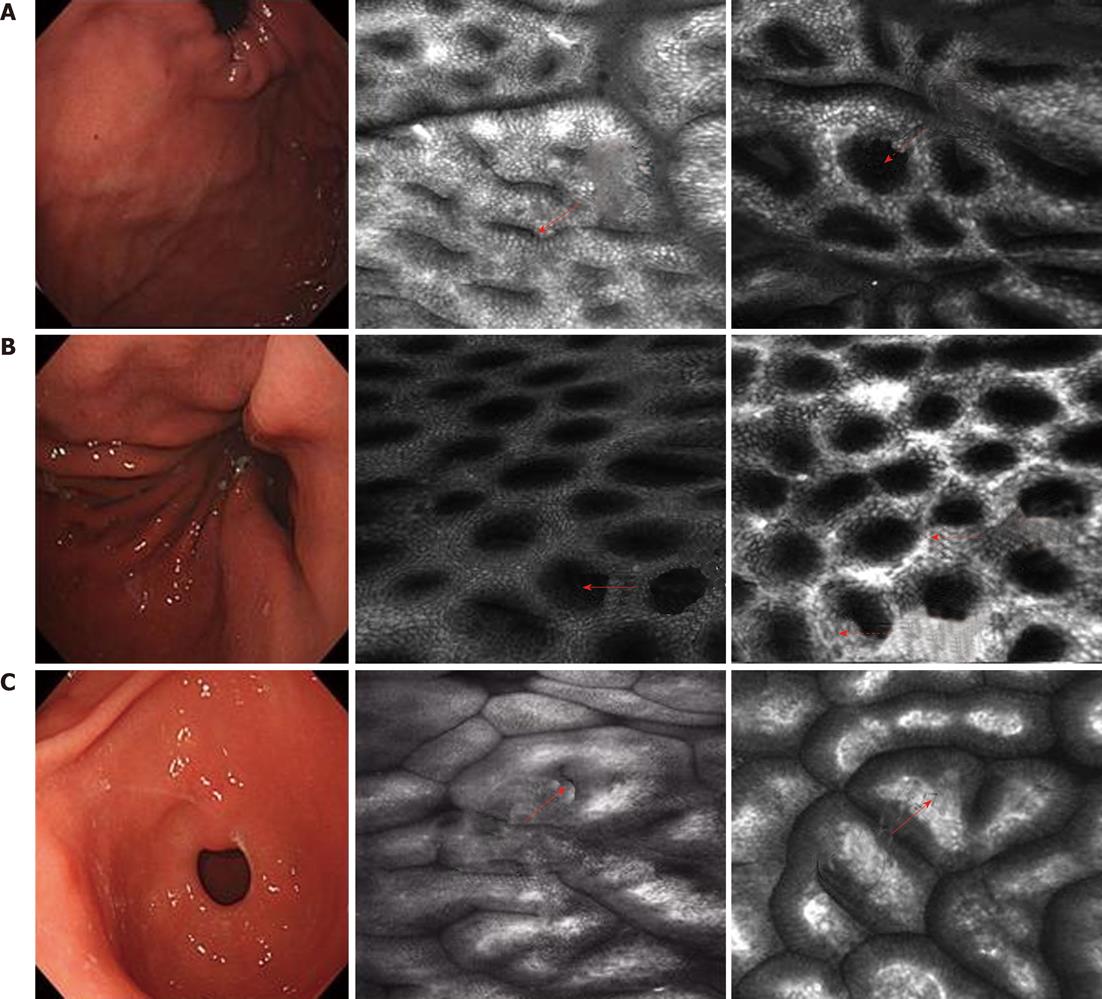
Gastric fundus: Superficial lamella gastric pits are round. Few pits compose one unit. The edge crack is the line type. Net-like subepithelial capillary network patterns surround the gastric pits (Figure 1A).
Gastric body: The gastric pits are round. Honeycomb-like subepithelial capillary network patterns surround the gastric pits (Figure 1B).
Gastric antrum: The gastric pits are line type. Coil-shaped subepithelial capillary network patterns surround the gastric pits (Figure 1C).
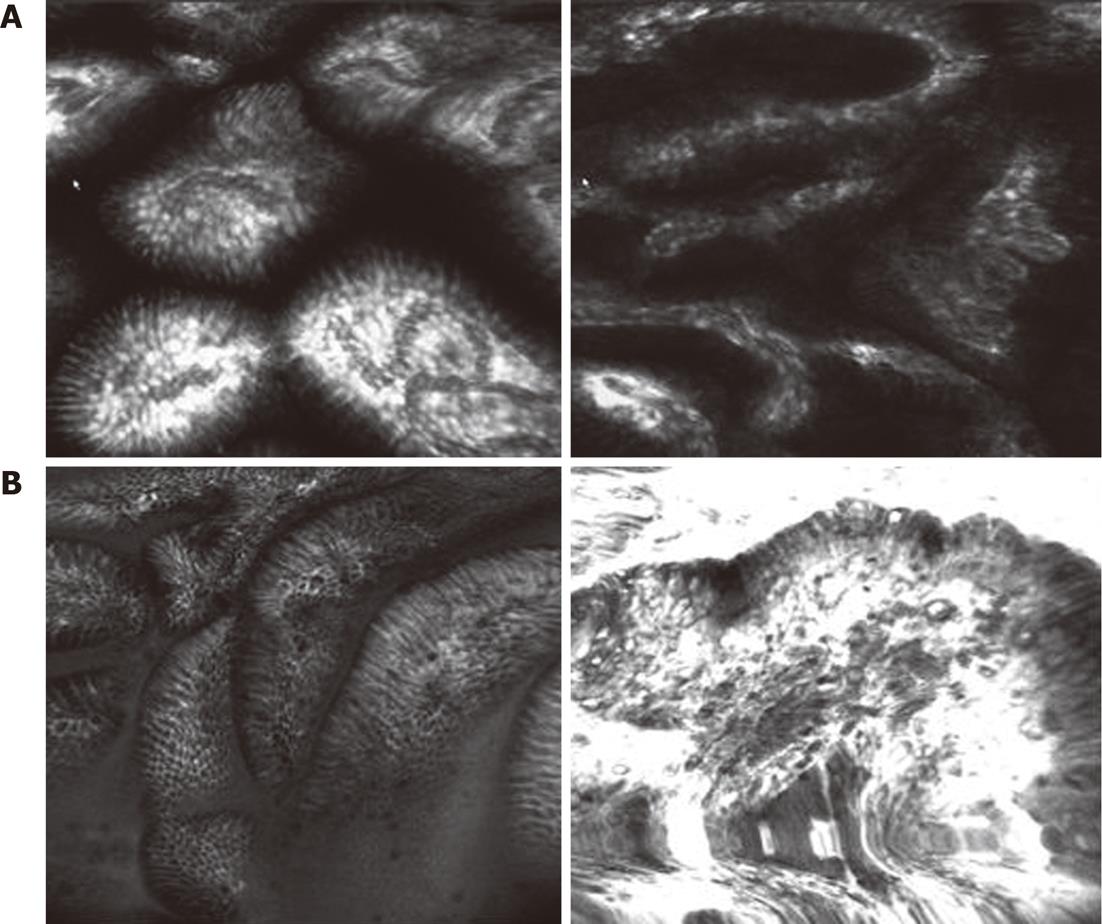
LIN showed that, for the different sizes of gastric pits, all capillary networks are thickening and circuitous (Figure 2A); HIN showed an abnormal arrangement of gastric pits. The thickening capillary network and the increasing branch present a mass shape (Figure 2B).
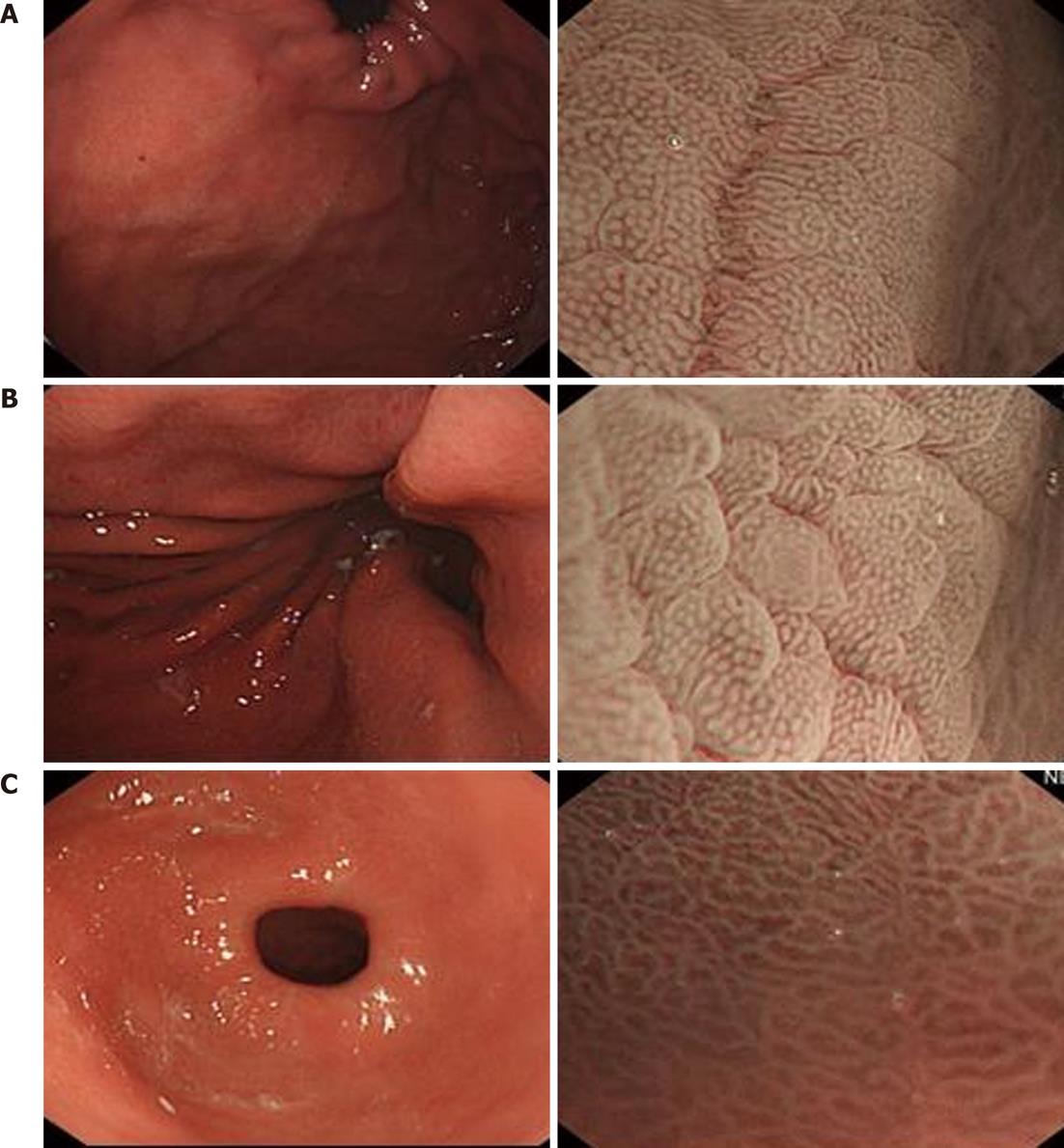
Gastric fundus: S: The individual morphology of the crypt epithelium shows a uniformly round/oval structure. Few structures are composed of one unit separated from the others. The width and length of the crypt epithelium are constant. The arrangement and distribution are regular and symmetrical; V: The mucosal capillaries have a uniform shape that can be closed-loop (polygonal). They have a consistent size and their arrangement and distribution are regular and symmetrical. Superficial lamella gastric pits are round (Figure 3A).
Gastric body: S: The individual morphology of the crypt epithelium shows a uniformly round structure. The width and length of the crypt epithelium are constant. The arrangement and distribution are regular and symmetrical; V: The mucosal capillaries have a uniform shape that can be closed-loop (polygonal). They have a consistent size and their arrangement and distribution are regular and symmetrical (Figure 3B).
Gastric antrum: S: The individual morphology of the crypt epithelium shows a uniformly round/linear structure. The width and length of the crypt epithelium are constant. The arrangement and distribution are regular and symmetrical; V: The mucosal capillaries have a uniform shape that can be open-loop. They have a consistent size and their arrangement and distribution are regular and symmetrical (Figure 3C).
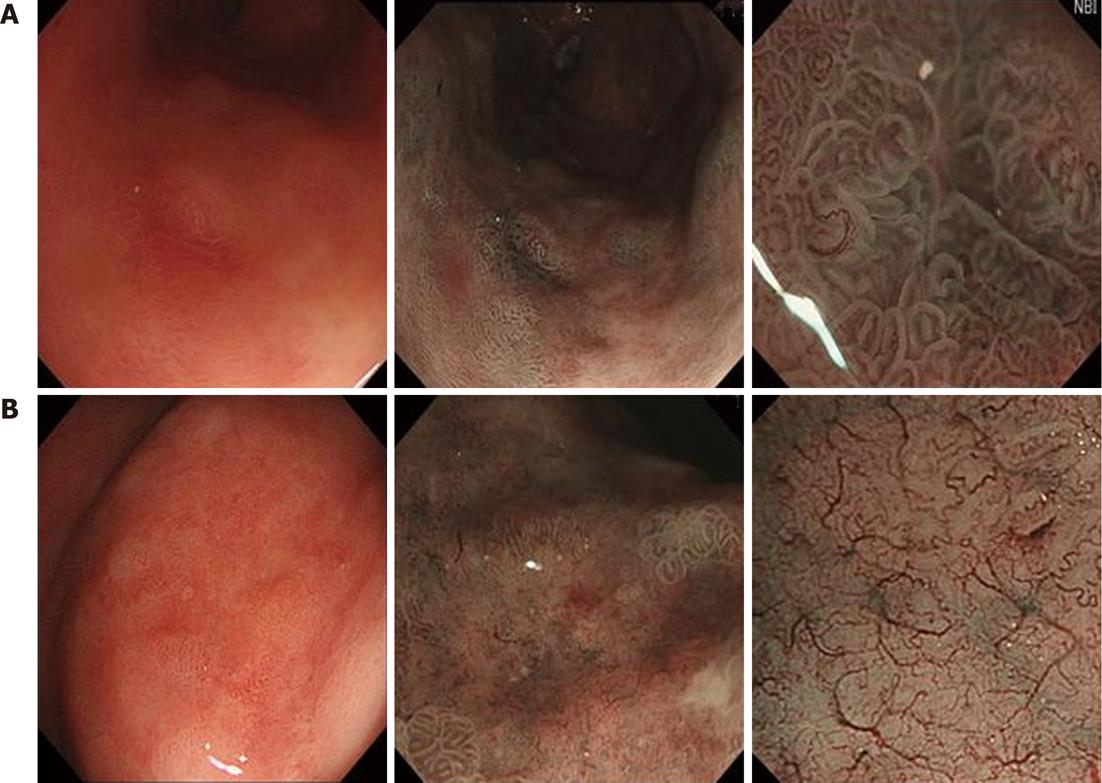
LIN presented irregular surface and MVPs with a clear demarcation line (Figure 4A). HIN presented irregular surface and MVPs with a demarcation line. Few areas showed as flat (mean absent MSP) with an obscured demarcation line (Figure 4B).
Irregular surface pattern: The individual morphology of the crypt epithelium shows an irregular tubular/linear/curved/papillary/villous structure. The width and length of the crypt epithelium varies. Arrangement and distribution are irregular and asymmetric.
Irregular MVP: The vessels differ in shape, being closed-loop (polygonal), open-loop, tortuous, branched, bizarrely-shaped, and with or without a network. The size of the vessels also varies and their arrangement and distribution are irregular and asymmetrical.
Among 58 patients with gastric IN, 22 lesions with HIN and 29 lesions with LIN were diagnosed by CLE. The results are summarized in Table 1. The sensitivity and specificity of CLE for predicting low grade gastric IN were 90.63% and 84.62%, respectively. The overall accuracy of the CLE diagnosis was 88% (95% CI: 78%-98%), and the kappa coefficient of agreement between the histopathology and the in vivo CLE imaging was 0.755. McNemar’s test (binomial distribution used) indicated good agreement between the final histopathology and the CLE imaging (P < 0.05).
| Histopathology (ESD) | LIN | HIN | Total |
| CLE | |||
| LIN | 29 | 4 | 33 |
| HIN | 3 | 22 | 25 |
| ME-NBI | |||
| LIN | 27 | 6 | 33 |
| HIN | 5 | 20 | 25 |
| ME-NBI + CLE | |||
| LIN | 32 | 8 | 40 |
| HIN | 0 | 18 | 18 |
| Total | 32 | 26 | 58 |
Among 58 patients of gastric IN, 20 lesions with HIN and 27 lesions with LIN were diagnosed by ME-NBI. The results are summarized in Table 1. The sensitivity and specificity of ME-NBI for predicting low grade gastric IN were 84.38% and 76.92% respectively. The overall accuracy of the ME-NBI diagnosis was 81% (95% CI: 69%-93%), and the kappa coefficient of agreement between the histopathology and the in vivo CLE imaging was 0.615. McNemar’s test (using binomial distribution) indicated good agreement between the final histopathology and the CLE imaging (P < 0.05).
Among 58 patients of gastric IN, 18 lesions with HIN and 32 lesions with LIN were diagnosed by ME-NBI with CLE. The results are summarized in Table 1. The sensitivity and specificity for predicting low grade gastric IN were 100% and 69.23% respectively. The overall accuracy of the ME-NBI diagnosis was 86.21% (95%CI: 73%-96%), and the kappa coefficient of agreement between the histopathology and the in vivo CLE imaging was 0.713. McNemar’s test (using binomial distribution) indicated good agreement between final histopathology and CLE imaging (P < 0.05).
Endoscopy plays an important role in the early detection of GI tract neoplasms., It has been difficult to assess pre-malignant and early neoplastic lesions precisely using conventional white light or dye-based image enhanced endoscopy. NBI is an innovative optical technology that can increase the contrast of the precise morphological changes in the mucosal surface and clearly visualize microvascular structures. It dramatically improves the detection of pre-malignant and early neoplastic lesions, particularly in combination with magnification endoscopy. These features allow the better targeting of biopsies, and improved and more appropriate treatment, thereby contributing to optimal quality of life and patient survival. ME-NBI has allowed brand new possibilities for the endoscopic diagnosis of GI tract diseases.
Mucosal morphology includes microvascular architecture, and changes in the course of malignant transformation. There have been many studies on the vasculature in cancerous mucosa using magnification endoscopes or NBI. Sano et al[12] first reported the clinical utility of NBI in the GI tract in 2001. Nakayoshi et al[1] classified the MVP of superficial gastric cancers based on their magnifying NBI images into three groups: (1) fine network pattern; (2) corkscrew pattern; and (3) unclassified pattern. They also compared the endoscopic pattern with the histological findings. Fine network and corkscrew patterns are useful for identifying differentiated adenocarcinoma and undifferentiated adenocarcinoma, respectively. At the same time, they imply that magnifying NBI is also useful for identifying the lateral extension of superficial gastric cancer. In their reports, they studied the microvascular networks of 165 patients with depressed-type early gastric cancer lesions using a magnification endoscope with NBI and demonstrated that 66.1% of differentiated adenocarcinoma possessed fine networks, while 85.7% of undifferentiated adenocarcinoma had a corkscrew network[13]. In 2004, the clinical value of ME-NBI in the diagnosis and treatment of gastric neoplasia was been reported by Sumiyama’s group. They came to the conclusion that it is important for the accurate assessment of the safety margin to ensure complete resection by endoscopic submucosal dissection or en bloc endoscopic mucosal resection for neoplastic lesions. In 2005, Yao et al[14,15] reported MVP is important for diagnosing early gastric carcinoma, as it provides 98.7% accuracy[16,17]. Then they implied the morphology of the white opaque substance (WOS) could be an alternative new optical sign for discriminating adenoma from carcinoma.
In our studies, we found the WOS was one of the phenotypes in NBI magnification images, but not the main discrimination. However, the MSP and microvascular structures differ between LIN and HIN. LIN showed an irregular MSP and MVP with a clear demarcation line. The individual morphology of the crypt epithelium showed the different shapes (tubular, linear, curved or papillary). The vessels differ in shape (closed-loop, open-loop, tortuous or branched). Both the MSP and MVP have the irregular arrangement and variation in width and length. In contrast with LIN, HIN showed as flat because of a few areas without a network. The accuracy, sensitivity and specificity of ME-NBI for predicting low grade gastric IN were 81.03%, 84.38% and 76.92%, respectively.
Gastric mucosa IN is acknowledged as precancerous, with early diagnosis and prevention being important. To date, the final diagnosis still depends on histopathology. CLE has been developed which is an integration of a confocal laser microscope in the distal tip of a conventional videoendoscope. The components enable confocal microscopy in addition to standard videoendoscopy, and the new device can provide real-time, high magnification, cross-sectional images of the GI epithelium during routine endoscopy without the need for biopsy. Thusly, histopathology has been termed optical biopsy. This endoscopic tool allows for the observation of images magnified up to 1000-fold, which enables the visualization of mucosal microvascular architecture in units as small as the capillaries in the mucosal layer. Compared with other new optic techniques, the greatest advantage of CLE is that it can enables surface and subsurface imaging of living cells in the mucosa during ongoing endoscopy.
The first publication about an integrated confocal fluorescence microscope into the distal tip of a conventional colonoscope (Pentax EC 3830FK, Tokyo, Japan) was made by Kiesslich et al[18] in 2004, showing that in vivo microscopy at a subcellular resolution (0.7 μm) simultaneously displayed to white light endoscopy was possible and achieved high accuracy. This permitted immediate diagnosis of colorectal INs using fluorescein or acriflavine as contrast agents. Nuclear changes can best be observed using acriflavine as a topical contrast agent, as shown by Kakeji et al[19].
The endoscopic detection of early gastric cancer is challenging. Endomicroscopy allows immediate microscopic analysis of the gastric mucosa during ongoing endoscopy. Fluorescein-guided endomicroscopy is particularly good at demonstrating blood vessel architecture, which can be used to differentiate between non-neoplastic and neoplastic lesions. Kitabatake et al[20] showed the differences of mucosal vasculature according to the grade of tumor differentiation using CLE. The accuracy for the diagnosis of gastric cancer was above 90%. Liu et al[9] reported that comparison of regular and altered gastric mucosa facilitates the interpretation of the confocal images. Changes in tissue and microscopic blood vessel architecture, as well as changes in cell morphology, are valuable endomicroscopic criteria in defining neoplasias. Zhang et al[21] investigated the pattern and in vivo architecture of gastritis and gastric cancer on 132 consecutive patients. They classified gastric pit-pattern cellular architecture into seven types: normal mucosa with fundic glands mainly showed type A (round pits); corporal mucosa with histologic gastritis showed type B (non-continuous short rod-like); normal mucosa with pyloric glands mainly showed type C (continuous short rod-like); antral mucosa with histologic gastritis showed type D (elongated and tortuous branch-like); type E showed in intestinal metaplasia mucosa (the Goblet cells could be easily identified); and the corresponding values of the type G pattern (corkscrew-like and irregular in quality) for predicting gastric cancer were 90.0% and 99.4%.
Our study showed that the pit pattern and capillary network in the gastric mucosal layer was made visible using the confocal endomicroscope. In normal gastric antrum mucosa, the gastric pits are line type and the capillary network patterns showed a coil-shaped subepithelial capillary network pattern which surrounded the gastric pits. In the gastric body mucosa, the gastric pits are round and the capillary network patterns are characterized by a honeycomb-like network which was situated along or between the mucosal ridges. However, in the gastric fundus mucosa, the findings were incompatible with previous studies on gastric pit pattern and vascular network[21]; the superficial lamella gastric pits are round. Few pits compose one unit. The edge crack is the line type. Net-like subepithelial capillary network patterns surround the gastric pits.
When we studied gastric IN, we classified the mucosa into two types and observed the differences between them. LIN showed the different sizes and abnormal arrangement of gastric pits. The capillary network was thickening and circuitous. However, in the confocal images of HIN, the gastric pits presented strange shape. Some of them were unrecognized. A black tumor substance substituted the gastric patterns in some areas. The thickening capillary network and the increasing branch present a mass shape. The accuracy, sensitivity and specificity of the CLE for predicting the low grade gastric IN were 87.93%, 90.63% and 84.62%, respectively. These findings do not conflict with the previous studies of Kitabatake et al[20].
The confocal images of normal gastric mucosa are similar to the NBI magnification images, but the MSP and MVP are more clearly visible by CLE than magnification endoscopy. The recent study of the diagnosis of the differentiation of gastric IN by the technique of CLE combined with ME-NBI has been lacking. Our study showed the accuracy, sensitivity and specificity of CLE combined with ME-NBI for predicting the low grade gastric IN were 86.21%, 100% and 69.23%, respectively. From the results, we know that the diagnostic accuracy and specificity for the differentiation of gastric IN by CLE are 87.93% and 84.62%, which is higher than the alternatives. CLE is the best technique for the diagnosis of the differentiation of gastric IN. However, the diagnostic sensitivity by CLE combined with ME-NBI is 100. This shows the consistency of the technique of CLE and NBI-ME for the diagnostic differentiation of gastric IN.
There are some limitations to CLE, in particular, the limited infiltration depth of the blue laser light. The depth of scanning is approximately 250 μm. Therefore the vessels in the submucosa were not visible by endomicroscopy. In addition, the collecting vein in gastric body mucosa was visible by magnifying NBI, but can not be discerned in confocal images. This is because CLE covers only a very small area while a collecting vein can be seen in about every 60 gastric pits. In conclusion, the CEM system provides precise and clear images of the microvascular architecture of gastric mucosa. There are distinct characteristics distinguishing normal and malignant superficial microvascular networks in the mucosal layer. Our findings suggest that changes in the MCV may indicate early endoscopically detectable signs of cancer, which might facilitate on-site diagnoses and enable better management.
When we analyzed the confocal images, we excluded some poor images. The main reason for this was the moving artifacts caused by GI motility, breath and heartbeats. In addition, excessive mucus often influenced laser scanning depth, which produced images without a clear microsurface or microvascular architecture. In clinical practice, both good and average images could be used for clinical analysis. The findings indicate that the images of normal mucosa were better than those of the cancerous mucosa. The reason for this is that the cancerous mucosa is not always flat. The confocal images of cancer were easily distinguishable from normal mucosa in our study.
We know the importance in using a contrast agent when performing CLE[19,22]. Fluorescein is inexpensive and non-mutagenic, and has been used in ophthalmology for decades. The subtle architecture of microvascular caliber vessel walls and even blood cells could be identified. The capillaries were much brighter than epithelial cells because of the flow of fluorescein in the vessels[20]. Nuclear changes can best be observed using acriflavine as a topical contrast agent. In our study, fluorescein was used as contrast agent, so that observation covered not only the surface structure, but also the deeper layer which displays the architecture of microvessels. However, we did not use the contrast agent acriflavine, so the nuclear changes could not be observed and analyzed in the confocal images.
We could not assess interobserver agreement about the mucosal patterns seen in magnification NBI and CLE in this study. Future studies could determine whether our results can be reproduced by using two blinded observers. Our study needs further longitudinal investigation. The other limitation of this study was the small number of cases. Further prospective blinded studies with a sufficient number of patients are therefore important.
In conclusion, these endoscopic techniques of magnifying endoscopy with NBI and CLE that can provide valuable information regarding normal gastric mucosa and should be used for evaluating the grade of gastric IN.
We thank the staff at the Department of Gastroenterology, General Hospital of the People’s Liberation Army, for their advice to Zhong-Sheng Lu during his training in endoscopic mucosal dissection from September 2008 to July 2009.
Diagnosis and localization of early gastric cancer is problematic because of the lack of any endoscopic characteristics, even with multiple random biopsies. In recent years, endoscopic image quality has improved. Magnifying endoscopy combined with narrow-band imaging (ME-NBI) has been used to achieve endoscopic pathology in gastric disease. Confocal laser endomicroscopy (CLE) is a newly-developed technology that has enabled endoscopists to collect real-time in vivo histological images or “virtual biopsies” of the gastrointestinal (GI) mucosa during endoscopy, and has stimulated significant interest in the application of this technique in clinical gastroenterology. The following attempts to concisely and accurately summarize the related background of the article and to enable the readers to gain some basic knowledge relevant to the article, thus helping them better understand its significance.
Any prognosis of GI cancer is closely related to the stage of the disease at diagnosis. Endoscopic submucosal dissection (ESD) and en bloc endoscopic mucosal resection (EMR) have been performed as curative treatments for many early-stage GI lesions in recent years. However, before engaging in endoscopic therapy, an accurate diagnosis is a precondition affecting the complete cure of the underlying malignancy or carcinoma in situ. For the past few years, many new types of endoscopic techniques, including ME-NBI and CLE, have emerged in many countries since these methods provide a strong indication of early lesions and are very useful in determining treatment options before ESD or EMR.
This study showed that the pit pattern and capillary network in the gastric mucosal layer was made visible using the confocal endomicroscopy. In normal gastric antrum mucosa, the gastric pits are of the line type and the capillary network patterns showed a coil-shaped subepithelial capillary network pattern which surrounded the gastric pits. In the gastric body mucosa, the gastric pits are round and the capillary network patterns are characterized by a honeycomb-like network which was situated along or between the mucosal ridges.
The diagnostic accuracy and specificity for the differentiation of gastric IN by CLE are 87.93% and 84.62%, which is higher than the alternatives. CLE is the best technique for the diagnosis of the differentiation of gastric intraepithelial neoplasia (IN). However, the diagnostic sensitivity of CLE combined with ME-NBI is 100%, which showed the consistency of CLE and NBI-ME for the diagnostic differentiation of gastric IN.
Gastric carcinoma is the second commonest cause of cancer deaths worldwide. Early detection and diagnosis of gastric cancer in the stomach is important for improving patient prognosis. CLE is a novel endoscopic modality that allows subsurface analysis of the gastric mucosa during ongoing endoscopy. ME-NBI is more accurate in the diagnosis of gastric cancer when the diagnostic triad of the disappearance of fine mucosal structure, microvascular dilation, and heterogeneity is used. Several studies have reported that this technique is of value in the diagnosis of premalignant lesions in the GI tract. Patients underwent an examination with CLE and magnifying NBI. Obtained image resolution was evaluated, and the morphology, pit patterns and blood capillary forms of lesions were analyzed. The results indicate the feasibility of these two techniques for the efficacious diagnostic classification of gastric INs.
For the study of gastric INs, the authors classified the mucosa into two types and observed the differences between them. Low grade gastric intraepithelial neoplasia (LIN) showed different sizes and an abnormal arrange of gastric pits. In the confocal images of high grade gastric intraepithelial neoplasias (HIN), the gastric pits presented strange shapes. The results showed that white opaque substance is one of the phenotypes in NBI magnification images, but not the main discrimination. The microsurface pattern and microvascular structures differ between LIN and HIN. LIN showed the irregular microsurface and microvascular pattern with a clear demarcation line. The study also showed that the pit pattern and capillary network in the gastric mucosal layer was made visible using a confocal endomicroscope. The method for CLE, ME-NBI is clearly stated.
Peer reviewers: Yu-Yuan Li, Professor, Department of Gastroenterology, First Municipal People’s Hospital of Guangzhou, Guangzhou Medical College, Guangzhou 510180, Guangdong Province, China; Dr. Hiroki Nakamura, Department of Gastroenterology and Hepatology, Koga Hospital 21, 3-3-8 Miyanojin, Kurume, Fukuoka 839-0801, Japan
S- Editor Gou SX L- Editor A E- Editor Xiong L
.
| 1. | Nakayoshi T, Tajiri H, Matsuda K, Kaise M, Ikegami M, Sasaki H. Magnifying endoscopy combined with narrow band imaging system for early gastric cancer: correlation of vascular pattern with histopathology (including video). Endoscopy. 2004;36:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 2. | Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am. 2008;18:451-66, viii. [PubMed] |
| 3. | Nguyen NQ, Leong RW. Current application of confocal endomicroscopy in gastrointestinal disorders. J Gastroenterol Hepatol. 2008;23:1483-1491. [PubMed] |
| 4. | Venkatesh K, Cohen M, Evans C, Delaney P, Thomas S, Taylor C, Abou-Taleb A, Kiesslich R, Thomson M. Feasibility of confocal endomicroscopy in the diagnosis of pediatric gastrointestinal disorders. World J Gastroenterol. 2009;15:2214-2219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Technology insight: confocal laser endoscopy for in vivo diagnosis of colorectal cancer. Nat Clin Pract Oncol. 2007;4:480-490. [PubMed] |
| 6. | Kiesslich R, Neurath MF. Chromoendoscopy and other novel imaging techniques. Gastroenterol Clin North Am. 2006;35:605-619. [PubMed] |
| 7. | Dixon MF. Gastrointestinal epithelial neoplasia: Vienna revisited. Gut. 2002;51:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 495] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 8. | Stolte M. The new Vienna classification of epithelial neoplasia of the gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003;442:99-106. [PubMed] |
| 9. | Liu H, Li YQ, Yu T, Zhao YA, Zhang JP, Zhang JN, Guo YT, Xie XJ, Zhang TG, Desmond PV. Confocal endomicroscopy for in vivo detection of microvascular architecture in normal and malignant lesions of upper gastrointestinal tract. J Gastroenterol Hepatol. 2008;23:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Yao K, Anagnostopoulos GK, Ragunath K. Magnifying endoscopy for diagnosing and delineating early gastric cancer. Endoscopy. 2009;41:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 11. | Yao K, Takaki Y, Matsui T, Iwashita A, Anagnostopoulos GK, Kaye P, Ragunath K. Clinical application of magnification endoscopy and narrow-band imaging in the upper gastrointestinal tract: new imaging techniques for detecting and characterizing gastrointestinal neoplasia. Gastrointest Endosc Clin N Am. 2008;18:415-33, vii-viii. [PubMed] |
| 12. | Sano Y, Kobayashi M, Hamamoto Y, Kato S, Fu KI, Yoshino T. New diagnostic method based on color imaging using narrow-band imaging (NBI) system for gastrointestinal tract [abstract]. Gastrointest Endosc. 2001;53:AB125. [DOI] [Full Text] |
| 13. | Sumiyama K, Kaise M, Nakayoshi T, Kato M, Mashiko T, Uchiyama Y, Goda K, Hino S, Nakamura Y, Matsuda K. Combined use of a magnifying endoscope with a narrow band imaging system and a multibending endoscope for en bloc EMR of early stage gastric cancer. Gastrointest Endosc. 2004;60:79-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Yao K, Iwashita A, Kikuchi Y, Yao T, Matsui T, Tanabe H, Nagahama T, Sou S. Novel zoom endoscopy technique for visualizing the microvascular architecture in gastric mucosa. Clin Gastroenterol Hepatol. 2005;3:S23-S26. [PubMed] |
| 15. | Yao K, Iwashita A, Tanabe H, Nagahama T, Matsui T, Ueki T, Sou S, Kikuchi Y, Yorioka M. Novel zoom endoscopy technique for diagnosis of small flat gastric cancer: a prospective, blind study. Clin Gastroenterol Hepatol. 2007;5:869-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Nakamura M, Shibata T, Tahara T, Yoshioka D, Okubo M, Mizoguchi Y, Kuroda M, Arisawa T, Hirata I. The usefulness of magnifying endoscopy with narrow-band imaging to distinguish carcinoma in flat elevated lesions in the stomach diagnosed as adenoma by using biopsy samples. Gastrointest Endosc. 2010;71:1070-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Yao K, Iwashita A, Tanabe H, Nishimata N, Nagahama T, Maki S, Takaki Y, Hirai F, Hisabe T, Nishimura T. White opaque substance within superficial elevated gastric neoplasia as visualized by magnification endoscopy with narrow-band imaging: a new optical sign for differentiating between adenoma and carcinoma. Gastrointest Endosc. 2008;68:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 559] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 19. | Kakeji Y, Yamaguchi S, Yoshida D, Tanoue K, Ueda M, Masunari A, Utsunomiya T, Imamura M, Honda H, Maehara Y. Development and assessment of morphologic criteria for diagnosing gastric cancer using confocal endomicroscopy: an ex vivo and in vivo study. Endoscopy. 2006;38:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Kitabatake S, Niwa Y, Miyahara R, Ohashi A, Matsuura T, Iguchi Y, Shimoyama Y, Nagasaka T, Maeda O, Ando T. Confocal endomicroscopy for the diagnosis of gastric cancer in vivo. Endoscopy. 2006;38:1110-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Zhang JN, Li YQ, Zhao YA, Yu T, Zhang JP, Guo YT, Liu H. Classification of gastric pit patterns by confocal endomicroscopy. Gastrointest Endosc. 2008;67:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (0)] |