Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4744
Revised: June 4, 2012
Accepted: June 15, 2012
Published online: September 14, 2012
AIM: To assess the prognostic significance of nuclear factor-κB (NF-κB) and its target genes in gastric cancer.
METHODS: The tumor tissues of 115 patients with gastric cancer were immunohistochemically evaluated using monoclonal antibodies against NF-κB RelA. Preoperative serum levels of vascular endothelial growth factor (VEGF), interleukin-6 (IL-6) were assessed via enzyme-linked immuno-sorbent assay. C-reactive protein (CRP) and serum amyloid A (SAA) were measured via immunotrubidimetry.
RESULTS: Positive rate of NF-κB RelA was 42.6%. NF-κB RelA expression in tumor tissues was also related to serum levels of IL-6 (P = 0.044) and CRP (P = 0.010). IL-6, SAA, CRP were related to depth of invasion, VEGF and SAA were correlated with lymph node metastasis. IL-6, VEGF, SAA and CRP were related to the stage. Univariate analysis demonstrated that immunostaining of NF-κB RelA, levels of IL-6, VEGF, SAA were significantly related with both disease free survival and overall survival (OS). Multivariate analysis verified that NF-κB RelA [hazard ratio (HR): 3.40, P = 0.024] and SAA (HR: 3.39, P = 0.045) were independently associated with OS.
CONCLUSION: Increased expression of NF-κB RelA and high levels of serum SAA were associated with poor OS in gastric cancer patients.
- Citation: Kwon HC, Kim SH, Oh SY, Lee S, Lee JH, Jang JS, Kim MC, Kim KH, Kim SJ, Kim SG, Kim HJ. Clinicopathologic significance of expression of nuclear factor-κB RelA and its target gene products in gastric cancer patients. World J Gastroenterol 2012; 18(34): 4744-4750
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4744.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4744
Gastric cancer continues to pose a significant health problem, despite its declining incidence in the West. It is the 4th most common cancer worldwide, accounting for 8.6% of all new cancer diagnoses in 2002[1]. In South Korea, gastric cancer is the most common carcinoma in men, and the third-most common cancer type in women[2]. Surgery is the only potentially curative treatment for localized gastric cancer. However, the majority of patients will relapse after definitive surgery, and the 5-year overall survival (OS) rates of this condition remain lower than 30% to 40% in Western countries[1]. These high levels of gastric cancer mortality can be attributed to the high incidence of serosal invasion, direct invasion into the adjacent organs, peritoneal seeding, lymph node metastasis, and distant metastasis of gastric cancer. The assessment of biological prognostic factors is of great clinical importance in gastric cancer. The widely used tumor node metastasis (TNM) staging exhibits high prognostic power, but cannot predict outcomes perfectly for particular individuals. The outcome of cancer patients may be affected by variability in tumor biology. Thus, tumors with similar clinical or pathologic characteristics frequently evidence different clinical outcomes. Gaining insight into the underlying molecular mechanisms of initiation and progression of gastric cancer will be a necessary step in definitively identifying groups of patients with poor prognoses. The transcription factor nuclear factor-κB (NF-κB) is a heterodimeric complex of the Rel-family proteins. The most common dimer is the RelA (p65)/p50 heterodimer, i.e., NF-κB[3]. NF-κB is sequestered in the cytoplasms in an inactive form via interaction with the inhibitor of nuclear factor-κB proteins (IκBs). A variety of stimuli, including cytokines and infectious agents, induce the phosphorylation, ubiquitination, and degradation of IκBs, and also allow the nuclear translocation of NF-κB[4]. Once in the nucleus, NF-κB binds to DNA at its response elements and activates the expression of genes. Recent studies using anti-RelA immunostaining have shown that NF-κB is constitutively activated in gastric cancer[5-7], Additionally, there is some evidence to suggest that NF-κB levels are higher in gastric cancer cells than in normal adjacent epithelial cells, and that NF-κB activation in gastric cancer is associated with lymphatic invasion, advanced stage, and poor clinical outcomes[5,7]. However, other investigators have reported conflicting data with regard to the relationship of NF-κB protein with patient survival[6].
The active NF-κB transcription factor promotes the expression of more than 150 target genes[8]. The majority of proteins encoded for by NF-κB target genes participate in host immune responses, whereas others appear to be involved in the proliferation, invasion, angiogenesis, and metastasis of cancer cells. NF-κB promotes the production of cytokines that function as growth factors for transformed enterocytes[9]. One of these growth factors was subsequently identified as interleukin-6 (IL-6), which is encoded for by a NF-κB target gene. IL-6 acts on a broad range of tissues and cell lines, and induces cell growth and differentiation, the production and expression of other cytokines, and acute-phase protein synthesis. IL-6 also promotes growth arrest[10], and promotes angiogenesis via the induction of vascular endothelial growth factor (VEGF) expression[11]. A previous study reported that the preoperative serum level of IL-6 was associated with lymph node metastasis, depth of invasion, advanced stage, and poor survival in gastric cancer patients[12].
C-reactive protein (CRP) and serum amyloid A (SAA) are nonspecific, acute-phase, hepatic proteins secreted in response to cytokines, including IL-1, IL-6, and tumor necrosis factor-α[13]. CRP is a representative marker for inflammatory conditions, and carries out a crucial anti-infection function in the immune system. In many cancers, chronic inflammation has reportedly been involved with malignant change, and the risks of colorectal cancer appear to be increased when pre-diagnostic CRP levels are high[14]. Cancer invasion begins with inflammation around cancer cells. Thus, serum CRP levels have generally been shown to be higher in cases of invasive cancer than in cases of non-invasive cancer[12].
An increasing body of in vitro evidence supports the involvement of SAA in carcinogenesis and neoplastic diseases[15]. An accumulating body of evidence has been collected to suggest that SAA might be included in a group of biomarkers for the detection of a pattern of physiological events that reflects the growth of malignancy and host response. There have also been some reports indicating that the SAA-induced upregulation of matrix metalloproteinase 9 was mediated via formyl peptide receptor like-1 and was achieved at the transcriptional level via NF-κB[16], and also that preoperative SAA was useful in predicting the survival of gastric cancer patients[17].
The expression of several angiogenic factors is also known to be regulated by NF-κB. Macrophages as well as tumor cells have been shown to generate VEGF under the control of NF-κB activation[18]. VEGF is the most important pro-angiogenic growth factor, and its stimulation under hypoxic conditions carries out critical functions in promoting the survival of malignant cells, in local tumor growth and invasion, and in the development of metastases[19]. We demonstrated that VEGF expression was correlated with the depth of invasion and NF-κB expression in stage III colorectal cancer patients[20].
The objective of the current investigation was to evaluate the prognostic significance of NF-κB RelA and its target gene products in gastric cancer patients.
From March 2005 to July 2006, a total of 115 patients with histologically-confirmed gastric carcinomas at the Dong-A University Hospital were enrolled in this study. After operation, the disease status of patients was evaluated via gastroendoscopy and abdominal computed tomography (CT). During the first 2 years, we checked CT and endoscopy at 6-mo intervals, and afterwards CT and endoscopy were conducted every 12 mo. We also carried out abdomen CT when we observed abdominal distension, acute abdominal pain, severe diarrhea and chest X-ray abnormalities suggestive of lung metastasis. Endoscopy was conducted in cases in which patients complained of unexplained abdominal discomfort and dyspepsia. On average, in the stage III and IV patients, the first evaluation was conducted within 3 mo after operation. All patients provided informed consent, and the hospital review board approved the study.
Two cores of 2-millimeters in diameter of formalin-fixed, paraffin-embedded tissue blocks were obtained from each gastric cancer patient’s sample and arranged in new recipient paraffin blocks using an UNITMA apparatus (UNITMA, Seoul, South Korea) after careful histological assessment. All cases except for one endoscopic biopsy sample and the normal control tissue, were embedded in 6-tissue microarray blocks. Tissue microarray blocks were serially cut into 4 μm sections and stained for routine hematoxylin and eosin staining and immunohistochemistry.
All immunohistochemistry processes were automatically performed using a Ventana autostainer (Benchmark; Ventana Medical Systems, Tuscon, Arizona, United States). For antigen retrieval, retrieval solution (Ventana) was automatically poured onto the sections, and heated for 60 min at 100 °C. Endogenous peroxide activity was blocked via immersion in 3% hydrogen peroxide for 4 min. With diluted primary antibody for NF-κB p65 (rabbit monoclonal antibody, 1:250, Epitomics, CA, United States), the tissue sections were incubated for 32 min at 36 °C. Immunoperoxidase staining was carried out using a DAB system (iView DAB detection kit, Ventana) and the sections were counterstained lightly with hematoxylin.
The percentage and intensity of the immunoreactive tumor cells in each core was recorded and the final values of the positive tumor cells were evaluated as the mean of the immunoreactivity in three cores. The presence of tumor tissue in at least two interpretable cores was required for the inclusion of a case in the statistical analysis. All of the slides were evaluated independently by an experienced pathologist (Kim SJ) who was blinded to all of the clinicopathologic data. Immunostaining for NF-κB RelA was regarded as positive when tumor cells evidenced granular cytoplasmic immunostaining of the lesion, with a minimal background. The percentage scoring of the immunoreactive tumor cells was as follows: 0 (0%), 1 (1%-10%), 2 (11%-50%) and 3 (> 50%). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak, if it was a blush), and 2 (strong, if it was obviously positive at × 20 magnification). A final score was obtained for each case by multiplying the percentage by the intensity score. Therefore, tumors with multiplied scores exceeding 4 (i.e., tumors with a strong intensity of > 10% of the tumor cells) were regarded as positively immunoreactive to NF-κB RelA; all other scores were considered negative.
Peripheral venous blood samples were obtained within 7 d prior to gastrectomy, collected in plain tubes, permitted to clot, and centrifuged for 10 min at 3000 r/min at 4 °C within one hour of collection to obtain the serum. The sera were aliquoted and stored at -80 °C until being assayed. The VEGF and IL-6 levels from the sera of patients and the standard solutions supplied were measured using a commercial system (Quantikine hVEGF, hIL-6 Immunoassay, R and D Systems, United States) in accordance with the manufacturer's instructions. All serum samples were measured by an investigator who was blinded to the clinical data. The blood samples for CRP analysis were collected in serum separation tubes, and the serum CRP levels were measured via immunoturbidimetry (Denka Seiken Co. Ltd., Japan). SAA protein was measured via latex agglutination turbidoimmunometric assays (Eiken Chemical Co., Japan).
The associations between NF-κB RelA expression and the relevant clinicopathological parameters were assessed via an χ2-test or Fisher’s exact test. Serum levels of VEGF, IL-6 and CRP were expressed as mean values. The Mann-Whitney U-test was used to evaluate the relationship between protein concentration and clinicopathological features. Disease-free survival (DFS) was defined as the length of time from surgery to initial recurrence of disease. OS was defined as the length of time from surgery to death. The Kaplan-Meier method was utilized to construct curves for DFS and OS. Data on patients who died without any evidence of disease recurrence were censored at the time of death for DFS calculations. The log-rank test was employed to compare distributions. In order to identify the independent factors that were significantly related to patient prognosis, we utilized Cox’s proportional hazard analysis via a stepwise procedure. All tests were two-sided, and P < 0.05 was considered statistically significant. Analyses were conducted using SPSS version 18.0 (SPSS, Chicago, IL, United States).
The mean age of the patients was 59 years (range: 24-75 years), and the study population included 47 (40.92%) females and 68 (59.1%) males. Thirty-six of the patients (31.3%) underwent radical total gastrectomy, 75 (65.2%) underwent radical subtotal gastrectomy, and four patients (3.5%) did not have operation due to distant metastasis. The majority of the tumors were located in the middle or lower stomach (72.1%), and were moderate to poorly differentiated carcinomas (75.7%). Thirty-four of the patients (30.6%) had pathological T3 (pT3) or pT4 tumors, 69 (63.2%) had lymph node metastases, and 26 patients (23.4%) had a positive lymph node ratio of ≥ 0.2. The postoperative stages of the patients were I, II, III and IV in 43 (37.4%), 23 (20.0%), 31 (27.0%) and 18 (15.7%) patients, respectively. With regard to the Lauren classifications, 46 (40.0%) patients were of the intestinal type, 43 (37.4%) of the diffuse type, and 15 (13.0%) of the mixed type. Sixteen patients (14.4%) had well, 28 (25.2%) had moderate, and 56 (50.5%) had poorly differentiated adenocarcinoma. Fifty-five patients (47.8%) received oral 5-fluorouracil, and 46 patients (40.0%) received cisplatin-based adjuvant chemotherapy after gastrectomy.
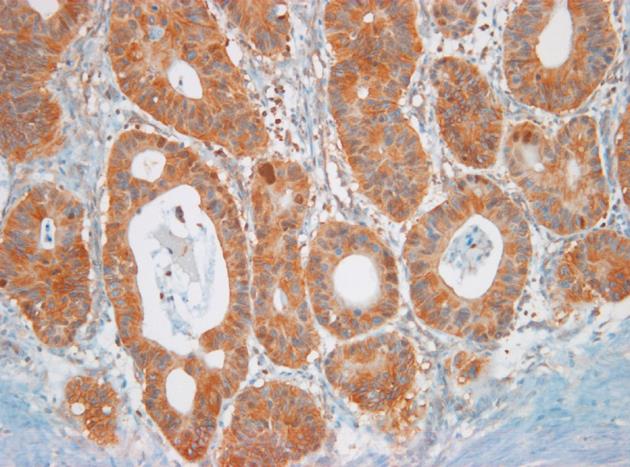
A representative tumor specimen evidencing increased NF-κB RelA expression in the cytoplasm is shown in Figure 1. NF-κB RelA expression was found to be significantly correlated with several findings (Table 1). NF-κB RelA expression was more common in cases of more than 5 cm (P = 0.045), deeper invasion (P = 0.012), lymph node metastases (P < 0.001), advanced stage (P < 0.001), positive lymphovascular invasion (P = 0.012), and increased Carbohydrate antigen 19-9 (P < 0.001). However, no significant correlations were noted between Nf-κB a RelA nd other parameters. We noted that VEGF, SAA levels were significantly correlated with tumor size (P = 0.022, P < 0.001, respectively). IL-6, SAA and CRP levels were related to the depth of invasion (P = 0.007, P < 0.001, P = 0.015, respectively). We also noted significant differences in serum VEGF and SAA levels between patients with lymph node metastasis and those without lymph node metastasis (P < 0.001, respectively). The mean levels of IL-6, VEGF, SAA and CRP were increased proportionally with the cancer stage (P = 0.031, P < 0.001, P < 0.001, P < 0.001, respectively). Additionally, NF-κB RelA expression was correlated positively with IL-6 (P = 0.044), and CRP (P = 0.010) levels (Table 2).
| NF-κB RelA (-) | NF-κB RelA (+) | P | |
| Sex | 1.000 | ||
| Male | 39 (57.4) | 29 (42.6) | |
| Female | 27 (57.4) | 20 (42.6) | |
| Age (yr) | 0.192 | ||
| < 60 | 38 (63.3) | 22 (36.7) | |
| ≥ 60 | 28 (50.9) | 27 (49.1) | |
| Site | 0.002 | ||
| Upper | 9 (37.5) | 15 (62.5) | |
| Middle | 9 (50.0) | 9 (50.0) | |
| Lower | 48 (72.7) | 18 (27.3) | |
| Diffuse | 0 (0) | 3 (100) | |
| Size (cm) | 0.045 | ||
| < 5 | 47 (67.1) | 23 (32.9) | |
| ≥ 5 | 19 (46.3) | 22 (53.7) | |
| Lauren | 0.988 | ||
| Intestinal | 27 (58.7) | 19 (41.3) | |
| Diffuse | 26 (57.8) | 19 (42.2) | |
| Mixed | 9 (60.0) | 6 (40.0) | |
| Differentiation | 0.631 | ||
| Well | 11 (68.8) | 5 (31.3) | |
| Moderate | 14 (50.0) | 14 (50.0) | |
| Poor | 34 (60.7) | 22 (39.3) | |
| Mucinous | 7 (63.6) | 4 (36.4) | |
| Lymphovascular invasion | 0.012 | ||
| - | 44 (69.8) | 19 (30.2) | |
| + | 22 (45.8) | 26 (54.2) | |
| T stage | 0.012 | ||
| 1-2 | 52 (67.5) | 25 (32.5) | |
| 3-4 | 14 (41.2) | 20 (58.8) | |
| Node | < 0.001 | ||
| - | 34 (81.0) | 8 (19.0) | |
| + | 32 (46.4) | 37 (53.6) | |
| Lymph node ratio | < 0.001 | ||
| < 0.2 | 60 (70.6) | 25 (29.4) | |
| ≥ 0.2 | 6 (23.1) | 20 (76.9) | |
| Stage | < 0.001 | ||
| 1 | 36 (83.7) | 7 (16.3) | |
| 2 | 14 (60.9) | 9 (39.1) | |
| 3 | 16 (51.6) | 15 (48.4) | |
| 4 | 0 (0) | 18 (100) | |
| CEA (ng/mL) | 0.134 | ||
| < 5 | 64 (59.3) | 44 (40.7) | |
| ≥ 5 | 2 (28.6) | 5 (71.4) | |
| CA19-9 (U/mL) | < 0.001 | ||
| < 37 | 63 (67.7) | 30 (32.3) | |
| ≥ 37 | 3 (13.6) | 19 (86.4) | |
| Nuclear factor-κB | P | ||
| - (n = 66) | + (n = 49) | ||
| IL-6 (pg/mL) | 8.1 ± 8.6 | 11.8 ± 10.7 | 0.044 |
| VEGF (pg/mL) | 117.2 ± 154.2 | 178.9 ± 202.7 | 0.066 |
| SAA (mg/mL) | 8.1 ± 20.3 | 14.5 ± 16.3 | 0.072 |
| CRP (mg/mL) | 0.1 ± 0.1 | 0.3 ± 0.4 | 0.010 |
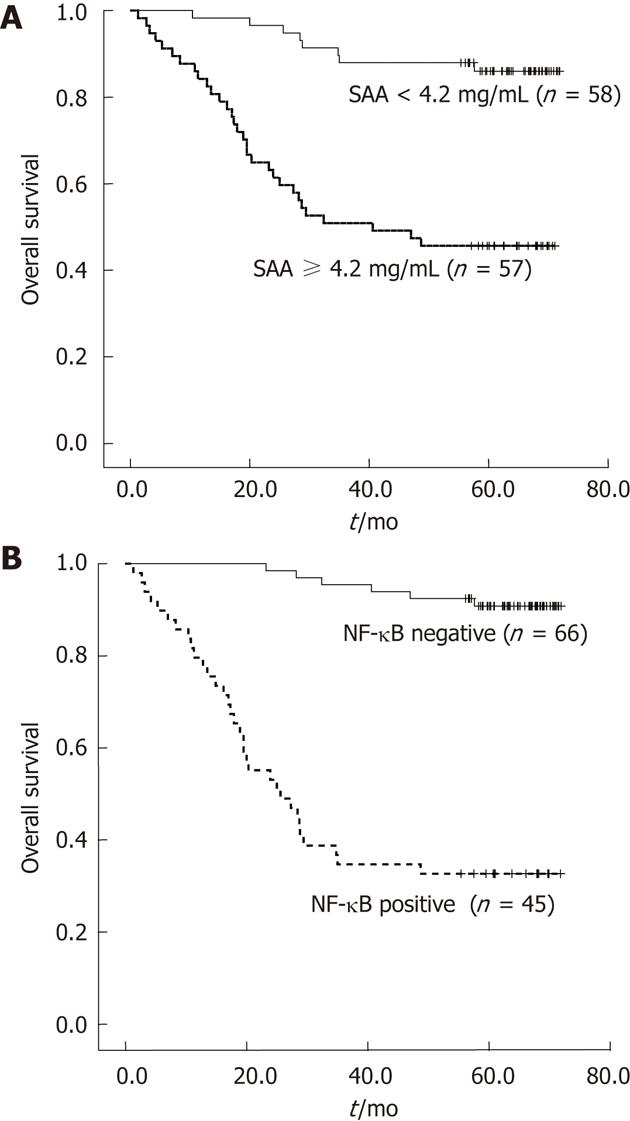
The median follow-up duration of the patients was 66.6 mo (range: 9.3-88.8 mo). Univariate analysis of clinicopathologic parameters and DFS and OS are provided in Table 3. Tumor size, location, lymphovascular invasion, stage, and positive lymph node ratio were significantly associated with both 5-year DFS and OS. Positive correlations were detected between immunostaining of NF-κB RelA, levels of IL-6, VEGF, SAA, and DFS and OS. CRP levels were not correlated with 5-year DFS or OS. The OS curves of SAA and NF-κB RelA are provided in Figure 2. In order to determine the independent prognostic values of these markers, we employed a multivariate Cox proportional hazard analysis to control for other prognostic factors (Table 4). Accordingly, stage was identified as an independent prognostic factor of both DFS [hazard ratio (HR): 7.83, 95% confidence interval (CI) = 2.44-25.18, P = 0.001], and OS (HR: 6.15, 95% CI = 1.97-19.22, P = 0.002). Positive lymph node ratio was also identified as a significant predictor of both DFS (HR: 1.68, 95% CI = 1.01-2.79, P = 0.043), and OS (HR: 2.46, 95% CI = 1.01-5.94, P = 0.047), after controlling for other clinicopathologic parameters. The expressions of NK-κB and SAA levels were shown to significantly affect OS on multivariate analysis (HR: 3.40, 95% CI = 1.17-9.87, P = 0.024; HR: 3.39, 95% CI = 1.02-11.23, P = 0.045, respectively).
| n | 5-yr DFS (%) | P | 5-yr OS (%) | P | |
| Sex | 0.824 | ||||
| Male | 68 | 64.7 | 0.827 | 65.8 | |
| Female | 47 | 68.1 | 70.2 | ||
| Age (yr) | 0.367 | ||||
| < 60 | 60 | 70 | 0.355 | 71.7 | |
| ≥ 60 | 65 | 61.8 | 62.7 | ||
| Site | < 0.001 | ||||
| Upper | 24 | 41.7 | < 0.001 | 45.8 | |
| Middle | 18 | 72.2 | 77.8 | ||
| Lower | 16 | 80.3 | 79.8 | ||
| Diffuse | 3 | 0 | 0 | ||
| Size (cm) | 0.005 | ||||
| < 5 | 70 | 77.1 | 0.006 | 78.1 | |
| ≥ 5 | 41 | 53.7 | 56.1 | ||
| Lauren | 0.301 | ||||
| Intestinal | 46 | 71.7 | 0.345 | 73.3 | |
| Diffuse | 43 | 60.5 | 62.8 | ||
| Mixed | 15 | 80 | 80.8 | ||
| Differentiation | 0.779 | ||||
| Well | 16 | 68.8 | 0.879 | 75 | |
| Moderate | 28 | 75.2 | 75 | ||
| Poor | 56 | 66.1 | 67 | ||
| Mucinous | 11 | 63.6 | 63.6 | ||
| Lymphovascular invasion | < 0.001 | ||||
| - | 63 | 82.5 | < 0.001 | 82 | |
| + | 48 | 50 | 54.2 | ||
| T stage | < 0.001 | ||||
| 1-2 | 77 | 79.2 | < 0.001 | 80 | |
| 3-4 | 34 | 44.1 | 47.1 | ||
| Node | < 0.001 | ||||
| - | 42 | 97.6 | < 0.001 | 97.6 | |
| + | 69 | 50.7 | 52.9 | ||
| Lymph node ratio | < 0.001 | ||||
| < 0.2 | 85 | 81.2 | < 0.001 | 81 | |
| ≥ 0.2 | 26 | 26.9 | 26.9 | ||
| Stage | <0.001 | ||||
| 1 | 43 | 100 | < 0.001 | 100 | |
| 2 | 23 | 60.9 | 63.2 | ||
| 3 | 31 | 54.8 | 58.1 | ||
| 4 | 18 | 11.1 | 11.1 | ||
| CEA (ng/mL) | < 0.001 | ||||
| < 5 | 108 | 69.4 | < 0.001 | 69.4 | |
| ≥ 5 | 7 | 14.3 | 14.3 | ||
| CA19-9 (U/mL) | < 0.001 | ||||
| < 37 | 93 | 73.1 | < 0.001 | 73 | |
| ≥ 37 | 22 | 36.4 | 36.4 | ||
| Nuclear factor-κB | < 0.001 | ||||
| - | 66 | 90.9 | < 0.001 | 90.8 | |
| + | 49 | 32.7 | 32.7 | ||
| IL-6 (pg/mL) | < 0.001 | ||||
| < 6.83 | 59 | 81.4 | < 0.001 | 81.4 | |
| ≥ 6.83 | 56 | 50 | 49.6 | ||
| VEGF (pg/mL) | 0.002 | ||||
| < 68.1 | 57 | 80.7 | 0.001 | 80.5 | |
| ≥ 68.1 | 58 | 52.6 | 52.6 | ||
| SAA (mg/mL) | < 0.001 | ||||
| < 4.2 | 58 | 86.2 | < 0.001 | 86 | |
| ≥ 4.2 | 57 | 45.6 | 45.6 | ||
| CRP (mg/mL) | 0.899 | ||||
| < 0.1 | 59 | 66.1 | 0.858 | 66.1 | |
| ≥ 0.1 | 56 | 66.1 | 65.9 | ||
| 5-yr disease free survival | 5-yr overall survival | |||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Stage | 7.83 | 2.44-25.18 | 0.001 | 6.15 | 1.97-19.2 | 0.002 |
| Site | 1.21 | 0.66-2.22 | 0.536 | 1.22 | 0.67-2.23 | 0.510 |
| Size | 0.48 | 0.188-1.27 | 0.142 | 0.577 | 0.22-1.41 | 0.218 |
| LN ratio | 1.68 | 1.01-2.79 | 0.043 | 2.46 | 1.01-5.94 | 0.047 |
| LVI | 0.79 | 0.30-2.11 | 0.650 | 1.02 | 0.41-2.52 | 0.951 |
| IL-6 | 1.40 | 0.53-3.66 | 0.488 | 1.19 | 0.45-3.14 | 0.726 |
| VEGF | 1.22 | 0.77-1.95 | 0.390 | 2.11 | 0.86-5.17 | 0.102 |
| SAA | 3.21 | 0.96-10.79 | 0.058 | 3.39 | 1.02-11.23 | 0.045 |
| CEA | 2.17 | 0.66-7.07 | 0.198 | 2.38 | 0.76-7.48 | 0.135 |
| CA19-9 | 0.93 | 0.35-2.45 | 0.898 | 1.96 | 0.76-5.02 | 0.158 |
| NF-κB RelA | 2.87 | 0.96-8.49 | 0.057 | 3.40 | 1.17-9.87 | 0.024 |
There have been a few reports regarding the expression of NF-κB in human gastric cancer assessed by immunohistochemistry using the same anti-RelA (p65) antibody[5,7]. The present study confirms constitutively active NF-κB RelA in gastric carcinoma, which is consistent with the findings of previous studies[5,7]. The expression rate of NF-κB RelA in gastric carcinoma is higher than that observed in normal adjacent epithelial cells, and NF-κB RelA expression was observed primarily in gastric carcinoma cytoplasm with an expression rate of 42.6%; this is somewhat higher than in other studies[5,7]. We showed that NF-κB RelA expression was correlated with tumor size, depth of invasion, lymph node metastasis, lymphovascular invasion and advanced stage. Furthermore, positive NF-κB RelA staining was associated with both DFS and OS, and NF-κB RelA was identified as an independent prognostic factor in multivariate analysis (HR: 3.40, 95% CI = 1.17-9.87, P = 0.024).
Sasaki et al[5] also reported that NF-κB activation was correlated with tumor size and lymphatic invasion. However, Lee et al[6] previously demonstrated that NF-κB activation was not correlated with poor patient prognosis, but rather was significantly associated with better prognosis of early stage gastric carcinoma patients. The absence of clear-cut criteria in the evaluation of NF-κB immunoexpression may be the major reason for the conflicts among the previously published data regarding the prognostic impact of this molecule in gastric cancer.
The activation of NF-κB regulates a variety of genes involved in the proliferation, invasion, angiogenesis, and metastasis of cancer cells[8]. In this study, NF-κB RelA expression was correlated positively with IL-6 and CRP levels. According to some studies attempting to evaluate the clinical significance of IL-6 in gastric cancer patients, IL-6 levels were higher in malignancies than in non-malignancies, increased with increasing tumor size and depth, and were associated with poor clinical outcomes[12,21]. We found significant correlations between elevated IL-6 levels and several conditions: large tumor size, deeper invasion depth, advanced stage, and poor DFS and OS.
Findings from studies of gastrointestinal cancer patients have demonstrated that those subjects with elevated serum CRP concentrations tended toward poorer prognoses than those whose CRP levels were not increases[14,22]. CRP was related to the depth of tumor invasion, tumor differentiation, and TNM stage. However, we found no significant associations between CRP and DFS or OS.
SAA is the most sensitive plasma protein indicative of inflammatory activity[23]. SAA is superior to CRP for the detection of inflammation associated with severe burns, viral infections, kidney transplantation, Crohn’s disease, and ulcerative colitis[23]. In this study, SAA was related to tumor size, depth of invasion, lymph node metastasis, clinical stage, and cancer recurrence. Similar results have been reported in other studies[17]. Those studies showed that gastric cancer patients had higher SAA levels than did patients with gastric ulcers and healthy individuals, and that SAA concentrations were correlated with prognosis[17]. SAA was related significantly to DFS and OS, and was determined to be an independent prognostic factor for OS in multivariate analysis (HR: 3.39, 95% CI = 1.02-11.23, P = 0.045).
Tumor VEGF expression was identified as a significant marker for tumor recurrence or reduced survival independent of conventional clinicopathological variables in gastric cancer[24,25]. However, the prognostic value of VEGF expression in gastric cancer remains unclear, and some studies have indicated that tumor VEGF expression may constitute an independent prognostic factor in DFS[24,25], whereas others have reported no such association[26]. In our study, VEGF expression was significantly correlated with tumor size, lymph node invasion and staging; however, our findings do not appear to confirm a significant association between VEGF expression and unfavorable prognosis in multivariate analysis.
Univariate analysis demonstrated that the immunostaining of NF-κB RelA and levels of IL-6, VEGF and SAA were all significantly related with both DFS and OS. Multivariate analysis results verified that NF-κB RelA and SAA were independently associated with OS. Although changes in the expression levels of several cytokines have been frequently reported, their proposed role in the pathogenesis and progression of gastric cancer remains controversial. The results of such studies may be confounded by several factors, including small patient sample size and variable therapeutic protocols which may potentially have affected patient outcomes. Well-designed large scale studies will be necessary to confirm our data.
The active nuclear factor-κB (NF-κB) transcription factor promotes the expression of target genes. The majority of proteins encoded for by NF-κB target genes participate in host immune responses, proliferation, invasion, angiogenesis, and metastasis of cancer cells.
The objective of the current investigation was to clarify the prognostic significance of NF-κB RelA and its target gene products in gastric cancer patients.
In the present study, NF-κB RelA expression in tumor tissues was related to increased serum levels of interleukin (IL)-6 (P = 0.044) and C reactive protein (CRP) (P = 0.010). Levels of serum IL-6, vascular endothelial growth factor (VEGF), serum amyloid A (SAA) and CRP were related to the stage. Univariate analysis demonstrated that immunostaining of NF-κB RelA, levels of IL-6, VEGF, SAA were significantly related with both disease free survival and overall survival (OS). Multivariate analysis verified that NF-κB RelA and SAA were independently associated with OS.
Increased expression of NF-κB RelA and high levels of serum SAA were associated with poor OS in gastric cancer patients.
The authors of this manuscript examined potential prognostic value of NF-κB RelA expression in gastric cancer patients and reported an inverse relationship between RelA expression and patient survival. In addition to RelA, the contribution of several inflammatory mediators/cytokines to the patient prognosis was also investigated. The study was well-designed and the data are convincing.
Peer reviewer: Dr. Fei Chen, Department of Pharmaceutical Sciences, Wayne State University, 259 Mack Avenue, Detroit, MI 48201, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13555] [Article Influence: 677.8] [Reference Citation Analysis (1)] |
| 2. | Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, Park EC, Ahn YO, Hwang IK, Lee DH, Choi JS, Kim WC, Lee TY, Yoo CI, Bae JM, Kim ON, Chung W, Kong IS, Lee DH, Lee JS. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009;41:122-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002;3:221-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2154] [Cited by in RCA: 2183] [Article Influence: 94.9] [Reference Citation Analysis (1)] |
| 4. | Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 5. | Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136-4142. [PubMed] |
| 6. | Lee BL, Lee HS, Jung J, Cho SJ, Chung HY, Kim WH, Jin YW, Kim CS, Nam SY. Nuclear factor-kappaB activation correlates with better prognosis and Akt activation in human gastric cancer. Clin Cancer Res. 2005;11:2518-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Levidou G, Korkolopoulou P, Nikiteas N, Tzanakis N, Thymara I, Saetta AA, Tsigris C, Rallis G, Vlasis K, Patsouris E. Expression of nuclear factor kappaB in human gastric carcinoma: relationship with I kappaB a and prognostic significance. Virchows Arch. 2007;450:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853-6866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3079] [Cited by in RCA: 3115] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 9. | Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1859] [Cited by in RCA: 1960] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 10. | Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1321] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 11. | Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 760] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 12. | Kim DK, Oh SY, Kwon HC, Lee S, Kwon KA, Kim BG, Kim SG, Kim SH, Jang JS, Kim MC. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer. 2009;9:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein, and fibrinogen. Semin Arthritis Rheum. 1990;20:129-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Kwon KA, Kim SH, Oh SY, Lee S, Han JY, Kim KH, Goh RY, Choi HJ, Park KJ, Roh MS. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010;10:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Malle E, Sodin-Semrl S, Kovacevic A. Serum amyloid A: an acute-phase protein involved in tumour pathogenesis. Cell Mol Life Sci. 2009;66:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Lee HY, Kim MK, Park KS, Bae YH, Yun J, Park JI, Kwak JY, Bae YS. Serum amyloid A stimulates matrix-metalloproteinase-9 upregulation via formyl peptide receptor like-1-mediated signaling in human monocytic cells. Biochem Biophys Res Commun. 2005;330:989-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Chan DC, Chen CJ, Chu HC, Chang WK, Yu JC, Chen YJ, Wen LL, Huang SC, Ku CH, Liu YC. Evaluation of serum amyloid A as a biomarker for gastric cancer. Ann Surg Oncol. 2007;14:84-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Kiriakidis S, Andreakos E, Monaco C, Foxwell B, Feldmann M, Paleolog E. VEGF expression in human macrophages is NF-kappaB-dependent: studies using adenoviruses expressing the endogenous NF-kappaB inhibitor IkappaBalpha and a kinase-defective form of the IkappaB kinase 2. J Cell Sci. 2003;116:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 192] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 19. | Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2468] [Cited by in RCA: 2569] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 20. | Kwon HC, Kim SH, Oh SY, Lee S, Kwon KA, Lee JH, Choi HJ, Park KJ, Lee HS, Roh MS. Clinicopathological significance of nuclear factor-kappa B, HIF-1 alpha, and vascular endothelial growth factor expression in stage III colorectal cancer. Cancer Sci. 2010;101:1557-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S, Aoki T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Crumley AB, McMillan DC, McKernan M, Going JJ, Shearer CJ, Stuart RC. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer. 2006;94:1568-1571. [PubMed] |
| 23. | Yamada T. Serum amyloid A (SAA): a concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999;37:381-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 137] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 24. | Seo HY, Park JM, Park KH, Kim SJ, Oh SC, Kim BS, Kim YH, Kim JS. Prognostic significance of serum vascular endothelial growth factor per platelet count in unresectable advanced gastric cancer patients. Jpn J Clin Oncol. 2010;40:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Karayiannakis AJ, Syrigos KN, Polychronidis A, Zbar A, Kouraklis G, Simopoulos C, Karatzas G. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 138] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Zheng S, Han MY, Xiao ZX, Peng JP, Dong Q. Clinical significance of vascular endothelial growth factor expression and neovascularization in colorectal carcinoma. World J Gastroenterol. 2003;9:1227-1230. [PubMed] |