Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4610
Revised: February 29, 2012
Accepted: June 8, 2012
Published online: September 7, 2012
AIM: To investigate whether the side population (SP) cells possess cancer stem cell-like characteristics in vitro and the role of SP cells in tumorigenic process in gastric cancer.
METHODS: We analyzed the presence of SP cells in different human gastric carcinoma cell lines, and then isolated and identified the SP cells from the KATO III human gastric cancer cell line by flow cytometry. The clonogenic ability and self-renewal were evaluated by clone and sphere formation assays. The related genes were determined by reverse transcription polymerase chain reaction. To compare tumorigenic ability, SP and non-side population (NSP) cells from the KATO III human gastric cancer cell line were subcutaneously injected into nude mice.
RESULTS: SP cells from the total population accounted for 0.57% in KATO III, 1.04% in Hs-746T, and 0.02% in AGS (CRL-1739). SP cells could grow clonally and have self-renewal capability in conditioned media. The expression of ABCG2, MDRI, Bmi-1 and Oct-4 was different between SP and NSP cells. However, there was no apparent difference between SP and NSP cells when they were injected into nude mice.
CONCLUSION: SP cells have some cancer stem cell-like characteristics in vitro and can be used for studying the tumorigenic process in gastric cancer.
- Citation: She JJ, Zhang PG, Wang X, Che XM, Wang ZM. Side population cells isolated from KATO III human gastric cancer cell line have cancer stem cell-like characteristics. World J Gastroenterol 2012; 18(33): 4610-4617
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4610.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4610
Cancer stem cells (CSC) have the characteristics of longevity, self-renewal and proliferation. It is believed that cancer stem cells are responsible for the heterogeneity and relapse of certain cancers. Flow cytometric analysis of cell surface antigens has served as the main tool to characterize CSC. CSC populations have already been identified in several solid tumors, including breast cancer[1], brain tumor[2], prostate cancer[3], melanoma[4], retinoblastoma[5], lung cancer[6], and colon cancer[7]. CSC often expresses the multi-drug resistant 1 (MDR1) or adenosine-triphosphate (ATP)-binding cassette (ABC) transporter genes, which help promote chemoresistance, a phenotype of side population (SP) cells[8].
SP cells can efflux the DNA-binding dye Hoechst33342 through an ABC membrane transporter. SP cells were initially investigated as part of the hematopoietic stem cells, and harbored stem cell-like characteristics[9]. Although conflicting results have been recently reported[10,11], most studies that investigated cell lines and tumors such as Cal-51[12], human breast carcinoma (MCF7)[13], human glioma (U373)[14], mouse ovarian carcinoma (MOVCAR 7)[15], human nasopharyngeal carcinoma (CNE-2)[16], Huh7 and PLC/PRF/5 hepatocellular carcinoma[15,16], 4T1 and NXS2 murine carcinoma[17] showed that these cell lines or tissues possessed SP cells, which were described to have stem cell-like fractions. Most studies suggested that SP cell analysis can be used to identify cancer stem cell populations[18].
Gastric cancer is the second most common cancer in the world. A total of 21 000 new cases of gastric cancer were diagnosed in the United States in 2010 with a projected five-year mortality rate exceeding 65%[19]. Aggressive surgery followed by chemotherapy resulted in a clinical response of 20%-35%. However, a majority of patients relapse and become drug-resistant. Various types of ABC transporters, especially ABC transporter genes 2 (ABCG2), contribute to drug resistance in cancers including gastric cancer, by pumping chemotherapeutic drugs out of cancer cells[20]. Therefore, SP cells could be used as a therapeutic target and for preventing relapse and drug-resistance in gastric cancer as well.
In this study, we analyzed the percentage of SP and non-side population (NSP) cells from several gastric cancer cell lines. By doing so, we sorted SP and NSP cells separately from the KATO III cell lines and estimated whether the SP cell fraction possessed cancer stem cell-like characteristics in vitro.
The human gastric cancer cell lines including KATO III (ATCC NO: HTB-103), Hs-746T (HTB-135) and AGS (CRL-1739) were procured from American Type Culture Collection. KATO III and Hs-746T were grown in DMEM supplemented with 100 mL/L fetal bovine serum (FBS) and penicillin/streptomycin at 37 °C in a humidified atmosphere with 50 mL/L CO2. AGS was grown in RPMI-1640 supplemented with 100 mL/L FBS and penicillin/streptomycin at 37 °C in a humidified atmosphere with 50 mL/L CO2. To analyze SP and NSP cell fractions, the cells were removed from their dishes with 2.5 g/L trypsin and 0.5 g/L ethylenediaminetetraacetic acid, centrifuged, washed with PBS and resuspended at 37 °C in Hank’s balanced salt solution (HBSS) containing 20ml/L FBS. Cells (1 × 106) were labeled in HBSS with 5.0 mg/L Hoechst33342 dye (Sigma, St. Louis, MO) either alone or in combination with 50 mol/L verapamil (Sigma, St. Louis, MO) at 37 °C for 90 min. After washing three times with PBS, the cells were resuspended in HBSS containing 20 mL/L FBS and 1mmol/L 4-(2-hydroxyethyl)-1-piperazine ethanesulphonic acid (HEPES), passed through a 40-μm mesh filter, then maintained at 4 °C until flow cytometric analysis. Then, 1 × 106 viable cells were analyzed and sorted using a FACS Vantage SE Cell Sorter (BD Biosciences, CA). The Hoechst dye was excited with a UV laser and its fluorescence was measured with both 675/20 (Hoechst Red) and 424/44 filters (Hoechst Blue). The analysis was repeated three times.
Following melting 10 g/L agar (DNA grade) in the microwave and warming 2 × Dulbecco’s modified eagle medium (DMEM) supplemented with 200 mL/L FBS to 40 °C in a water bath, equal volumes of the two solutions were mixed to yield a new solution of 5 g/L agar, 1 × DMEM and 100 mL/L FBS. Next, 1.0 mL of mixed solution was added to each well of a 6-well plate to form the base agar. Then, 7 g/L agar (DNA grade) was melted in the microwave and cooled to 40 °C in a water bath; similarly, 2 × DMEM and 200 mL/L FBS were warmed to the same temperature. Freshly sorted SP and NSP cells from KATO III were passed through a 40-μm filter to provide a single cell suspension and were counted. Three mL DMEM, 1.5 mL 2 × DMEM containing 200 mL/L FBS and 1.5 mL agar including 400 cells were then mixed together, and a 1.5 mL cell suspension of this solution was placed into each well of a 6-well plate as the top agar. Finally, 100 cells were seeded into each well, and were incubated at 37 °C in a humidified incubator for 2-3 wk. Colonies were either left unstained, or were stained with 5 g/L MTT (Sigma, St. Louis, MO) for no more than one hour, and counted under a dissecting microscope. The procedure was repeated three times. After SP cells were seeded in soft agar assays, colonies containing more than 50 cells (primary colony) were removed from soft agar with sterile Pasteur pipettes, treated with trypsin and mechanically dissociated into single cells. Then, 100 cells were seeded into each well at 37 °C in a humidified incubator for another 2-3 wk. Colonies (secondary colony) were stained with 5 g/L MTT for no more than one hour, and counted under a dissecting microscope. The procedure was repeated three times.
Plasmid DNA was kept at a 3:3:1 ratio [Vector (EGFP): ∆8.91: VSV-G] according to the lipofectamine protocol (Invitrogen). Next, 2.5 μg plamid DNA was diluted in 500 μL serum-free DMEM for each well, and 2.5 μL Mix PLUS reagent and 6.25 μL Lipofectamine™ LTX reagent were added to the diluted DNA and incubated for 30 min at room temperature. Then, approximately 500 μL of the DNA-Lipofectamine™ LTX complex was added to each well containing 293FT cells, which were 40%-50% confluent. The cells were incubated at 37 °C in a CO2 incubator for six hours and the medium was changed for transfected cells. After 24 h, the media from 293FT cells was collected, and 8 mg/L polybrene was added and transferred to target cells. The transfer of media from 293 FT to KATO III cells was repeated after 24 h and 48 h.
To test sphere formation in suspension, sorted SP and NSP cells from KATO III were passed through a 40-μm filter to provide a single cell suspension. Next, 4 mL medium containing 100 cells were added to the 6-well plates with serum-free media including F12 with EGF (10 μg/L), insulin (20 mg/L) and basic fibroblast growth factor (bFGF) (10 μg/L). Aliquots of epidermal growth factor (EGF), insulin and bFGF were added twice a week. After 10-14 d, plates were visually assayed for the formation of floating spheres. To assess the ability of primary spheres to form secondary spheres, colonies containing more than 30 cells were collected by centrifugation and trypsinized to yield single cells. After passing through a 40-μm filter, 4 mL serum-free media containing 100 cells were added to each 6-well plate and were cultured 10-14 d for secondary sphere formation. Spheres were counted under a dissecting microscope.
1 × 105 SP and NSP cells from the KATO III total population were collected in a separate centrifuge tube with 350 μL RLT buffer containing 10 g/L 2-mercaptoethanol, total RNA was extracted from these cells using a RNeasy Mini Kit (Qiagen, CA) according to the protocol provided by the manufacturer. RNA was transcribed into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen, CA). RT-PCR was performed using a SuperScript One-Step kit (Invitrogen, CA). The primers were as follows: Glyceraldehyde-3-phosphate dehydrogenase, 5-CTG CAC CAC CAA CTG CTT AG-3 and 5-AGG TCC ACC ACT GAC ACG TT-3; ABCG2, 5-GGG TTC TCT TCT TCC TGA CGA CC-3 and 5-TGG TTG TGA GAT TGA CCA ACA GAC C-3; MDR1, 5-GCC TGG CAG CTG GAA GAC AAA TAC-3 and 5-ATG GCC AAA ATC ACA AGG GTT AGC-3; B-cell-specific Moloney murine leukemia virus insertion site 1 (Bmi-1), 5-AGC AGA AAT GCA TCG AAC AA-3 and 5-CCT AAC CAG ATG AAG TTG CTG A-3; Octamer-binding transcription factor 4 (Oct-4), 5-GAG AAT TTG TTC CTG CAG TGC-3 and 5-GTT CCC AAT TCC TTC CTT AGT G-3. The PCR products were separated by electrophoresis on a 20 g/L agarose gel.
Sorted SP and NSP cells from KATO III were resuspended in 50 μL HBSS, ranging in density from 104 cells to 103 cells, and then mixed with 50 μL Matrigel (Becton Dickinson, NJ) to prevent injected cell dispersion and loss. Then, cells were subcutaneously injected into 6- or 7-wk old nude mice on the day of sorting. Nude mice were obtained from the Animal Institute of the Xi’an Jiaotong University, China (XJTU). All experiments were approved by the Animal Care Committee of XJTU. Mice were monitored every day to assess tumor formation for 6-8 wk after transplantation.
All values were expressed as mean ± SD. Any significant difference among mean values was further evaluated by the Student’s t test.
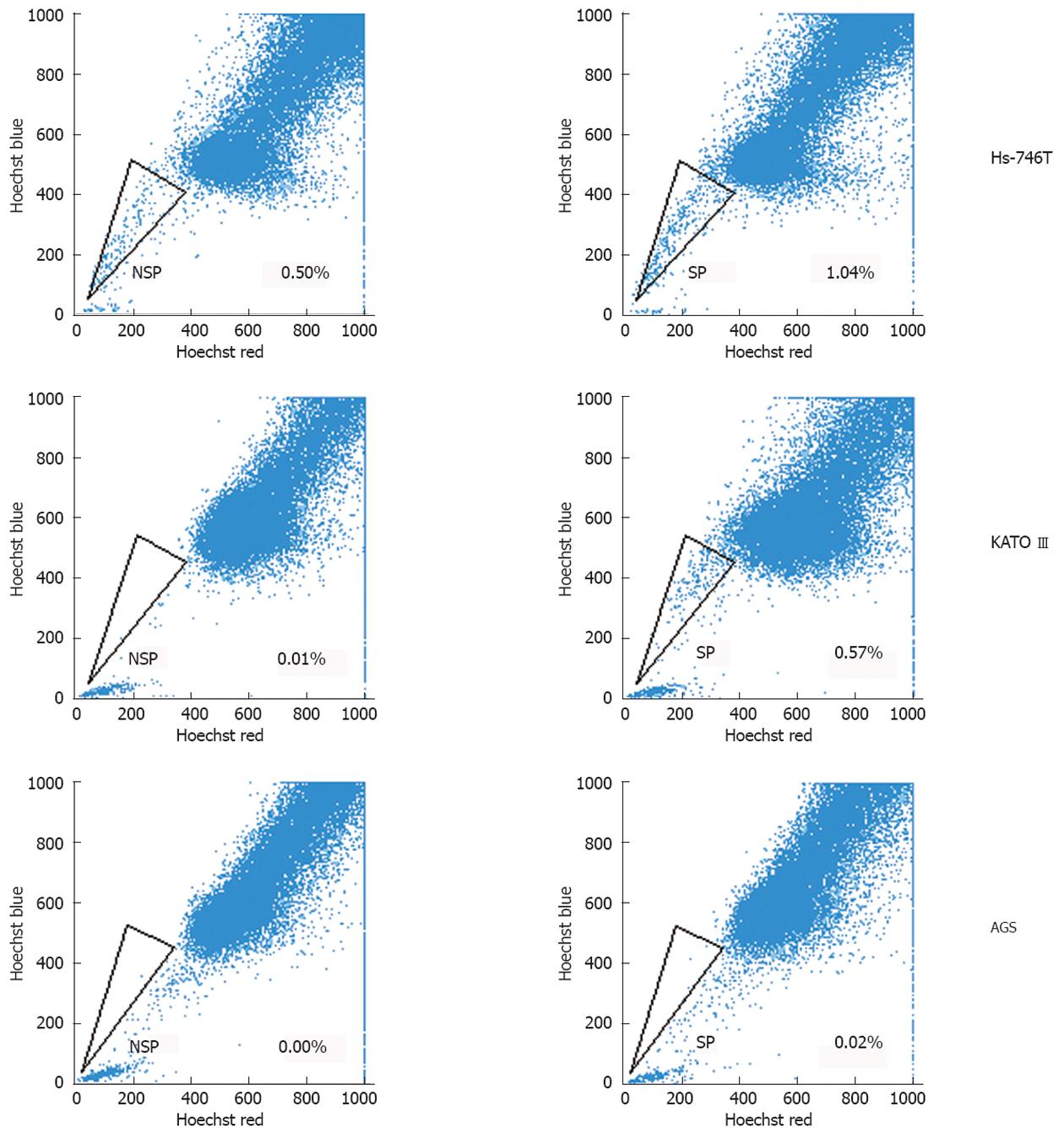
SP was identified through their fluorescence profile in a dual-wavelength analysis by flow cytometry (Hoechst red 675/20; Hoechst blue 424/44), and were shown as a characteristic tail separated from the complete population.
The percentage of SP cells in human gastric cancer cell lines was 0.57% in KATO III, 1.04% in Hs-746T, and 0.02% in AGS (Figure 1). SP cells decreased in number following treatment with verapamil, an inhibitor of the ABC transporter. To further investigate the function of SP and NSP cells in these gastric cancer cell lines, in vitro and in vivo tumorigenicity was studied mainly among KATO III cells.
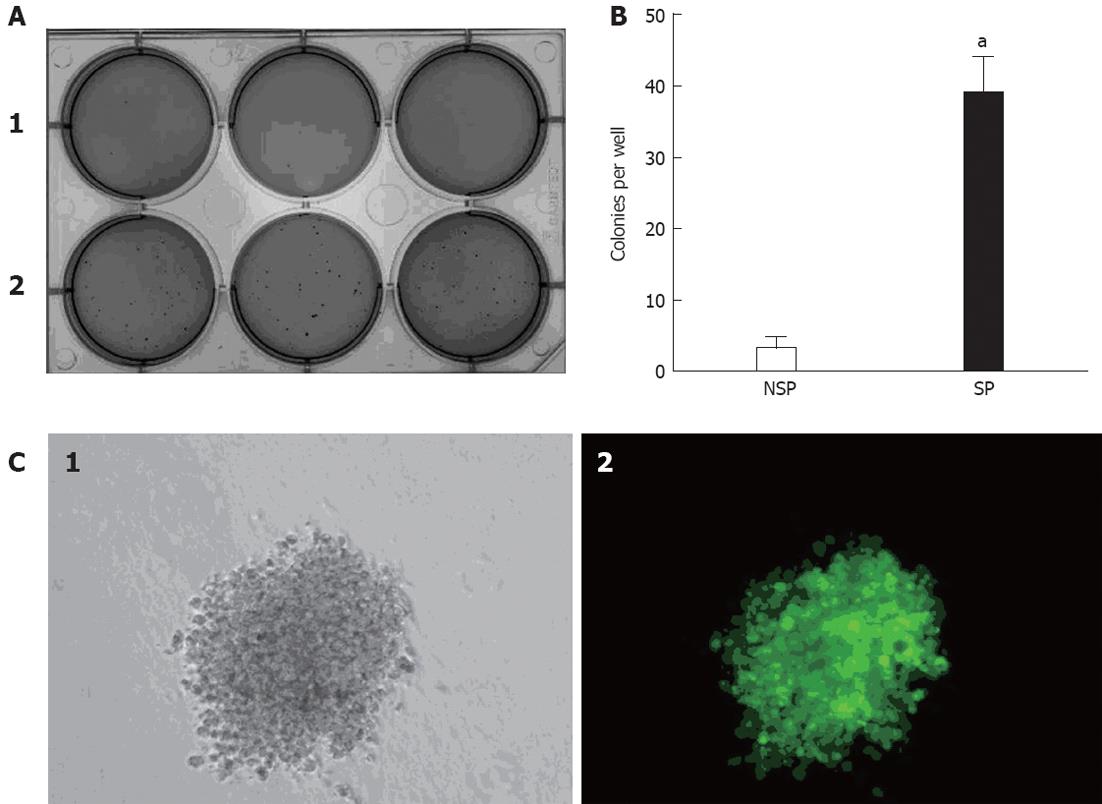
The clonogenic ability of SP and NSP cells were examined when seeded as single cells. The clonogenic efficiency of SP cells was significantly higher than NSP cells in soft agar assays (Figure 2A and B). Moreover, most SP cells could divide into colonies of more than 50 cells in soft agar after three weeks. There was no apparent difference between NSP cells and the complete population. The self-renewal ability of SP cells was also examined in serial soft agar assays. There was an increase in clonogenic efficiency from primary to secondary colonies. Moreover, secondary colonies were similar in size to the primary colonies, thus demonstrating that SP cells can maintain and expand themselves in serial soft agar assays. To ascertain whether the colony comes from a single cell, KATO III was infected with enhanced green fluorescent protein-expressing (EGFP) lentivirus and equal numbers of KATO III-GFP and KATO III cells were seeded from primary to second passage into serial soft agar assays. It was revealed that KATO III-GFP cells expressed strong green fluorescence and showed a similar growth rate compared to non-infected KATO III. Moreover, there were no mixed colonies (Figure 2C), indicating that the colonies came from single cells.
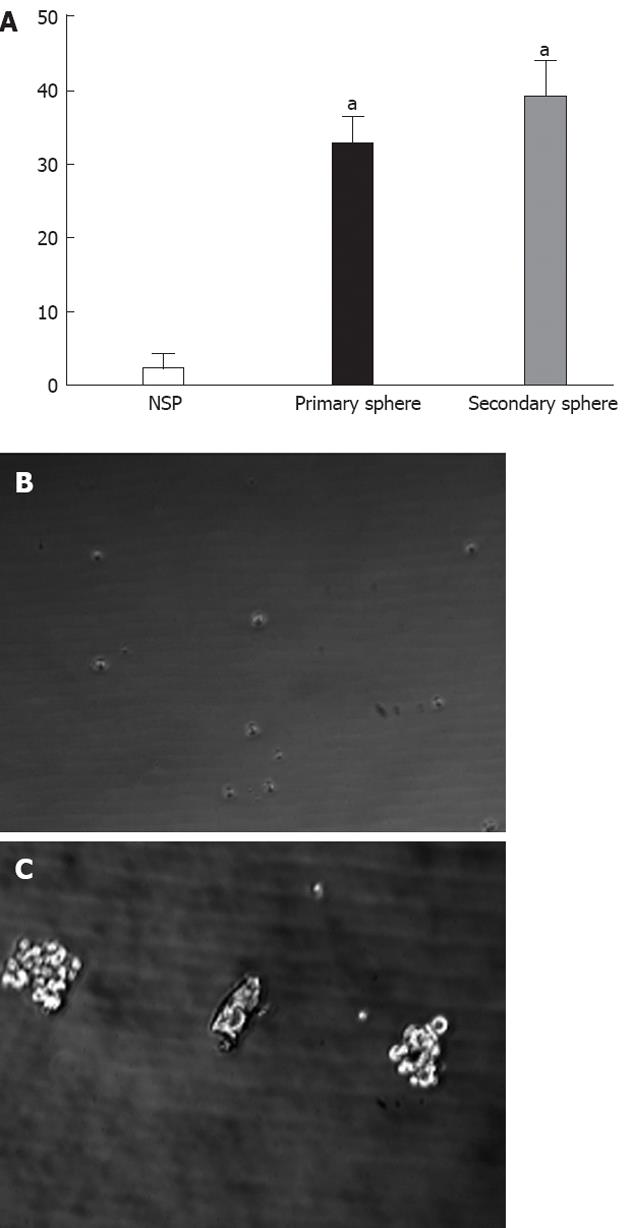
The ability of SP and NSP cells to generate spherical clones and self-renewal was evaluated by sphere formation assays (Figure 3A). It was difficult to observe floating spheres from NSP cells (Figure 3B). SP cells started to form floating spheres after 2-3 d of seeding, and became primary spheres of more than 30 cells after 10-14 d (Figure 3C). The secondary sphere occurred slightly more quickly and frequently than the primary spheres, suggesting that SP cells have self-renewal ability.
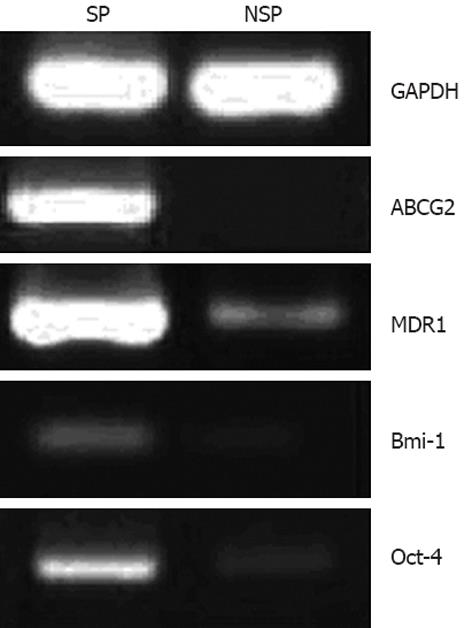
First, the expression of ABC transporters, including ABCG2 and MDR1, was observed, which was significantly higher in SP than in NSP cells (Figure 4). To further determine whether SP cells have stem cell-like characteristics, SP and NSP cells were examined for their expression of stem cell markers. The results demonstrated that the expression of Oct-4 and Bmi-1 in SP cells was higher than in NSP cells (Figure 4), suggesting that SP cells had some of the characteristics of undifferentiated stem cells.
To compare tumorigenic ability, SP and NSP cells from the KATO III human gastric cancer cell line were subcutaneously injected into the nude mice. Each mouse was individually injected with different amounts of SP and NSP cells. Six weeks after injection, although tumor diameter was smaller in NSP than in SP cells, four out of six mice could form tumors when injected either with SP or NSP cells (Table 1). All remaining mice could form tumors after eight weeks.
| Mouse | Cell | Cell dose | Tumor diameter (cm) |
| A | SP | 1 × 104 | 2.0 |
| 1 × 103 | 1.0 | ||
| NSP | 1 × 104 | 2.0 | |
| 1 × 103 | 0.5 | ||
| B | SP | 1 × 104 | 2.4 |
| 1 × 103 | 1.3 | ||
| NSP | 1 × 104 | 2.0 | |
| 1 × 103 | Not tested | ||
| C | SP | 1 × 104 | 2.0 |
| 1 × 103 | 1.1 | ||
| NSP | 1 × 104 | 2.0 | |
| 1 × 103 | 0.9 | ||
| D | SP | 1 × 104 | 2.2 |
| 1 × 103 | 1.2 | ||
| NSP | 1 × 104 | 1.9 | |
| 1 × 103 | 1.0 | ||
| E | SP | 1 × 104 | 1.8 |
| 1 × 103 | 1.2 | ||
| NSP | 1 × 104 | 1.8 | |
| 1 × 103 | Not tested | ||
| F | SP | 1 × 104 | 2.0 |
| 1 × 103 | 1.0 | ||
| NSP | 1 × 104 | 1.8 | |
| 1 × 103 | 1.0 |
To our knowledge, there has been no report identifying SP cells from the gastric cancer cell lines. As such, in this paper we analyzed SP and NSP cells in the KATO III, Hs-746T and AGS human gastric cancer cell lines. In the KATO III and Hs-746T cell lines, which have a high tumorigenic ability, SP cells accounted for 0.57% and 1.04% of the total population, moreover, they occupied 0.02% in the high tumorigenic AGS cell line. Therefore, it is challenging to draw a conclusion from the percentage of SP cells in different cell lines to compare their tumorigenic ability. These data are consistent with the C6 glioma cell line, which was mainly composed of cancer stem cells, although many of these cells were neither CD133+ nor a side population[21]. Further studies are therefore needed to compare the relationship between SP prevalence and tumorigenicity.
Single cells that grow anchorage-independently in soft agar are known to be malignant. Thus, cell lines should contain those cells which could form colonies and those that did not form colonies in soft agar assays. As such, soft agar assays can be used to investigate this tumorigenic ability in vitro. By seeding the mixed cells of KATO III-GFP and KATO III into soft agar, we could conclusively determine that colonies came from single cells. The clonogenic ability of SP cells isolated from KATO III was higher than that of NSP cells in soft agar and sphere forming assays. This is in agreement with the reports for the MCF7[13] and ARO[11] cell lines. In original animal transplant experiments, SP of KATO III were injected into nude mice subcutaneously, ranging in density from 104 to 102 cells. The tumor forms in mice by injecting 104 (2 out of 2) and 103 cells (2 out of 2) SP in 6 wk. The other mice did not form tumor by injecting 5 × 102 (0 out of 2) and 1 × 102 cells (0 out of 2) SP until week 10. In tumor formation assays, SP and NSP of KATO III ranging from 104 to 103 cells were injected. In our study, 12 injection sites for SP cells could form tumors in six weeks. However, 12 injection sites for NSP cells could also form tumors as well, although the observation period had to be increased to eight weeks. There was no significant difference in tumorigenicity in vivo between SP and NSP cells, which suggested that although CSC was enriched in SP cells, it may contain both SP and NSP cells.
Indefinite self-renewal is one of the essential properties of stem cells. A stem cell could undergo asymmetric division continuously, producing one cell that retains self-renewal ability and differentiates into a mature cell. Stem cells and progenitor cells have the property of anchorage-independent growth[22]. Therefore, soft agar and sphere formation assays can be used to test the self-renewal ability of stem cells in vitro[23,24]. In serial soft agar and sphere formation assays, single cells isolated from primary colonies coming from SP cells could form a secondary colony, suggesting that SP cells have the self-renewal capacity.
Bmi-1 is a transcriptional repressor belonging to the polycomb group of transcription factors. It has been shown to be important in the self-renewal of both normal and leukemic stem cells, as well as neuronal stem cells[25]. Oct-4 is a POU homeodomain transcription factor that is a key regulator of self-renewal in embryonic stem cells[26]. Cellular expression of Oct-4 is believed to have the capacity for self-renewal[27]. Moreover, the self-renewal ability of cancer stem cells could drive tumorigencity[28]. Oct-4 and Bmi-1 overexpression in SP cells isolated from KATO III was observed, which might reflect the property of self-renewal of SP cells.
While ABCG2 can efflux the DNA binding dye Hoechst 33342, which could induce an SP phenotype, MDR1 is the best-studied member of the ABC transporter superfamily of genes[29]. The expression level of ABCG2 and MDR1 mRNA was higher in SP than in NSP cells as determined by RT-PCR analysis, which contributes to the chemotherapeutic resistance of SP cells, and thus may be a target for cancer therapy.
Gastric cancer is the second leading cause of cancer-related deaths worldwide. Cancer stem cells have the characteristics of longevity, self-renewal and proliferation. It is believed that cancer stem cells are responsible for the heterogeneity and relapse of certain cancers including gastric cancer.
Side population (SP) cells can efflux the DNA-binding dye Hoechst33342 through an adenosine-triphosphate-binding cassette (ABC) membrane transporter and harbor stem cell-like characteristics. Recent studies suggested that SP cell analysis can be used to identify cancer stem cell populations. However, there has been no report identifying SP cells from the gastric cancer cell lines.
This is the first study to analyze SP and non-side population (NSP) cells in several human gastric cancer cell lines. The clonogenic ability of SP cells isolated from KATO III was higher than that of NSP cells in soft agar and sphere forming assays. Oct-4 and Bmi-1 overexpression in SP cells isolated from KATO III might reflect the property of self-renewal of SP cells. Moreover, ABCG2 and MDR1 overexpression contributes to the chemotherapeutic resistance of SP cells, and thus may be a target for cancer therapy.
SP cells could be used as a therapeutic target and for preventing relapse and drug-resistance in gastric cancer.
Cancer stem cells are characterized by its self-renewal capacity, differentiation potential, and cancer-initiating ability. Side population cells expressing the ABC transporter were distinguished from whole cell population. Recent studies demonstrated that SP cells could be characterized as cancer stem cells in primary tissues and tumor cell lines, therefore, it was postulated to be responsible for tumor development and recurrence.
Overall, this paper is very well written with good English and solid methodology. The biggest issue is that the content is not new. Basically, what the authors did was to confirm and characterize the side population cells in gastric cancer cell lines, which is not surprising. With that said, it is believed no one has done it in these cells, and it still may somewhat be informative to readers.
Peer reviewers: Kazuaki Takabe, MD, PhD, Assistant Professor of Surgery and Assistant Professor of Biochemistry and Molecular Biology, Surgical Oncology, Virginia Commonwealth University Massey Cancer Center, Medical College of Virginia, Virginia Commonwealth University, PO Box 980011, Richmond, VA 23298-0011, United States; Hikaru Nagahara MD, PhD, Professor, Aoyama Hospital, Tokyo Women’s Medical University, 2-7-13 Kita-Aoyama, Minatoku, Tokyo 107-0061, Japan
S- Editor Cheng JX L- Editor Ma JY E- Editor Zhang DN
| 1. | Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983-3988. [PubMed] |
| 2. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [PubMed] |
| 3. | Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946-10951. [PubMed] |
| 4. | Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65:9328-9337. [PubMed] |
| 5. | Seigel GM, Campbell LM, Narayan M, Gonzalez-Fernandez F. Cancer stem cell characteristics in retinoblastoma. Mol Vis. 2005;11:729-737. [PubMed] |
| 6. | Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823-835. [PubMed] |
| 7. | Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111-115. [PubMed] |
| 8. | Kayo H, Yamazaki H, Nishida H, Dang NH, Morimoto C. Stem cell properties and the side population cells as a target for interferon-alpha in adult T-cell leukemia/lymphoma. Biochem Biophys Res Commun. 2007;364:808-814. [PubMed] |
| 9. | Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797-1806. [PubMed] |
| 10. | Platet N, Mayol JF, Berger F, Hérodin F, Wion D. Fluctuation of the SP/non-SP phenotype in the C6 glioma cell line. FEBS Lett. 2007;581:1435-1440. [PubMed] |
| 11. | Mitsutake N, Iwao A, Nagai K, Namba H, Ohtsuru A, Saenko V, Yamashita S. Characterization of side population in thyroid cancer cell lines: cancer stem-like cells are enriched partly but not exclusively. Endocrinology. 2007;148:1797-1803. [PubMed] |
| 12. | Christgen M, Ballmaier M, Bruchhardt H, von Wasielewski R, Kreipe H, Lehmann U. Identification of a distinct side population of cancer cells in the Cal-51 human breast carcinoma cell line. Mol Cell Biochem. 2007;306:201-212. [PubMed] |
| 13. | Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res. 2005;65:6207-6219. [PubMed] |
| 14. | Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, Maclaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proc Natl Acad Sci USA. 2006;103:11154-11159. [PubMed] |
| 15. | Wang J, Guo LP, Chen LZ, Zeng YX, Lu SH. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716-3724. [PubMed] |
| 16. | Haraguchi N, Utsunomiya T, Inoue H, Tanaka F, Mimori K, Barnard GF, Mori M. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells. 2006;24:506-513. [PubMed] |
| 17. | Kruger JA, Kaplan CD, Luo Y, Zhou H, Markowitz D, Xiang R, Reisfeld RA. Characterization of stem cell-like cancer cells in immune-competent mice. Blood. 2006;108:3906-3912. [PubMed] |
| 18. | Hadnagy A, Gaboury L, Beaulieu R, Balicki D. SP analysis may be used to identify cancer stem cell populations. Exp Cell Res. 2006;312:3701-3710. [PubMed] |
| 19. | Available from: http: //www.cancer.gov/cancertopics/types/stomach.. |
| 20. | Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22:7340-7358. [PubMed] |
| 21. | Zheng X, Shen G, Yang X, Liu W. Most C6 cells are cancer stem cells: evidence from clonal and population analyses. Cancer Res. 2007;67:3691-3697. [PubMed] |
| 22. | Dontu G, Wicha MS. Survival of mammary stem cells in suspension culture: implications for stem cell biology and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:75-86. [PubMed] |
| 23. | Chumsri S, Phatak P, Edelman MJ, Khakpour N, Hamburger AW, Burger AM. Cancer stem cells and individualized therapy. Cancer Genomics Proteomics. 2007;4:165-174. [PubMed] |
| 24. | Farnie G, Clarke RB. Mammary stem cells and breast cancer--role of Notch signalling. Stem Cell Rev. 2007;3:169-175. [PubMed] |
| 25. | Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature. 2003;423:255-260. [PubMed] |
| 26. | Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435-439. [PubMed] |
| 27. | Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372-376. [PubMed] |