Published online Sep 14, 2012. doi: 10.3748/wjg.v18.i34.4736
Revised: April 12, 2012
Accepted: April 20, 2012
Published online: September 14, 2012
AIM: To delineate indications and limitations for "extended" radical cholecystectomy for gallbladder cancer: a procedure which was instituted in our department in 1982.
METHODS: Of 145 patients who underwent a radical resection for gallbladder cancer from 1982 through 2006, 52 (36%) had an extended radical cholecystectomy, which involved en bloc resection of the gallbladder, gallbladder fossa, extrahepatic bile duct, and the regional lymph nodes (first- and second-echelon node groups). A retrospective analysis of the 52 patients was conducted including at least 5 years of follow up. Residual tumor status was judged as no residual tumor (R0) or microscopic/macroscopic residual tumor (R1-2). Pathological findings were documented according to the American Joint Committee on Cancer Cancer Staging Manual (7th edition).
RESULTS: The primary tumor was classified as pathological T1 (pT1) in 3 patients, pT2 in 36, pT3 in 12, and pT4 in 1. Twenty-three patients had lymph node metastases; 11 had a single positive node, 4 had two positive nodes, and 8 had three or more positive nodes. None of the three patients with pT1 tumors had nodal disease, whereas 23 of 49 (47%) with pT2 or more advanced tumors had nodal disease. One patient died during the hospital stay for definitive resection, giving an in-hospital mortality rate of 2%. Overall survival (OS) after extended radical cholecystectomy was 65% at 5 years and 53% at 10 years in all 52 patients. OS differed according to the pT classification (P < 0.001) and the nodal status (P = 0.010). All of 3 patients with pT1 tumors and most (29 of 36) patients with pT2 tumors survived for more than 5 years. Of 12 patients with pT3 tumors, 8 who had an R1-2 resection, distant metastasis, or extensive extrahepatic organ involvement died soon after resection. Of the remaining four pT3 patients who had localized hepatic spread through the gallbladder fossa and underwent an R0 resection, 2 survived for more than 5 years and another survived for 4 years and 2 mo. The only patient with pT4 tumor died of disease soon after resection. Among 23 node-positive patients, 11 survived for more than 5 years, and of these, 10 had a modest degree of nodal disease (one or two positive nodes).
CONCLUSION: Extended radical cholecystectomy is indicated for pT2 tumors and some pT3 tumors with localized hepatic invasion, provided that the regional nodal disease is limited to a modest degree (up to two positive nodes). Extensive pT3 disease, pT4 disease, or marked nodal disease appears to be beyond the scope of this radical procedure.
- Citation: Shirai Y, Sakata J, Wakai T, Ohashi T, Hatakeyama K. "Extended" radical cholecystectomy for gallbladder cancer: Long-term outcomes, indications and limitations. World J Gastroenterol 2012; 18(34): 4736-4743
- URL: https://www.wjgnet.com/1007-9327/full/v18/i34/4736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i34.4736
The history of radical surgery for gallbladder cancer started with cholecystectomy combined with wedge resection of the gallbladder fossa (without regional lymphadenectomy) in the early 20th century[1,2]. However, outcomes after this procedure were disappointing even in tumors limited to the wall of the gallbladder, probably due to the lack of regional lymphadenectomy[1,2]. In 1954, Glenn et al[3] first proposed a radical resection procedure with intended regional lymphadenectomy (portal lymph node dissection), designated as “radical cholecystectomy” (Glenn operation), for localized gallbladder cancer. Pack et al[4] in 1955 and Fahim et al[5] in 1962 advocated radical resection consisting of hepatectomy and portal lymph node dissection, and this strategy remains the one recommended by the National Comprehensive Cancer Network (NCCN) for pathological T1b (pT1b) or more advanced gallbladder cancer[6]. Outcomes after such radical resections, however, remain unsatisfactory, particularly for node-positive patients[7-10].
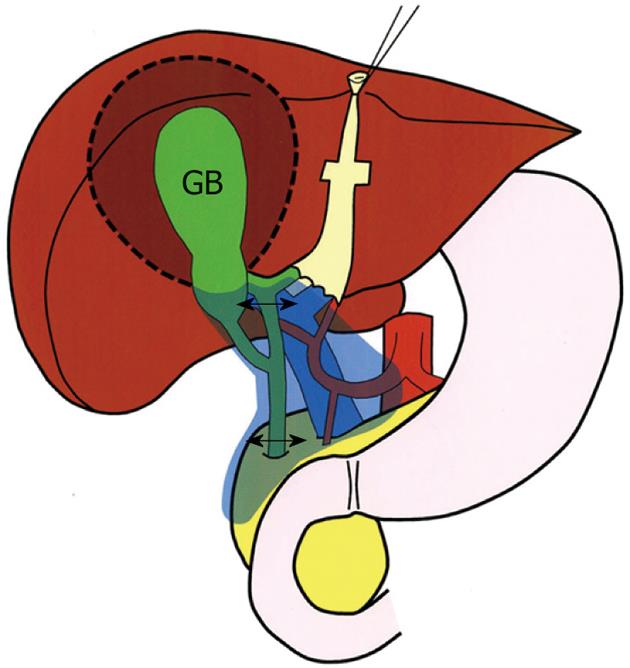
In Japan, an aggressive attitude toward gallbladder cancer emerged and gradually grew among specialized hepatobiliary surgeons in the 1970s and 1980s[11]. In this context, "extended" radical cholecystectomy (designated as the modified Glenn operation) was instituted in 1982 in our department (Figure 1)[11-13], as a modification of the radical cholecystectomy described by Glenn et al[3,14]. The procedure involves en bloc resection of the gallbladder, gallbladder fossa, extrahepatic bile duct, and the regional lymph nodes as defined previously[11,15]. Although this procedure has been widely used for gallbladder cancer among Japanese surgeons[11,16-18], indications and limitations remain to be established.
This study aimed to delineate indications and limitations for the extended radical cholecystectomy procedure for gallbladder cancer by retrospectively analyzing the long-term outcomes of 52 patients who underwent this procedure in the study department. The study goal was to definitively establish the role of this particular procedure in surgical management strategies for gallbladder cancer.
From May 1982 through December 2006, 145 consecutive patients underwent a variety of radical resection procedures for gallbladder cancer (including cancer arising in the cystic duct[19]) in the study department (Table 1). A radical resection was defined as an en bloc resection of both the primary tumor and the regional lymph nodes. This study period was selected in order to evaluate long-term outcomes after resection; the follow-up period was at least 5 years in all the patients. Of these, 52 (36%) who underwent an extended radical cholecystectomy (modified Glenn operation; Figure 1) were selected as our study cohort. They comprised 39 women and 13 men ranging in age from 43 to 84 years (median, 66.5 years).
Among the 52 patients, 34 underwent the extended radical cholecystectomy as an initial radical resection and 18 underwent this procedure as a radical second resection after a prior simple cholecystectomy for presumed benign disease. Although early (pT1) tumors do not warrant radical resection[20], three patients with pT1 tumor underwent the extended radical cholecystectomy because pT2 or more advanced disease was not ruled out before resection.
Extended radical cholecystectomy involves a cholecystectomy, wedge resection of the gallbladder fossa with a rim of non-neoplastic liver tissue (approximately 2 cm in thickness or more), resection of the suprapancreatic segment of the extrahepatic bile duct (bile duct resection), and regional lymph node dissection in an en bloc fashion (Figure 1). The main differences between this procedure and the original radical cholecystectomy described by Glenn et al[3,14] comprise the extent of regional lymphadenectomy and the presence or absence of bile duct resection.
The regional lymph nodes of the gallbladder include the cystic duct, pericholedochal, posterior superior (posterosuperior) pancreaticoduodenal, retroportal, right celiac, and hepatic artery node groups[11,15]. The cystic duct and pericholedochal node groups have been regarded as the first-echelon nodes of the gallbladder, whereas the other node groups as the second-echelon nodes[11,15]. Extended radical cholecystectomy harvests both the first- and second-echelon nodes en bloc (Figure 1).
We perform a bile duct resection to facilitate the regional lymphadenectomy, to remove pericholedochal lymphatic vessels and nodes simultaneously, to remove the possible presence of ductal (periductal) involvement[21], and to avoid the occurrence of ischemic biliary stricture after aggressive periductal nodal dissection[22].
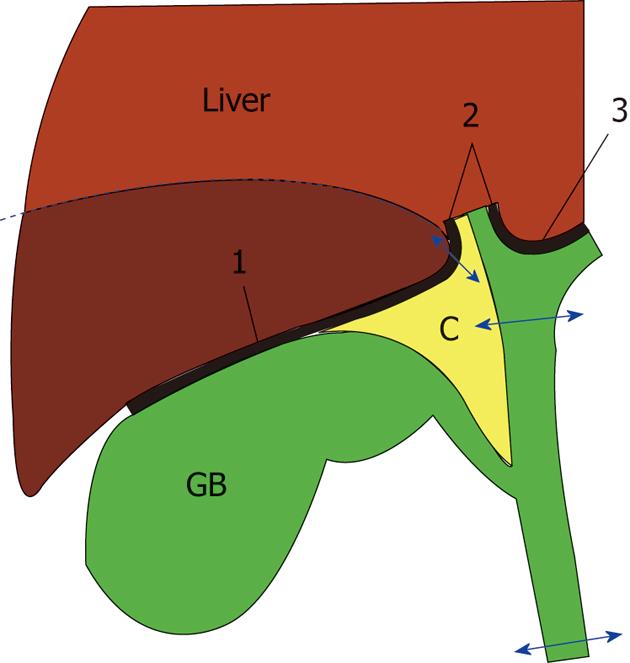
The extent of hepatectomy appears to be similar between the extended procedure and the original described by Glenn et al[3,14]. With regard to resection of the gallbladder fossa for invasive tumors, we emphasize the need for complete excision of the cystic plate to avoid violating the subserosal tumor plane (Figure 2).
Among the 52 patients who underwent an extended radical cholecystectomy, 19 with suspected (or confirmed) regional nodal disease also underwent a dissection of the paraaortic lymph nodes (cephalad to the origin of the inferior mesenteric artery). Paraaortic nodal disease was classified as N2 disease according to the American Joint Committee on Cancer (AJCC) Cancer Staging Manual (7th edition)[19].
Immediately after resection, the surgeon(s) retrieved lymph nodes from the node-bearing adipose tissues of the fresh surgical specimen. A total of 871 regional lymph nodes (excluding paraaortic nodes) were retrieved from the 52 patients (median 16, range 6 to 36 per patient). Resected specimens together with the retrieved lymph nodes were submitted for histological examination. Residual tumor status was judged as no residual tumor (R0) or microscopic/macroscopic residual tumor (R1-2). All pathological findings were documented according to the AJCC Cancer Staging Manual (7th edition)[19].
Adjuvant chemotherapy was administered to 23 patients at the discretion of the individual surgeon. No patients received adjuvant radiotherapy. Patients discharged to home were followed regularly in outpatient clinics every 1-6 mo for at least 5 years, with a median follow-up period of 188 mo (range: 82-351 mo).
To determine prognostic factors after extended radical cholecystectomy, the following 15 conventional variables were tested univariately and multivariately in 52 patients who underwent the procedure (Table 2): age, gender, gallstone (absent vs present), timing of radical resection (initial resection vs second resection), adjuvant chemotherapy (absent vs present), size of the primary tumor (≤ 60 mm vs > 60 mm), pT classification, pathological regional lymph nodes (pN) classification, pathological distant metastasis (pM) classification, histological type, histological grade, lymphatic vessel invasion, venous invasion, perineural invasion, and residual tumor status.
| Variable | n | Survival rate (%) | Univariate analysis | Multivariate analysis | ||
| 5-yr | 10-yr | P value | Relative risk (95% CI) | P value | ||
| Age (yr) | 0.002 | |||||
| ≤ 65 | 20 | 80 | 80 | |||
| > 65 | 32 | 50 | 35 | |||
| pT classification1 | < 0.001 | 0.001 | ||||
| pT1 plus pT2 | 39 | 82 | 70 | 1.000 | ||
| pT3 plus pT4 | 13 | 15 | 0 | 5.947 (2.054-17.221) | ||
| pN classification1 | 0.037 | |||||
| pN0 | 29 | 79 | 67 | |||
| pN1 | 12 | 58 | 33 | |||
| pN2 | 11 | 36 | 36 | |||
| pM classification1 | < 0.001 | 0.002 | ||||
| pM0 | 48 | 71 | 57 | 1.000 | ||
| pM1 | 4 | 0 | 0 | 20.068 (3.083-130.620) | ||
| Histological type1 | < 0.001 | 0.001 | ||||
| Adenocarcinoma | 48 | 71 | 57 | 1.000 | ||
| Others | 4 | 0 | 0 | 16.919 (3.015-94.939) | ||
| Histological grade1 | 0.021 | |||||
| G1 | 17 | 88 | 73 | |||
| G2 plus G3 plus G4 | 35 | 54 | 43 | |||
| Lymphatic vessel invasion (L)1 | 0.016 | |||||
| L0 | 20 | 85 | 73 | |||
| L1 | 32 | 53 | 40 | |||
| Venous invasion (V)1 | 0.004 | |||||
| V0 | 24 | 83 | 74 | |||
| V1 | 28 | 50 | 34 | |||
| Perineural invasion | 0.041 | 0.084 | ||||
| Absent | 36 | 72 | 62 | 1.000 | ||
| Present | 16 | 50 | 31 | 2.396 (0.889-6.460) | ||
| Residual tumor status1 | < 0.001 | 0.071 | ||||
| R0 | 47 | 72 | 58 | 1.000 | ||
| R1 plus R2 | 5 | 0 | 0 | 3.585 (0.897-14.329) | ||
Medical records and survival data were obtained for all 52 patients. Overall survival (OS) was defined as the interval between the date of definitive resection and the date of last follow up or death from any cause. The Kaplan-Meier method was used to estimate cumulative OS rates, and the log rank test was used to evaluate differences between groups. The Cox proportional hazards regression model using a step-backward fitting procedure was applied to identify independent prognostic factors. In this model, a step-wise selection was used for variable selection with entry and removal limits of P < 0.05 and P > 0.10, respectively.
The IBM SPSS Statistics 19 software (IBM Japan, Tokyo, Japan) was used for all statistical evaluations. All tests were two-tailed, and P < 0.05 was taken to indicate statistical significance.
The pT was classified as pT1 in 3 patients, pT2 in 36, pT3 in 12, and pT4 in 1. Twenty-three patients had lymph node metastases, with the number of positive nodes per patient ranging from 1 to 41 (median, 2); 11 patients had a single positive node, 4 had two positive nodes, and 8 had three or more positive nodes. None of the three patients with pT1 tumors had nodal disease, whereas 23 of 49 (47%) with pT2 or more advanced tumors had nodal disease. One patient who underwent a radical second resection died during the hospital stay for definitive resection, giving an in-hospital mortality rate of 2%.
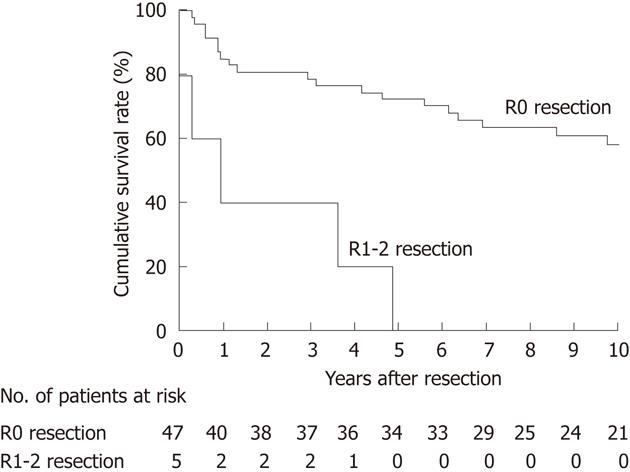
Of the 52 patients who underwent an extended radical cholecystectomy, 34 survived for more than 5 years and 18 died within 5 years. OS following extended radical cholecystectomy was 65% at 5 years and 53% at 10 years (median survival time, not reached) across the study cohort. OS was significantly worse in the 5 patients undergoing a noncurative (R1-2) resection than in the 47 patients undergoing a potentially curative (R0) resection (P < 0.001; Figure 3); the R1-2-resected patients died of recurrence within 5 years. This result indicated that R0 resection is a prerequisite for long-term survival after extended radical cholecystectomy.
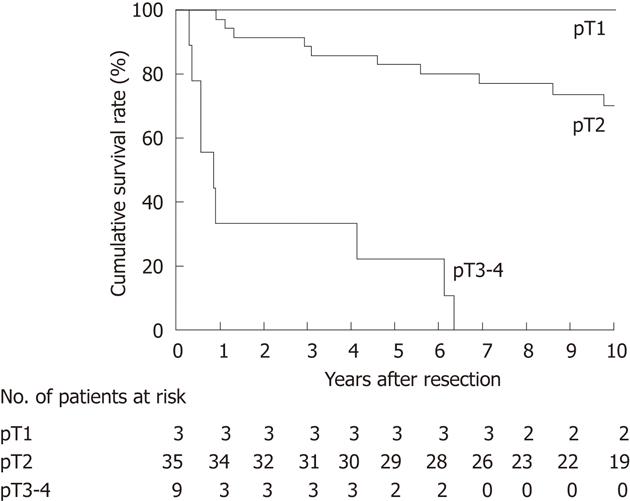
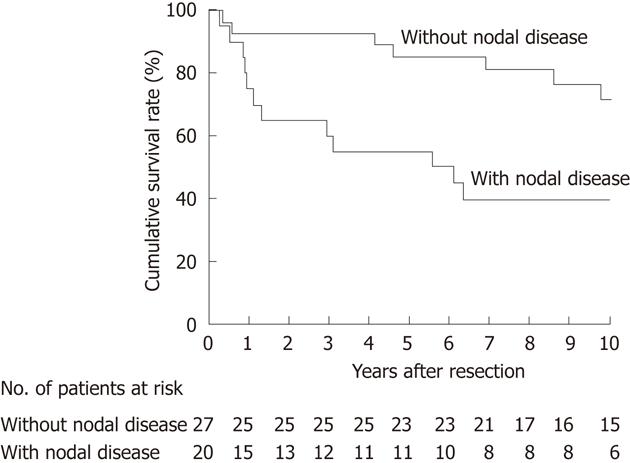
We then focused on the subgroup of 47 patients who had undergone an R0 resection for subsequent survival analyses. OS after R0 resection differed according to the pT classification (P < 0.001; Figure 4) and nodal status (P = 0.010; Figure 5). At the time of last follow-up, 11 patients with node-positive disease had survived for more than 5 years, suggesting that regional lymphadenectomy could achieve an acceptable rate of long-term survival for node-positive patients given an R0 resection (Figure 5).
All patients with pT1 tumor and most (29 of 36) patients with pT2 tumor survived for more than 5 years, while most (10 of 12) patients with pT3 tumor and the only patient with pT4 tumor died within 5 years (Table 3).
| Extent of tumor spread1 | No. of 5-yr survivors (n = 34) | No. of non-5-yr survivors (n = 18) |
| pT1 (n = 3) | ||
| pT1a pN0 pM0 (Stage I) | 2 | 0 |
| pT1b pN0 pM0 (Stage I) | 1 | 0 |
| pT2 (n = 36) | ||
| pT2 pN0 pM0 (Stage II) | 20 | 1 |
| pT2 pN1 pM0 (Stage IIIB) | 5 | 2 |
| pT2 pN2 pM0 (Stage IVB) | 4 | 3 |
| pT2 pN2 pM1 (Stage IVB) | 0 | 1 |
| pT3 (n = 12) | ||
| pT3 pN0 pM0 (Stage IIIA) | 0 | 3 |
| pT3 pN1 pM0 (Stage IIIB) | 2 | 3 |
| pT3 pN2 pM0 (Stage IVB) | 0 | 1 |
| pT3 pN0 pM1 (Stage IVB) | 0 | 2 |
| pT3 pN2 pM1 (Stage IVB) | 0 | 1 |
| pT4 (n = 1) | ||
| pT4 pN2 pM0 (Stage IVB) | 0 | 1 |
Of the 12 patients with pT3 tumors, 3 had an R1-2 resection, 2 had distant metastasis (pM1) in the liver, 2 had extensive extrahepatic organ involvement, and 1 had both an R1-2 resection and pM1 in the liver. All of these patients died within 5 years. Of the remaining four pT3 patients who had localized hepatic spread through the gallbladder fossa and underwent an R0 resection, 2 survived for more than 5 years, 1 survived for 4 years and 2 mo, and another succumbed to the cancer 4 mo after resection. Taken together, the above observations indicated that extended radical cholecystectomy was effective for most T1-2 lesions and some pT3 lesions with localized hepatic spread.
Among the eleven 5-year survivors with node-positive disease (Table 4), the number of positive lymph nodes was limited to one or two in 10 patients (patient No. 11 underwent an aggressive paraaortic lymphadenectomy for a total of nine positive paraaortic nodes), suggesting that extended radical cholecystectomy was effective in patients with up to a modest degree of regional nodal disease (up to two positive nodes).
| Patient No. | Age (yr) | Sex | pT | Lymph node status | |
| Location of positive nodes | No. of positive nodes | ||||
| 1 | 58 | M | 2 | Hepatic artery | 1 |
| 2 | 67 | F | 2 | Cystic duct | 1 |
| 3 | 68 | F | 2 | Cystic duct | 1 |
| 4 | 77 | M | 3 | Cystic duct | 1 |
| 5 | 75 | F | 3 | Pericholedochal | 1 |
| 6 | 62 | F | 2 | Cystic duct | 1 |
| 7 | 63 | F | 2 | Cystic duct and posterosuperior pancreaticoduodenal | 2 |
| 8 | 60 | F | 2 | Cystic duct | 2 |
| 9 | 67 | F | 2 | Retroportal | 2 |
| 10 | 55 | F | 2 | Cystic duct and hepatic artery | 2 |
| 11 | 57 | F | 2 | Paraaortic | 91 |
Among the 15 conventional variables, age, pT classification, pN classification, pM classification, histological type, histological grade, lymphatic vessel invasion, venous invasion, perineural invasion, and residual tumor status were identified as significant prognostic factors on univariate analyses (Table 2). Timing of radical resection (initial resection vs second resection) did not affect OS rates after resection (P = 0.404).
The 10 univariately significant variables were then entered into multivariate analyses, with pT classification, pM classification, and histological type identified as independently significant variables (Table 2).
A standardized resection procedure has not been established for gallbladder cancer due to the diverse modes of spread possible with this disease[5,23]. Thus, the scope of radical resection when treating gallbladder cancer should be tailored based on the extent of spread in individual patients. Here, we analyzed 52 patients undergoing extended radical cholecystectomy and found it to be effective for pT1-2 tumors and some pT3 tumors with localized hepatic invasion, provided that the regional nodal disease was absent or only present to a modest degree (one or two positive lymph nodes).
In 1954, Glenn et al[3] first proposed a radical surgical procedure, designated as “radical cholecystectomy”, where the gallbladder fossa and the node-bearing tissues within the hepatoduodenal ligament (portal lymph node dissection) were excised en bloc at the time of cholecystectomy. Extended radical cholecystectomy in our department (Figure 1) differs from the original Glenn and Hays procedure in the extent of lymphadenectomy and the presence or absence of bile duct resection. The extent of lymphadenectomy in the former procedure includes both the first- and the second-echelon node groups[11,15], but that in the original procedure was more limited[3,6,14]. The difference in the extent of lymphadenectomy may explain, in part, the acceptable rate of long-term survival (55%) achieved in our node-positive patients (Figure 5). Bile duct resection provides better local control by removing the possible presence of ductal (periductal) spread[21] as well as better clearance of nodal disease (especially periductal nodal disease). Thus, we believe that extended radical cholecystectomy confers oncological advantages over the original radical procedure.
The main modes of hepatic spread from resectable gallbladder cancer involve both direct invasion and portal tract invasion (lesions within the portal tracts of adjacent liver), the latter of which features intrahepatic lymphatic invasion[5,23-25]. We previously reported that the portal tract invasion is seen only in the vicinity (within about 1 cm) of the advancing margin of direct liver invasion[24,25]. Thus, a hepatectomy margin of approximately 2 cm or more in our extended radical cholecystectomy appears to be sufficient. The fact that local recurrence in hepatectomy margins did not occur in any of our patients (data not shown) also supports the validity of our 2 cm hepatectomy margin. When performing a wedge hepatectomy for invasive tumor, the entire cystic plate should be resected (Figure 2) because incomplete excision of the cystic plate violates the subserosal plane of the gallbladder and thus may leave behind tumor cells in this plane. Also, complete excision of the cystic plate facilitates removal of the adipose tissue within the triangle of Calot, which usually contains cystic duct node(s).
The current study showed that most patients with pT2 gallbladder cancer who underwent an extended radical cholecystectomy survived for more than 5 years (Figure 4), suggesting that pT2 tumors are the best candidates for this procedure. Regarding pT3-4 tumors, we cannot draw a definitive conclusion due to the small number of patients analyzed in this study. However, the fact that three of the four pT3 patients with localized invasion of the liver through the gallbladder fossa survived for more than 4 years suggests that such pT3 patients may benefit from extended radical cholecystectomy, provided that they have no distant metastases and undergo an R0 resection. Our results also suggest that a considerable proportion of node-positive patients may benefit from this procedure (Figure 5). By analyzing 5-year survivors with nodal disease (Table 4), we realized that this procedure might clear only a modest degree of nodal disease (one or two positive nodes in our cohort). Taken together, the above observations indicate that the extended radical cholecystectomy undertaken in our department provides survival benefit for most patients with pT2 tumors and some patients with pT3 tumors with localized hepatic invasion, provided that the nodal disease is limited to a modest degree and an R0 resection is feasible.
In 2007, Yokomizo et al[26] argued that hepatectomy and/or bile duct resection could be withheld in radical surgery for pT2 gallbladder cancer provided that negative resection margins are achieved. The current NCCN guidelines[6] recommend hepatectomy and lymphadenectomy for pT1b or greater tumors, whereas bile duct resection is suggested as optional. We previously reported that a considerable proportion (40%) of pT2 patients could survive for more than 5 years after cholecystectomy alone if the resection margins are negative[12]. The above observations indicate that bile duct resection, hepatectomy, and even lymphadenectomy could be omitted for some pT2 patients. Indeed, some of our pT2 patients with advanced age or comorbid disease(s) had a less aggressive resection by omitting bile duct resection and/or hepatectomy (Table 1). However, considering the excellent outcomes after extended radical cholecystectomy for pT2 tumors (Figure 4), we now usually recommend application of the extended radical cholecystectomy to robust patients with pT2 gallbladder cancer.
The authors’ policy on radical surgery for gallbladder cancer is to select a resection procedure for each patient based on the extent of tumor spread in the patient. Although extended radical cholecystectomy has been considered the standard procedure for fairly localized tumors in our department, only one-third (52 of 145) of our patients underwent this procedure (Table 1). The current study implies that extensive pT3 disease, pT4 disease, or marked nodal disease is beyond the scope of extended radical cholecystectomy. Such tumors may require more extensive resections (major hepatectomy, pancreaticoduodenectomy, etc.) for clearance of the locoregional disease[11]. Adequate selection of a resection procedure in individual patients is mandatory to improve survival for gallbladder cancer.
The main limitations of the current study include the retrospective nature of the analysis and the small number of patients spanning a long period of time. We believe, however, that these limitations did not greatly affect the results of the study as the differences between the groups were too marked to have resulted from bias. In addition, the role of extended radical cholecystectomy in surgical management strategy for gallbladder cancer is now more clearly defined than previously[11,12,16]. Our results thus provide useful information for selecting an adequate resectional procedure for individual patients with gallbladder cancer.
In conclusion, extended radical cholecystectomy is indicated for gallbladder cancer patients with pT2 tumors and for some with pT3 tumors with localized hepatic invasion, provided that the regional nodal disease is limited to a modest degree (up to two positive nodes). Extensive pT3 disease, pT4 disease, or marked nodal disease appears to be beyond the scope of this procedure and thus may require more extensive resection. This study confirms that extended radical cholecystectomy plays a key role in the surgical management of invasive gallbladder cancer if the spread is moderate.
In 1954, Glenn and Hays first proposed “radical cholecystectomy”, which has been used worldwide for localized gallbladder cancer. “Extended” radical cholecystectomy (designated as the modified Glenn operation) was instituted in the study department in 1982. Although the authors’ procedure has been widely used among Japanese surgeons, indications for this particular procedure have not yet been established.
As a standardized resection procedure has not been defined for gallbladder cancer, the scope of radical resection should be tailored based on the extent of spread in individual patients. However, adequate selection of a resection procedure in individual patients remains yet to be established.
The current study clearly defines the role of extended radical cholecystectomy in surgical management strategy for gallbladder cancer. Briefly, extended radical cholecystectomy is indicated for pathological T2 (pT2) tumors and for some pT3 tumors with localized hepatic invasion, provided that the nodal disease is limited to a modest degree (up to two positive nodes). Extensive pT3 disease, pT4 disease, or marked nodal disease appears to be beyond the scope of this procedure and thus may require more extensive resection.
The current study provides practical information for selecting an adequate resectional procedure for individual patients with gallbladder cancer.
Radical cholecystectomy (Glenn operation): en bloc excision of the gallbladder, gallbladder fossa, and the node-bearing tissues within the hepatoduodenal ligament; “Extended” radical cholecystectomy (modified Glenn operation): en bloc excision of the gallbladder, gallbladder fossa, extrahepatic bile duct, and the regional lymph nodes (first- and second-echelon node groups).
Overall, this paper is well written, concise and informative. “Extended” radical cholecystectomy involves more extensive regional lymphadenectomy compared with the “original” radical cholecystectomy proposed by Glenn and Hays. This has led to achievement of good long-term survival in node-positive patients.
Peer reviewers: Abdul-Wahed Meshikhes, MD, Department of Surgery, King Fahad Specialist Hospital, Amir Bin Thabit St, Dammam 31444, Eastern Province, Saudi Arabia; Pankaj Garg, Dr., Consultant, Department of General Surgery, Fortis Super Speciality Hospital, Mohali, Punjab, Panchkula 134112, India
S- Editor Lv S L- Editor Logan S E- Editor Zheng XM
.
| 1. | Sheinfeld W. Cholecystectomy and partial hepatectomy for carcinoma of the gall bladder with local liver extension. Surgery. 1947;22:48-58. [PubMed] |
| 2. | Glenn F, Hays DM. Carcinoma of the extrahepatic biliary tract. Surg Clin North Am. 1953;479-492. [PubMed] |
| 3. | Glenn F, Hays DM. The scope of radical surgery in the treatment of malignant tumors of the extrahepatic biliary tract. Surg Gynecol Obstet. 1954;99:529-541. [PubMed] |
| 4. | Pack GT, Miller TR, Brasfield RD. Total right hepatic lobectomy for cancer of the gallbladder; report of three cases. Ann Surg. 1955;142:6-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Fahim RB, McDonald JR, Richards JC, Ferris DO. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg. 1962;156:114-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 221] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 6. | Benson AB, Abrams TA, Ben-Josef E, Bloomston PM, Botha JF, Clary BM, Covey A, Curley SA, D'Angelica MI, Davila R. NCCN clinical practice guidelines in oncology: hepatobiliary cancers. J Natl Compr Canc Netw. 2009;7:350-391. [PubMed] |
| 7. | Bartlett DL, Fong Y, Fortner JG, Brennan MF, Blumgart LH. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann Surg. 1996;224:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Benoist S, Panis Y, Fagniez PL. Long-term results after curative resection for carcinoma of the gallbladder. French University Association for Surgical Research. Am J Surg. 1998;175:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Donohue JH, Nagorney DM, Grant CS, Tsushima K, Ilstrup DM, Adson MA. Carcinoma of the gallbladder. Does radical resection improve outcome? Arch Surg. 1990;125:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 169] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Frena A, La Guardia G, Martin F. Outcome of radical surgery for carcinoma of the gallbladder according to the tumor node metastasis and Japanese Society of Biliary Surgery stages. J Gastrointest Surg. 2004;8:580-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Shirai Y, Wakai T, Hatakeyama K. Radical lymph node dissection for gallbladder cancer: indications and limitations. Surg Oncol Clin N Am. 2007;16:221-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Shirai Y, Yoshida K, Tsukada K, Muto T. Inapparent carcinoma of the gallbladder. An appraisal of a radical second operation after simple cholecystectomy. Ann Surg. 1992;215:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 202] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 13. | Shirai Y, Yoshida K, Tsukada K, Muto T, Watanabe H. Radical surgery for gallbladder carcinoma. Long-term results. Ann Surg. 1992;216:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 140] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Glenn F. Radical cholecystectomy for carcinoma of the gallbladder. New York: The Macmillan Company 1963; 86-88. |
| 15. | Shirai Y, Yoshida K, Tsukada K, Ohtani T, Muto T. Identification of the regional lymphatic system of the gallbladder by vital staining. Br J Surg. 1992;79:659-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 16. | Shimada H, Endo I, Togo S, Nakano A, Izumi T, Nakagawara G. The role of lymph node dissection in the treatment of gallbladder carcinoma. Cancer. 1997;79:892-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Chijiiwa K, Nakano K, Ueda J, Noshiro H, Nagai E, Yamaguchi K, Tanaka M. Surgical treatment of patients with T2 gallbladder carcinoma invading the subserosal layer. J Am Coll Surg. 2001;192:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Yamaguchi K, Chijiiwa K, Saiki S, Nishihara K, Takashima M, Kawakami K, Tanaka M. Retrospective analysis of 70 operations for gallbladder carcinoma. Br J Surg. 1997;84:200-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York: Springer 2010; . |
| 20. | Wakai T, Shirai Y, Yokoyama N, Nagakura S, Watanabe H, Hatakeyama K. Early gallbladder carcinoma does not warrant radical resection. Br J Surg. 2001;88:675-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 21. | Shimizu Y, Ohtsuka M, Ito H, Kimura F, Shimizu H, Togawa A, Yoshidome H, Kato A, Miyazaki M. Should the extrahepatic bile duct be resected for locally advanced gallbladder cancer? Surgery. 2004;136:1012-107; discussion 1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Ishizuka D, Shirai Y, Hatakeyama K. Ischemic biliary stricture due to lymph node dissection in the hepatoduodenal ligament. Hepatogastroenterology. 1998;45:2048-2050. [PubMed] |
| 23. | Cooper WA. Carcinoma of the gallbladder. Arch Surg. 1937;35:431-448. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Shirai Y, Tsukada K, Ohtani T, Watanabe H, Hatakeyama K. Hepatic metastases from carcinoma of the gallbladder. Cancer. 1995;75:2063-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Wakai T, Shirai Y, Sakata J, Nagahashi M, Ajioka Y, Hatakeyama K. Mode of hepatic spread from gallbladder carcinoma: an immunohistochemical analysis of 42 hepatectomized specimens. Am J Surg Pathol. 2010;34:65-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Yokomizo H, Yamane T, Hirata T, Hifumi M, Kawaguchi T, Fukuda S. Surgical treatment of pT2 gallbladder carcinoma: a reevaluation of the therapeutic effect of hepatectomy and extrahepatic bile duct resection based on the long-term outcome. Ann Surg Oncol. 2007;14:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |