Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4597
Revised: February 27, 2012
Accepted: March 20, 2012
Published online: September 7, 2012
AIM: To determine the pattern of secreted mucin expression in Helicobacter pylori (H. pylori)-related, nonsteroidal anti-inflammatory drug (NSAID)-related and idiopathic gastric ulcers.
METHODS: We randomly selected 92 patients with H. pylori-associated (n = 30), NSAID-associated (n = 18), combined H. pylori and NSAID-associated gastric ulcers (n = 24), and patients with idiopathic gastric ulcers (n = 20). Immunohistochemistry for T-cell CD4/CD8, and for mucin 5AC (MUC5AC) and mucin 6 (MUC6), was performed on sections of the mucosa from the ulcer margin. Inflammation score was assessed according to the Sydney system.
RESULTS: MUC5AC was expressed on the surface epithelium (98.9%) and neck glands (98.9%) with minimal expression in the deep glands (6.5%). MUC6 was strongly expressed in the deep glands (97.8%), variable in the neck glands (19.6%) and absent in the surface epithelium (0%). The pattern of mucin expression in idiopathic ulcer margins was not different from the expression in ulcers associated with H. pylori, NSAIDs, or combined H. pylori and NSAIDs. CD4/CD8 ratio was higher in H. pylori-positive patients (P = 0.009). Idiopathic ulcers are associated with hospitalized patients and have higher bleeding and mortality rates.
CONCLUSION: Idiopathic ulcers have a unique clinical profile. Gastric mucin expression in idiopathic gastric ulcers is unchanged compared with H. pylori and/or NSAID-associated ulcers.
- Citation: Boltin D, Halpern M, Levi Z, Vilkin A, Morgenstern S, Ho SB, Niv Y. Gastric mucin expression in Helicobacter pylori-related, nonsteroidal anti-inflammatory drug-related and idiopathic ulcers. World J Gastroenterol 2012; 18(33): 4597-4603
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4597.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4597
Helicobacter pylori (H. pylori) infection and non-steroidal anti-inflammatory drugs (NSAIDs) are the leading causes of peptic ulcer[1-5]. However, in up to 39% of cases neither of these risk factors is identified[6]. While it may be prudent to exclude rarer causes of gastric ulcer such as malignancy, Zollinger-Ellison syndrome or systemic mastocytosis, in many cases H. pylori/NSAID-negative ulcers are apparently idiopathic.
Mucins are high-molecular-weight glycoproteins, which are heavily decorated with O-linked oligo-saccharides and n-glycan chains, linked to a protein backbone. There are 21 mucin (MUC) genes known in the human genome. These genes encode 2 groups of mucins: secreted mucins and membrane-bound mucins. The main mucins expressed in the stomach are MUC 1 (membrane-bound) and MUC5AC and MUC6 (secreted mucins). It has been proposed that defects in gastric mucin quality or quantity play a role in the pathogenesis of H. pylori/NSAID-negative ulcers[7]. MUC5AC, forming the bulk of the adherent unstirred mucous layer, is secreted by surface foveolar cells, whereas MUC6 is secreted by neck and gland cells, and both are strongly expressed in normal gastric mucosa[8]. These two mucin proteins remain segregated within the mucous gel in a laminated linear arrangement[9]. NSAIDs disrupt the production of prostaglandin-E2 which mediates mucin secretion. H. pylori similarly decreases mucin synthesis via inhibition of galactosyltransferase[10], this despite mucin’s inherent antibacterial properties[11].
The pattern of mucin secretion in idiopathic peptic ulcer disease has yet to be determined. The aim of the present study is to identify the clinical and endoscopic features, and gastric mucin secretion patterns, in peptic ulcer disease positive and negative for H. pylori infection and/or NSAID therapy.
Approval was granted by the ethics committee of Rabin Medical Center. Non-consecutive patients who underwent routine or emergency upper endoscopy at the Department of Gastroenterology, Rabin Medical Center, Beilinson Hospital, and who were assigned an endoscopic diagnosis of gastric ulcer, between 2003 and 2009, were randomly identified using an established computerized endoscopy reporting system. Clinical parameters were recorded, including patient age and gender, major indication for upper endoscopy, concomitant diseases and the use of aspirin and NSAIDs in the previous 3 mo. Endoscopic parameters were recorded, including ulcer site, size and number. H. pylori status was determined via histological detection on biopsies taken from the ulcer margins, gastric body or antrum and/or the rapid urease test and/or 13C-urea breath test performed within 3 mo. Exclusion criteria included cases where no biopsies were taken from the ulcer margin, and where biopsies revealed neoplasia. Patients with clinical or histological evidence of Zollinger-Ellison syndrome, gastrointestinal malignancy, eosinophilic gastroenteritis, systemic mastocytosis, and patients receiving bisphosphonates, potassium salts or iron, were excluded.
Formalin-fixed, paraffin-embedded tissue samples from ulcer margins were obtained from the Pathology Department. Where additional biopsies of gastric body or antrum were taken at endoscopy, these too were obtained. Paraffin-embedded blocks were cut into 4 μm thick sections. Slides were deparaffinized in xylene and rehydrated using a graded ethanol series. Antigen was retrieved by boiling the slides in a microwave oven for 15 min in 0.01 mol/L citrate buffer (pH 6.0). Endogenous peroxidase was blocked with a 3% H2O2-methanol solution, and the slides were incubated in 10% normal goat serum for 30 min to prevent nonspecific staining. The tissue sections were then incubated overnight at 4 °C with primary antibody (MUC5AC or MUC6, 1:100; Santa Cruz, CA). The standard biotin-streptavidin-peroxidase method was then used, and the sections were lightly counterstained with hematoxylin. Histologically normal gastric biopsies were used as positive controls for MUC5AC and MUC6. The sections incubated with phosphate-buffered saline (0.01 mol/L, pH 7.4) instead of primary antibody were used as negative controls. Cytoplasm staining was assessed in at least 10 high-power fields by two blinded observers at 3 sites: the foveola, the mucous neck cells, and the glands. The range of cytoplasmic staining (0: 0%-5%; 1: 6%-30%; 2: 31%-60%; and 3: 61%-100%) and the intensity of staining (0: No staining; 1: Weak staining; 2: Intermediate staining; 3: Strong staining) were assessed, and averages of the grades were taken. The final staining score was defined as the product of scores for the range and intensity of cytoplasmic staining. Staining was defined as negative if the staining score was 0 or 1, intermediate for 2, 3 or 4, and high for 6 or 9[12]. All specimens were scored blindly.
Immunohistochemistry with monoclonal antibodies to T-cell CD4 and CD8 antigens was performed for 5 cases from each group using a technique previously described[13]. Tissue sections were cut 4 μm thick from routinely processed formalin-fixed and paraffin-embedded blocks. The slides were oven dried overnight at 60 °C. The slides were then put inside the Ventana (Benchmark, United States). The Ventana was activated by loading the pre-programmed recipe file for the appropriate antibody. For CD4 we used polyclonal antibody (Spring, CA, United States), and for CD8 a monoclonal antibody, clone SP16 (DBS, CA, United States). Immunohistochemical staining was performed by the I-view DAB detection kit of Ventana. Dark brown staining was defined as positive, and no staining was defined as negative. Staining was scored as follows: 0 (no detectable staining); 1 (1%-10% positive cells); 2 (11%-50%); 3 (51%-80%); and 4 (more than 80%). In cells with positive staining, the staining was intense and uniform, so intensity was not factored into the scoring. The ratio of CD4/CD8 intraepithelial/mucosal lymphocytes was assessed for ten low-power microscopic fields. Inflammation score was measured according to the Sydney system, and compared between the groups[14]. Sydney score for inflammation is the sum of 5 criteria: H. pylori status, atrophy, intestinal metaplasia, lymphocytic infiltration and polymorphonuclear cell infiltration. Every criterion has 0 to 3 score (none exists, mild, moderate, severe) thus the range is 0 to 15.
Statistical analysis was performed using statistical package for the social sciences software 19.0 (SPSS, Inc.). Patient groups were compared using the Pearson χ2 test, F test and Duncan test. P values were considered significant when ≤ 0.05.
Ulcer biopsies from 92 patients were included in the final set analysis, including 30 H. pylori-associated ulcers, 18 NSAID-associated, 24 associated with combined H. pylori/NSAID and 20 idiopathic, neither associated with H. pylori nor NSAID use. Patient characteristics are summarized in Table 1.
| H. pylori+/NSAID- | H. pylori-/NSAID+ | H. pylori+/NSAID+ | H. pylori-/NSAID- | Total | |
| n = 30 (32.6%) | n = 18 (19.6%) | n = 24 (26.1%) | n = 20 (21.7%) | n = 92 | |
| Age, yr [mean (SD, range)] | 58.6 (18.58, 18-88) | 72.17 (10.44, 56-89) | 69.46 (13.08, 24-83) | 70.3 (12.64, 42-95) | 66.6 (15.5, 18-95) |
| Gender (male) n (%) | 10 (33) | 9 (50) | 13 (54.2) | 12 (60) | 44 (47.8) |
| Ethnicity n (%) | |||||
| Israeli (Jewish) | 11 (36.7) | 9 (50) | 12 (50) | 6 (30) | 38 (41.3) |
| Israeli (Arab) | 3 (10) | 0 (0) | 2 (8.3) | 1 (5) | 6 (6.5) |
| Western Europe/United States | 7 (23.3) | 0 (0) | 1 (4.2) | 2 (10) | 10 (10.9) |
| Eastern Europe/FSU | 2 (6.7) | 6 (33.3) | 6 (25) | 6 (30) | 20 (21.7) |
| Middle east/Africa | 7 (23.3) | 2 (11.1) | 3 (12.5) | 4 (20) | 16 (17.4) |
| South America | 0 (0) | 1 (5.6) | 0 (0) | 1 (5) | 2 (2.2) |
| Inpatient n (%) | 11 (36.7) | 12 (60) | 10 (41.7) | 16 (80) | 49 (53.3) |
| Comorbid disease n (%) | |||||
| ASCVD | 3 (10) | 12 (66.7) | 11 (45.8) | 6 (30) | 32 (34.8) |
| COPD | 2 (6.7) | 4 (22.2) | 2 (8.3) | 7 (35) | 15 (16.3) |
| Diabetes | 4 (13.3) | 4 (22.2) | 12 (50) | 6 (30) | 26 (28.3) |
| Current malignancy | 1 (3.3) | 2 (11.1) | 2 (8.3) | 2 (10) | 7 (7.6) |
| Alcohol abuse | 0 (0) | 0 (0) | 0 (0) | 3 (15) | 3 (3.3) |
| Other significant systemic disease | 1 (3.3)3 | 1 (5.6)4 | 1 (4.2)4 | 5 (25)5 | 8 (8.7) |
| Total1 | 7 (23.3) | 15 (83.3) | 19 (79.2) | 14 (70) | 55 (59.8) |
| Primary indication for endoscopy n (%) | |||||
| Iron deficiency anemia | 9 (30) | 5 (27.8) | 6 (25) | 1 (5) | 21 (22.8) |
| Epigastric pain/GERD | 8 (26.7) | 6 (33.3) | 4 (16.5) | 4 (20) | 22 (23.9) |
| Upper GI bleeding | 7 (23.3) | 6 (33.3) | 6 (25) | 9 (45) | 28 (30.4) |
| Fecal occult blood | 2 (6.7) | 0 (0) | 1 (4.2) | 1 (5) | 4 (4.3) |
| Weight loss | 2 (6.7) | 1 (5.6) | 5 (20.8) | 0 (0) | 8 (8.7) |
| Screening for gastric cancer | 2 (6.7) | 0 (0) | 0 (0) | 0 (0) | 2 (2.2) |
| Esophageal varices | 0 (0) | 0 (0) | 0 (0) | 2 (10) | 2 (2.2) |
| Vomiting | 0 (0) | 0 (0) | 0 (0) | 1 (5) | 1 (1.1) |
| Other2 | 0 (0) | 0 (0) | 2 (8.3) | 2 (10) | 4 (4.3) |
| Hemoglobin, g/dL [mean (SD, range)] | 11.0 (3.22, 3.4-16.7) | 10.6 (2.77, 5.5-15.5) | 11.0 (2.84, 5.7-14.7) | 11.1 (2.70, 6.2-15.3) | 10.9 (2.98, 3.4-16.7) |
| Died within 12 mo of endoscopy | 3 (10) | 2 (11.1) | 2 (8.3) | 5 (25) | 12 (13.0) |
Forty-four patients were male, with no significant differences between the 4 groups (P = 0.24). Mean age was 66.6 years. Patients with H. pylori-associated ulcers not receiving NSAIDs were significantly younger compared to the other 3 groups (α = 0.05, Duncan test). There was no significant difference in the patients’ origin between the groups. Although significant co-morbidities were observed in all groups, 80% (16/20) of patients with H. pylori/NSAID-negative ulcers were inpatients at the time of their endoscopy, compared to 45.8% (33/72) in other groups (P = 0.007). Furthermore, idiopathic ulcers were associated with decreased survival, where 25% (5/20) died within 12 mo, compared to 9.7% (7/72) in other groups (all cause mortality, P = 0.04).
Patients with H. pylori/NSAID-negative ulcers more often presented with upper gastrointestinal bleeding: 45% (9/20) compared with 26.4% (19/72) in the other groups (P = 0.11). Subacute and asymptomatic presentations (iron deficiency anemia, weight loss, fecal occult blood and screening) were less common in those with H. pylori/NSAID-negative ulcers (2/20, 10%, compared to 34/72, 47.2%, P = 0.003).
No difference between groups was observed regarding ulcer number or size. H. pylori-negative/NSAID-positive ulcers tended to be more often located in the gastric body: 6/18 (33.3%) compared with other groups 19/74 (25.7%) (P = 0.51). The presence of intestinal metaplasia did not differ between groups (Table 2).
| H. pylori+/NSAID- | H. pylori-/NSAID+ | H. pylori+/NSAID+ | H. pylori-/NSAID- | Total | |
| n = 30 (32.6%) | n = 18 (19.6%) | n = 24 (26.1%) | n = 20 (21.7%) | n = 92 | |
| Ulcer number (per procedure) n (%) | |||||
| 1 | 21 (70) | 10 (55.6) | 13 (54.2) | 14 (70) | 58 (63.0) |
| 2 | 4 (13.3) | 3 (16.7) | 5 (20.8) | 3 (15) | 15 (16.3) |
| ≥ 3 | 5 (16.7) | 5 (27.8) | 6 (25) | 3 (15) | 19 (20.7) |
| Ulcer size (n)1 | |||||
| ≤ 5 mm | 18 | 14 | 27 | 14 | 73 |
| 6-10 mm | 7 | 8 | 10 | 11 | 36 |
| 11-20 mm | 5 | 3 | 4 | 4 | 16 |
| > 20 mm | 4 | 0 | 0 | 2 | 6 |
| Not specified | 2 | 2 | 2 | 0 | 6 |
| Ulcer location n (%) | |||||
| Antrum | 19 (63.3) | 10 (55.6) | 18 (75) | 16 (80) | 63 (68.5) |
| Body | 9 (30) | 6 (33.3) | 6 (25) | 4 (20) | 25 (27.2) |
| Cardia | 2 (6.7) | 2 (11.1) | 0 (0) | 0 | 4 (4.3) |
| Ulcer H. pylori positive n (%) | 28 (93.3)2 | 0 (0) | 20 (83.3) | 0 (0) | 48 (52.2) |
| Ulcer IM n (%) | 5 (16.7) | 4 (22.2) | 3 (16.7) | 4 (20) | 16 (17.4) |
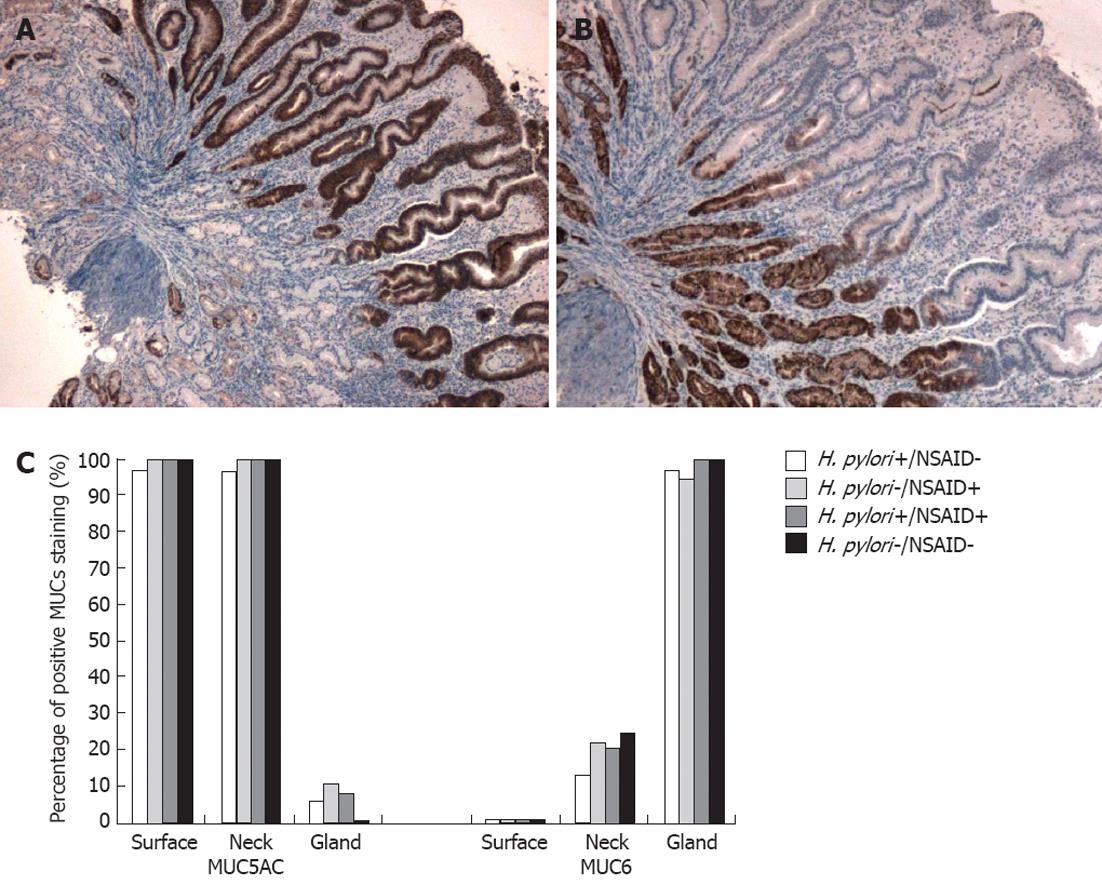
Virtually all biopsies had positive (intermediate or high) staining for MUC5AC in the surface and neck cells (Figure 1). Staining for MUC5AC was minimal in gland cells (Tables 3 and 4). Although no significant difference between groups was observed there was a trend towards decreased expression of MUC5AC in the deep glands of idiopathic ulcers (P = 0.09). Including for analysis only cells with high mucin expression (extent and intensity score products of 6 or 9), and separate analyses of staining extent and intensity, did not significantly alter the results. All but two biopsies stained positively (intermediate or high) for MUC6 in the gland cells (Figure 1), and staining was uniformly negative in the surface cells. Staining for MUC6 was variable in the neck cells. Again, including only cells with high mucin expression (extent and intensity score products of 6 or 9), and separate analyses of staining extent and intensity, did not significantly alter the results.
| Mucin 5AC | Mucin 6 | ||||||
| Surface cell positivity | Neck cell positivity | Gland cell positivity | Surface cell positivity | Neck cell positivity | Gland cell positivity | ||
| H. pylori+/NSAID- | a | 29 (96.7) | 29 (96.7) | 2 (6.7) | 0 (0) | 4 (13.3) | 29 (96.7) |
| (n = 30) | b | 29 (96.6) | 27 (90) | 0 (0) | 0 (0) | 1 (3.3) | 25 (83.3) |
| H. pylori-/NSAID+ | a | 18 (100) | 18 (100) | 2 (11.1) | 0 (0) | 4 (22.2) | 17 (94.4) |
| (n = 18) | b | 16 (88.9) | 15 (83.3) | 0 (0) | 0 (0) | 2 (11.1) | 14 (77.8) |
| H. pylori+/NSAID+ | a | 24 (100) | 24 (100) | 2 (8.3) | 0 (0) | 5 (20.8) | 24 (100) |
| (n = 24) | b | 24 (100) | 23 (95.8) | 2 (8.3) | 0 (0) | 2 (8.3) | 22 (91.7) |
| H. pylori-/NSAID- | a | 20 (100) | 20 (100) | 0 (0) | 0 (0) | 5 (25) | 20 (100) |
| (n = 20) | b | 19 (95) | 16 (80) | 0 (0) | 0 (0) | 1 (5) | 19 (95) |
| Total | a | 91 (98.9) | 91 (98.9) | 6 (6.5) | 0 (0) | 18 (19.6) | 90 (97.8) |
| (n = 92) | b | 88 (95.7) | 81 (88.0) | 2 (2.2) | 0 (0) | 6 (6.5) | 80 (97.0) |
| H. pylori+ | H. pylori- | P value | NSAID+ | NSAID- | P value | |
| MUC5AC | ||||||
| Surface cell positivity | 53/54 (98.1) | 38/38 (100) | 0.39 | 42/42 (100) | 49/50 (98) | 0.36 |
| Neck cell positivity | 53/54 (98.1) | 38/38 (100) | 0.39 | 42/42 (100) | 49/50 (98) | 0.36 |
| Gland cell positivity | 4/54 (7.4) | 2/38 (5.3) | 0.68 | 4/42 (9.5) | 2/50 (4) | 0.29 |
| MUC6 | ||||||
| Surface cell positivity | 0/54 (0) | 0/38 (0) | NA | 0/42 (0) | 0/50 (0) | NA |
| Neck cell positivity | 9/54 (16.7) | 9/38 (23.7) | 0.40 | 9/42 (21.4) | 9/50 (18) | 0.68 |
| Gland cell positivity | 53/54 (98.1) | 37/38 (97.4) | 0.80 | 41/42 (97.6) | 49/50 (98) | 0.90 |
T-cell CD4/CD8 ratio was significantly lower in the NSAID-positive groups when compared to the H. pylori-positive groups (P = 0.009, Table 5). As expected, the Sydney inflammation score was significantly lower in the H. pylori-negative/NSAID-negative group than in the H. pylori-positive/NSAID-negative group (P = 0.017, Table 6).
| Group | CD4+/CD8+ | CD8+/HPF | CD4+/HPF |
| H. pylori+/NSAID- | 3.15 | 22.0 ± 4.4 | 69.6 ± 38.2a |
| H. pylori-/NSAID+ | 0.55 | 14.0 ± 9.4 | 8.4 ± 12.5 |
| H. pylori+/NSAID+ | 2.99 | 26.6 ± 20.9 | 50.0 ± 25.2 |
| H. pylori-/NSAID- | 1.15 | 29.0 ± 11.4 | 38.0 ± 27.7 |
| Group | H. pylori | Atrophy | Intestinal metaplasia | Lymphocytes | PMN | Score |
| H. pylori+/NSAID- | 2.20 ± 0.44 | 0.60 ± 0.54 | 0.40 ± 0.54 | 1.80 ± 0.83 | 1.20 ± 1.30 | 6.20 ± 2.48a |
| H. pylori-/NSAID+ | 0.00 ± 0.00 | 0.40 ± 0.54 | 0.60 ± 1.34 | 1.40 ± 0.89 | 0.20 ± 0.44 | 2.60 ± 2.19 |
| H. pylori+/NSAID+ | 1.80 ± 1.09 | 0.80 ± 0.83 | 0.40 ± 0.89 | 2.00 ± 0.70 | 1.40 ± 0.89 | 6.40 ± 3.13 |
| H. pylori-/NSAID- | 0.00 ± 0.00 | 0.20 ± 0.44 | 0.20 ± 0.44 | 1.40 ± 0.54 | 0.40 ± 0.54 | 2.20 ± 1.64 |
Gastric secreted mucin expression has not been previously studied in the setting of the idiopathic ulcer. In the present study, the distribution of immunohistochemical staining for MUC5AC and MUC6 in the margins of H. pylori/NSAID-negative ulcers did not significantly differ from staining patterns in the margins of ulcers associated with H. pylori and/or NSAIDS.
H. pylori interacts with gastric mucins in various manners, in order to facilitate its colonization. It has been established that H. pylori disrupts the assembly of the mucin molecule via inhibition of galactosyltransferase[10,12]. Furthermore, H. pylori reduces gastric mucous viscosity by elevating pH through urease secretion, thereby enhancing its motility within gastric mucous[15]. Kobayashi et al[16] demonstrated that BabA and SabA adhesins on H. pylori bind to Lewis B blood group antigens on MUC5AC, facilitating colonization.
On the other hand, gastric mucins have antimicrobial properties which are directed against H. pylori. Kawakubo et al[11] demonstrated that unique -glycans in MUC6 inhibit bacterial biosynthesis of cholesteryl-α-D-glucopyranoside, a major cell wall component. Lindén et al[17] suggest that mucins decorated with Leb (the binding site for the H. pylori BabA adhesin) effectively bind H. pylori, thereby impairing its colonization of the mucosal surface. Similarly, in Trichuris muris infection MUC5AC is aberrantly expressed in the intestine and plays a key role in expulsion of the nematode[18]. Byrd et al[19] demonstrated that gastric biopsies from H. pylori-related gastritis patients frequently expressed the aberrant location of MUC6 staining in the surface foveolar cells. In the present study, cytoplasmic mucin staining of ulcers associated with H. pylori was unchanged. One reason for this discrepancy is the fact that the present study only included patients with gastric ulcers, and sampled gastric mucosa immediately adjacent to the edge of a gastric ulcer. These patients represent a distinct subgroup of patients with H. pylori infection, and our findings imply that alterations in secreted gastric mucins do not play a role in the pathogenesis of gastric ulcer in patients with H. pylori infection. This is supported by Marques et al[20] who found that although topographic expression of MUC5AC and MUC6 mucins was altered in H. pylori-related gastritis, the expression of these mucins was unchanged in mucosa adjacent to patients with H. pylori-related gastric ulcer.
An interesting finding in our study was that many patients with H. pylori/NSAID-negative ulcers had multiple comorbidities, were more often inpatients at the time of endoscopy, had fewer subacute presentations, and had a poorer survival. This concurs with Chan et al[21] who noted that three-quarters of patients with acutely bleeding H. pylori/NSAID-negative ulcers have significant comorbidity including major organ failure and malignancy. A large prospective study found that concomitant diseases and the absence of epigastric pain are independent risk factors for H. pylori/NSAID-negative ulcers in the duodenum[22]. A higher number of comorbidities were associated with increased ulcer size and depth, and more bleeding complications. Furthermore, our data are consistent with previous findings that idiopathic ulcer is an independent risk factor associated with long-term mortality[23,24].
In this study, H. pylori/NSAID-negative gastric ulcers were associated with underlying systemic disease, which could be severe. This association has been reported previously in idiopathic ulcers[24], and suggests the possible role of ischemic or non-specific inflammatory factors in their pathogenesis[25]. Despite the heterogeneity of possible pathogenic factors of these idiopathic H. pylori/NSAID-negative cases, our results indicate virtually no difference in qualitative MUC5AC and MUC6 staining in these ulcers compared with definite H. pylori-positive, NSAID-positive, and combined H.pylori/NSAID-positive gastric ulcers .
H. pylori-positive ulcer is associated with a high inflammation rate, thus groups 2 and 4 had significantly lower Sydney inflammation score than groups 1 and 3 (P = 0.017). In addition, we found a low ratio of T-cell CD4/CD8 in the groups negative for H. pylori; but when NSAID use was also negative the result did not reach significance. Similar findings were described by Strömberg et al[26]. In peptic ulcer patients positive for H. pylori, the number of intraepithelial T-cell CD4+ was higher than in patients with H. pylori infection but without ulcer or in healthy controls negative for H. pylori. Thus, H. pylori infection recruits CD4+ lymphocytes.
A limitation of our study is the retrospective nature of the data collection, which precluded elimination of false negative tests for H. pylori (probably due to proton pump inhibitor, bismuth or antibiotics), and cases of surreptitious or unreported NSAID use, which would result in misclassification of ulcers as H. pylori/NSAID-negative. This could only be overcome using a prospective study design, by performing multiple tests for H. pylori and assaying serum salicylate and plasma thromboxane, respectively.
In conclusion, patterns of mucin secretion in H. pylori/NSAID-negative ulcers need to be further studied in well-designed, prospective studies which minimize cross-contamination of groups. Idiopathic peptic ulcers are an increasingly encountered entity, with unique clinical and endoscopic features. Future efforts should focus on identifying genetic and epigenetic factors which regulate mucin secretion in this setting, as well as characterizing a potential role of the membrane-bound mucins and other mucosal protective factors.
In health, a mucin layer protects the stomach from the harmful effects of gastric acid and ulceration. The main causes of peptic ulcer disease are Helicobacter pylori (H. pylori) infection and aspirin or nonsteroidal anti-inflammatory drug (NSAID) therapy. However, up to 39% of peptic ulcer disease is idiopathic. Idiopathic ulcers are often associated with poorer outcomes.
Alterations in secreted gastric mucins have been described in H. pylori infection and NSAID treatment, and may have a role in ulcer pathogenesis. This is the first study to look at gastric mucin secretion in idiopathic ulcers.
Mucin 5AC (MUC5AC) and MUC6 are equally expressed by H. pylori-induced, NSAID-induced and idiopathic ulcers. The number of CD4 and CD8 lymphocytes is highest in H. pylori-induced ulcers reflecting a greater inflammatory component. Inflammation score is lowest in NSAID-associated ulcers.
Idiopathic peptic ulcer disease is more aggressive than peptic ulcer disease induced by H. pylori or NSAIDs. Elucidating the pathogenesis of idiopathic ulcers will lead the way to finding appropriate therapies.
Mucin is the major component of gastric mucous and is synthesized and secreted by specialized cells. The two primary gastric mucin molecules are coined MUC5AC and MUC6; H. pylori is a ubiquitous bacterium which may cause gastric ulcer; NSAIDs include a range of over-the-counter medications commonly used for analgesia and rheumatic disease, and similarly cause gastric ulceration. Idiopathic ulcers are ones with no identifiable cause.
The manuscript is interesting and has potential to enhance understanding on MUC5AC and MUC6 expression in gastric mucosa in H. pylori-, NSAID- and idiopathic-ulcers.
Peer reviewer: Waliul Khan, MBBS, PhD, Mcmaster Uni-versity, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
S- Editor Gou SX L- Editor Logan S E- Editor Li JY
| 1. | Hyvärinen H, Salmenkylä S, Sipponen P. Helicobacter pylori-negative duodenal and pyloric ulcer: role of NSAIDs. Digestion. 1996;57:305-309. [PubMed] |
| 2. | Gisbert JP, Blanco M, Mateos JM, Fernández-Salazar L, Fernández-Bermejo M, Cantero J, Pajares JM. H. pylori-negative duodenal ulcer prevalence and causes in 774 patients. Dig Dis Sci. 1999;44:2295-2302. [PubMed] |
| 3. | Tsuji H, Kohli Y, Fukumitsu S, Morita K, Kaneko H, Ohkawara T, Minami M, Ueda K, Sawa Y, Matsuzaki H. Helicobacter pylori-negative gastric and duodenal ulcers. J Gastroenterol. 1999;34:455-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Elitsur Y, Lawrence Z. Non-Helicobacter pylori related duodenal ulcer disease in children. Helicobacter. 2001;6:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | McColl KE, el-Nujumi AM, Chittajallu RS, Dahill SW, Dorrian CA, el-Omar E, Penman I, Fitzsimons EJ, Drain J, Graham H. A study of the pathogenesis of Helicobacter pylori negative chronic duodenal ulceration. Gut. 1993;34:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Jyotheeswaran S, Shah AN, Jin HO, Potter GD, Ona FV, Chey WY. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol. 1998;93:574-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Niv Y. H. pylori/NSAID--negative peptic ulcer--the mucin theory. Med Hypotheses. 2010;75:433-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 209] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Ho SB, Takamura K, Anway R, Shekels LL, Toribara NW, Ota H. The adherent gastric mucous layer is composed of alternating layers of MUC5AC and MUC6 mucin proteins. Dig Dis Sci. 2004;49:1598-1606. [PubMed] |
| 10. | Tanaka S, Mizuno M, Maga T, Yoshinaga F, Tomoda J, Nasu J, Okada H, Yokota K, Oguma K, Shiratori Y. H. pylori decreases gastric mucin synthesis via inhibition of galactosyltransferase. Hepatogastroenterology. 2003;50:1739-1742. [PubMed] |
| 11. | Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science. 2004;305:1003-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 12. | Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94:166-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 14. | Price AB. The Sydney System: histological division. J Gastroenterol Hepatol. 1991;6:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 654] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 15. | Celli JP, Turner BS, Afdhal NH, Keates S, Ghiran I, Kelly CP, Ewoldt RH, McKinley GH, So P, Erramilli S. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc Natl Acad Sci USA. 2009;106:14321-14326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 282] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 16. | Kobayashi M, Lee H, Nakayama J, Fukuda M. Roles of gastric mucin-type O-glycans in the pathogenesis of Helicobacter pylori infection. Glycobiology. 2009;19:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Lindén S, Semino-Mora C, Liu H, Rick J, Dubois A. Role of mucin Lewis status in resistance to Helicobacter pylori infection in pediatric patients. Helicobacter. 2010;15:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011;208:893-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 19. | Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997;113:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Marques T, David L, Reis C, Nogueira A. Topographic expression of MUC5AC and MUC6 in the gastric mucosa infected by Helicobacter pylori and in associated diseases. Pathol Res Pract. 2005;201:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Chan HL, Wu JC, Chan FK, Choi CL, Ching JY, Lee YT, Leung WK, Lau JY, Chung SC, Sung JJ. Is non-Helicobacter pylori, non-NSAID peptic ulcer a common cause of upper GI bleeding? A prospective study of 977 patients. Gastrointest Endosc. 2001;53:438-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Xia HH, Wong BC, Wong KW, Wong SY, Wong WM, Lai KC, Hu WH, Chan CK, Lam SK. Clinical and endoscopic characteristics of non-Helicobacter pylori, non-NSAID duodenal ulcers: a long-term prospective study. Aliment Pharmacol Ther. 2001;15:1875-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Wong GL, Wong VW, Chan Y, Ching JY, Au K, Hui AJ, Lai LH, Chow DK, Siu DK, Lui YN. High incidence of mortality and recurrent bleeding in patients with Helicobacter pylori-negative idiopathic bleeding ulcers. Gastroenterology. 2009;137:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Gisbert JP, Calvet X. Review article: Helicobacter pylori-negative duodenal ulcer disease. Aliment Pharmacol Ther. 2009;30:791-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 25. | McColl KE. How I manage H. pylori-negative, NSAID/aspirin-negative peptic ulcers. Am J Gastroenterol. 2009;104:190-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Strömberg E, Lundgren A, Edebo A, Lundin S, Svennerholm AM, Lindholm C. Increased frequency of activated T-cells in the Helicobacter pylori-infected antrum and duodenum. FEMS Immunol Med Microbiol. 2003;36:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |