Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4517
Revised: March 15, 2012
Accepted: March 29, 2012
Published online: September 7, 2012
Pregnancy associated with chronic hepatitis B (CHB) is a common and important problem with unique challenges. Pregnant women infected with CHB are different from the general population, and their special problems need to be considered: such as the effect of hepatitis B virus (HBV) infection on the mother and fetus, the effect of pregnancy on replication of the HBV, whether mothers should take HBV antiviral therapy during pregnancy, the effect of these treatments on the mother and fetus, how to carry out immunization of neonates, whether it can induce hepatitis activity after delivery and other serious issues. At present, there are about 350 million individuals with HBV infection worldwide, of which 50% were infected during the perinatal or neonatal period, especially in HBV-endemic countries. Currently, the rate of HBV infection in the child-bearing age group is still at a high level, and the infection rate is as high as 8.16%. Effective prevention of mother-to-child transmission is an important means of reducing the global burden of chronic HBV infection. Even after adopting the combined immunization measures, there are still 5%-10% of babies born with HBV infection in hepatitis B e antigen positive pregnant women. As HBV perinatal transmission is the main cause of chronic HBV infection, we must consider how to prevent this transmission to reduce the burden of HBV infection. In this population of chronic HBV infected women of childbearing age, specific detection, intervention and follow-up measures are particularly worthy of attention and discussion.
- Citation: Han GR, Xu CL, Zhao W, Yang YF. Management of chronic hepatitis B in pregnancy. World J Gastroenterol 2012; 18(33): 4517-4521
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4517.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4517
Chronic hepatitis B (CHB) in pregnancy is an important and pervasive issue with unique challenges. However, for pregnant women with chronic hepatitis B virus (HBV) infection, unlike the general population, many special problems need to be considered, such as the influence of HBV infection on the mother and fetus, influence of pregnancy on HBV replication, effects of antiviral treatment on maternal and neonatal outcomes, immunization of newborns and the possible flare of hepatitis after delivery. Approximately 350 million people are infected with HBV worldwide and 50% of them have acquired their infection in the perinatal or neonatal period, especially in countries where HBV has a high prevalence[1,2]. In these countries, women of childbearing age have a higher hepatitis B e antigen (HBeAg)-positive rate and a higher probability of mother-to-infant transmission, the younger they are when infected with HBV, the higher the risk of developing CHB[1,3,4]. The rate of HBV infection among women of childbearing age is still at a high level (7.18%) in China[5-7], which leads to an increased risk of HBV vertical transmission and damage due to CHB in the mother and fetus in pregnancy. Therefore, the effective prevention of vertical transmission of HBV is an important approach in reducing the global burden of CHB. Specific hepatitis B immunoglobulin (HBIG) is available for passive protection and is normally used in combination with hepatitis B vaccine to confer immediate cover (passive immunity) and long-lasting protection (active immunity) in newborns, which is administered as an effective prophylactic measure to prevent mother-to-infant transmission of HBV, however, 5%-10% infants of HBeAg-positive mothers are still infected with HBV[8-10].
Overall, no severe effects due to CHB are found in pregnancy. Studies have shown that chronic HBV infection is associated with gestational diabetes mellitus, antepartum hemorrhage, threatened premature labor and lower Apgar score. Mothers with seriously abnormal liver function complications are prone to postpartum hemorrhage, puerperal infection, low body weight infants, fetal distress, premature birth, fetal death and neonatal asphyxia[11-21]. A series of physiological changes occur during pregnancy, including vigorous metabolism and increased nutrient consumption. These changes occur to promote the metabolic needs of the mother as well as the needs of the growing fetus. Abundant sex hormone produced by the mother needs to be metabolized and inactivated in the liver, and metabolism and detoxification in the fetus also depend on the mother’s liver, which correlates with aggravation of pre-existing liver diseases and exacerbation of liver damage[22,23]. Alanine aminotransferase (ALT) in late pregnancy and the postpartum period shows an increasing tendency, however, HBV replication in the gestational period is not noticeably different[24-28]. Some women appear to undergo HBeAg seroconversion in the initial months after delivery if immune activation occurs, with a seroconversion rate of 12.5% to 17%, which is correlated with an obvious decrease in adrenal cortex hormone[29,30]. Although HBV infection during pregnancy can often be tolerated, severe hepatitis and hepatic failure induced by perinatal hepatic flare reactions still occur, and can have an unfavorable outcome[29].
As relatively safe assays for the diagnosis of HBV infection are available and effective treatment strategies for CHB have been developed, the screening of HBV infection in the perinatal period has become standard care, which can identify newborns that require prophylaxis with hepatitis B vaccine and HBIG as well as pregnant women who require antiviral therapy. In addition, it is beneficial to advise patients with hepatitis B about sexual and family contacts. Screening and vaccination are key factors in the successful prevention and control of HBV infection.
Women with CHB should actively plan their pregnancy and undergo baseline evaluations, such as hepatitis B surface antigen (HBsAg), HBeAg, antibody to HBeAg (anti-HBe), HBV DNA, severity of liver disease and the presence of other viral infections, are suggested before pregnancy. The ability to sustain pregnancy and the risk of vertical transmission of HBV in women with CHB should also be evaluated. All pregnant women should be screened for HBV infection at the first prenatal examination, and HBsAg-positive patients should be transferred to hospitals with experience in the management of CHB for easier monitoring of mothers in pregnancy, delivery and the postpartum period as well as newborns, and appropriate prevention of mother-to-infant transmission of HBV based on an individual’s condition should be conducted.
The treatment goals for CHB in pregnancy are to achieve stabilization of liver function in mothers and prevent HBV infection in newborns. Regular monitoring of liver function and HBV DNA level should be performed in the gestational period to determine whether liver disease is progressing and antiviral therapy is needed in mothers. Sinha et al[11] from India made several suggestions aimed at Asian HBV carriers when planning a pregnancy: First, in patients with lower HBV DNA level at baseline (HBV DNA < 106 copies in HBeAg-positive patients and HBV DNA < 105 in HBeAg-negative patients) and no obvious fibration, antiviral therapy may be delayed, but monitoring should be performed during pregnancy. In patients with HBV DNA > 107 copies/mL repeated in the late trimester of pregnancy, or prior delivery history of a HBV-positive infant and HBV DNA > 106 copies/mL, antiviral therapy should be administered; Second, in patients with higher HBV DNA level at baseline and obvious fibration, but without liver cirrhosis, antiviral therapy is suggested. If a response is sustained after off-treatment, pregnancy is feasible, monitoring should be performed, and management is the same as that outlined in the first scenario; If a response is not sustained after drug withdrawal, management is the same as the third scenario; Third, If patients have liver cirrhosis before pregnancy, antiviral therapy [lamivudine (LAM), tenofovir (TDF) or telbivudine (LdT)] is suggested first, and one of these drugs should be continued during pregnancy, and monitoring should also be conducted.
To effectively prevent HBV infection in infants, delivery methods are also believed to be potential risk factors for mother-to-infant transmission[31,32], however, there is no clear evidence to show that delivery methods are correlated with reduced vertical transmission of HBV[33-35]. Immediate vaccination with HBVac in combination with HBIG in infants born to CHB mothers can effectively prevent infection in the labor and postnatal period, but has no effect on intrauterine infection[36-38], which is the primary cause leading to failure of vaccination. As both HBV intrauterine and perinatal transmission is significantly correlated with HBV DNA level in pregnant women[39-45], currently most attention is focused on oral antiviral drugs in late pregnancy, which reduce HBV intrauterine transmission by decreasing HBV DNA titers in peripheral blood before delivery[46-48].
The current difficulty in preventing mother-to-infant transmission of HBV is the prevention of intrauterine transmission. High HBV DNA level is the most important independent risk factor for intrauterine transmission, thus pregnant woman can take oral antiviral drugs in late pregnancy to reduce HBV DNA titers in peripheral blood before delivery and decrease HBV intrauterine transmission.
Due to inhibition of cell proliferation, interferon is contraindicated in pregnant women. For patients using interferon, pregnancy is practicable after discontinuation of interferon for 6 mo. LdT and TDF are authorized category B antiviral drugs by the Food and Drug Administration for use in pregnancy. After reviewing the increasing safety data on LAM in clinical practise[49-52], LAM was elevated to a category B antiviral drug in pregnancy by NIH, that is, the category B antiviral drugs in pregnancy include LAM, LdT and TDF[33,53].
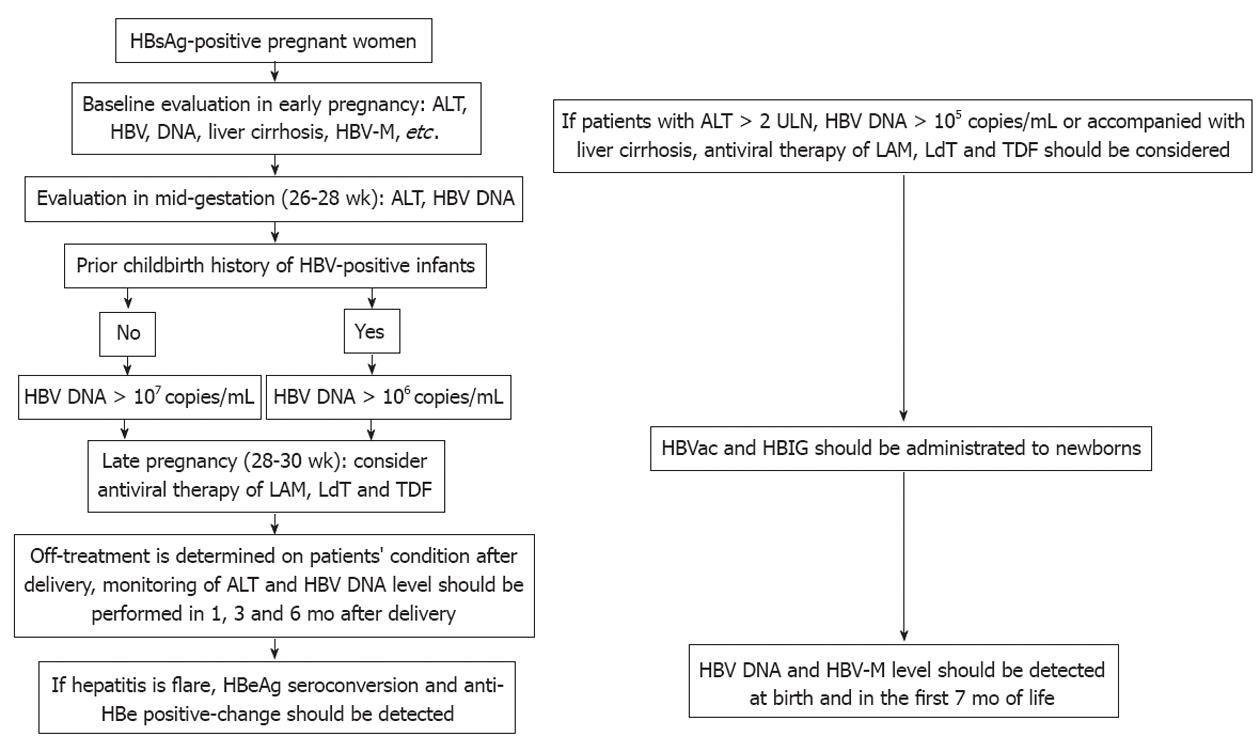
For HBsAg-positive pregnant women by screening, baseline evaluation, such as HBV-M (HBsAg, HBeAg, anti-Hbe), HBV DNA, hepatitis activity and severity of hepatic fibrosis/liver cirrhosis, are suggested in early pregnancy[54-56]. In patients with higher HBV DNA level and hepatitis activity (ALT > 2 upper limit of normal, HBV DNA > 105 copies/mL) at baseline or accompanied by liver cirrhosis, antiviral therapy should be administered during early pregnancy. For patients with normal liver function, ALT and HBV DNA level should be reevaluated in mid-gestation (26-28 wk). For patients with HBV DNA > 107 copies/mL or prior delivery history of HBV-positive infants and HBV DNA > 106 copies/mL, antiviral therapy (LAM, TDF or LdT) should be given at 28-30 wk until 4 wk after delivery, then it should be determined whether the above therapy is to be continued on the basis of the patient’s condition; otherwise antiviral therapy should not be given[57]. ALT and HBV DNA level should be monitored in all HBsAg-positive pregnant women at 1, 3 and 6 mo after delivery. In a hepatitis flare, HBeAg seroconversion and anti-Hbe positive-change should be detected[58]. Active-passive immunization should be performed in all newborns on schedule; HBV-M (HBsAg, HBeAg, anti-HBe) and HBV DNA level should also be detected at birth and in the first 7 mo of life in newborns[59]. In patients with liver cirrhosis before pregnancy, antiviral therapy (LAM, TDF or LdT) are suggested first, one of these drugs should be continued during pregnancy, and monitoring should also be conducted (Figure 1).
For women with an unplanned pregnancy during the course of antiviral therapy for CHB, individualized management is performed according to the actual condition of the patient. There are two options for patients: one is temporal off-treatment and whole course monitoring of HBV DNA and ALT level, in addition, antiviral therapy is based on the patient’s actual condition after pregnancy, which is suitable for patients with mild hepatitis and a lower risk of recurrence and disease progression; the other option is sequential use of LAM, TDF or LdT as antiviral therapy during the whole course[11,36]. Although active-passive immunity should be given to all newborns of HBsAg-positive pregnant women, breast feeding does not increase the risks of HBV infection. However, there is not enough safety data on these drugs with regard to newborn exposure during breast feeding to assess whether patients receiving antiviral therapy should breastfeed their children [57].
Overall, HBV perinatal transmission is a major cause of chronic HBV infection. To reduce the burden of HBV infection, we must consider how to prevent HBV transmission. For women of childbearing age, HBV detection and intervention deserves special attention and investigation.
Peer reviewers: Epameinondas Tsianos, Professor of Internal Medicine, Department of Medicine, University of Ionnina, 45110 Ioannina, Greece; Dr. Richard A Rippe, National Institutes of Health, 5635 Fishers Lane, Rockville, MD 20852, United States; Stephan Menne, Research Associate Professor, Microbiology and Immunology, Georgetown University, Georgetown University Medical Center, 3900 Reservoir Rd., Washington, WA 20057, United States
S- Editor Gou SX L- Editor Webster JR E- Editor Li JY
| 1. | Alter MJ. Epidemiology of hepatitis B in Europe and worldwide. J Hepatol. 2003;39 Suppl 1:S64-S69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Gambarin-Gelwan M. Hepatitis B in pregnancy. Clin Liver Dis. 2007;11:945-963, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 4. | Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y. Epidemiological serosurvey of hepatitis B in China--declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550-6557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 710] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 6. | Ni YH, Chang MH, Huang LM, Chen HL, Hsu HY, Chiu TY, Tsai KS, Chen DS. Hepatitis B virus infection in children and adolescents in a hyperendemic area: 15 years after mass hepatitis B vaccination. Ann Intern Med. 2001;135:796-800. [PubMed] |
| 7. | Zhang L, Xu A, Yan B, Song L, Li M, Xiao Z, Xu Q, Li L. A significant reduction in hepatitis B virus infection among the children of Shandong Province, China: the effect of 15 years of universal infant hepatitis B vaccination. Int J Infect Dis. 2010;14:e483-e488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Jonas MM. Hepatitis B and pregnancy: an underestimated issue. Liver Int. 2009;29 Suppl 1:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ. 2006;332:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 281] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 10. | Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology. 2010;53:20-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Sinha S, Kumar M. Pregnancy and chronic hepatitis B virus infection. Hepatol Res. 2010;40:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Kwon CI, Hwang SG, Shin SJ, Chang SW, Kim SY, Ko KH, Hong SP, Park PW, Rim KS, Kang MS. Occult hepatitis B virus infection in pregnant woman and its clinical implication. Liver Int. 2008;28:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Alexiou VG, Ierodiakonou V, Peppas G, Falagas ME. Antimicrobial prophylaxis in surgery: an international survey. Surg Infect (Larchmt). 2010;11:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 14. | Li XM, Ma L, Yang YB, Shi ZJ, Zhou SS. Analyses of prognostic indices of chronic liver failure caused by hepatitis virus. World J Gastroenterol. 2005;11:2841-2843. [PubMed] |
| 15. | Bhatia V, Singhal A, Panda SK, Acharya SK. A 20-year single-center experience with acute liver failure during pregnancy: is the prognosis really worse? Hepatology. 2008;48:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Hieber JP, Dalton D, Shorey J, Combes B. Hepatitis and pregnancy. J Pediatr. 1977;91:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61-69. [PubMed] |
| 18. | Reinus JF, Leikin EL. Viral hepatitis in pregnancy. Clin Liver Dis. 1999;3:115–130. [DOI] [Full Text] |
| 19. | Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156-S165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 20. | Wong HY, Tan JY, Lim CC. Abnormal liver function tests in the symptomatic pregnant patient: the local experience in Singapore. Ann Acad Med Singapore. 2004;33:204-208. [PubMed] |
| 21. | Tse KY, Ho LF, Lao T. The impact of maternal HBsAg carrier status on pregnancy outcomes: a case-control study. J Hepatol. 2005;43:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 22. | ter Borg MJ, Leemans WF, de Man RA, Janssen HL. Exacerbation of chronic hepatitis B infection after delivery. J Viral Hepat. 2008;15:37-41. [PubMed] |
| 23. | Buster EH, van Erpecum KJ, Schalm SW, Zaaijer HL, Brouwer JT, Gelderblom HC, de Knegt RJ, Minke Bakker C, Reesink HW, Janssen HL. Treatment of chronic hepatitis B virus infection - Dutch national guidelines. Neth J Med. 2008;66:292-306. [PubMed] |
| 24. | Söderström A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Wang Z, Zhang J, Yang H, Li X, Wen S, Guo Y, Sun J, Hou J. Quantitative analysis of HBV DNA level and HBeAg titer in hepatitis B surface antigen positive mothers and their babies: HBeAg passage through the placenta and the rate of decay in babies. J Med Virol. 2003;71:360-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Ngui SL, Andrews NJ, Underhill GS, Heptonstall J, Teo CG. Failed postnatal immunoprophylaxis for hepatitis B: characteristics of maternal hepatitis B virus as risk factors. Clin Infect Dis. 1998;27:100-106. [PubMed] |
| 27. | Sangfelt P, Reichard O, Lidman K, von Sydow M, Forsgren M. Prevention of hepatitis B by immunization of the newborn infant--a long-term follow-up study in Stockholm, Sweden. Scand J Infect Dis. 1995;27:3-7. [PubMed] |
| 28. | Vranckx R, Alisjahbana A, Meheus A. Hepatitis B virus vaccination and antenatal transmission of HBV markers to neonates. J Viral Hepat. 1999;6:135-139. [PubMed] |
| 29. | Lin HH, Chen PJ, Chen DS, Sung JL, Yang KH, Young YC, Liou YS, Chen YP, Lee TY. Postpartum subsidence of hepatitis B viral replication in HBeAg-positive carrier mothers. J Med Virol. 1989;29:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Lin HH, Wu WY, Kao JH, Chen DS. Hepatitis B post-partum e antigen clearance in hepatitis B carrier mothers: Correlation with viral characteristics. J Gastroenterol Hepatol. 2006;21:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Lee SD, Lo KJ, Tsai YT, Wu JC, Wu TC, Yang ZL, Ng HT. Role of caesarean section in prevention of mother-infant transmission of hepatitis B virus. Lancet. 1988;2:833-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Yang J, Zeng XM, Men YL, Zhao LS. Elective caesarean section versus vaginal delivery for preventing mother to child transmission of hepatitis B virus--a systematic review. Virol J. 2008;5:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Tran TT. Management of hepatitis B in pregnancy: weighing the options. Cleve Clin J Med. 2009;76 Suppl 3:S25-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Ahn J, Salem SB, Cohen SM. Evaluation and management of hepatitis B in pregnancy: a survey of current practices. Gastroenterol Hepatol (N Y). 2010;6:570-578. [PubMed] |
| 35. | Song YM, Sung J, Yang S, Choe YH, Chang YS, Park WS. Factors associated with immunoprophylaxis failure against vertical transmission of hepatitis B virus. Eur J Pediatr. 2007;166:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | del Canho R, Grosheide PM, Mazel JA, Heijtink RA, Hop WC, Gerards LJ, de Gast GC, Fetter WP, Zwijneberg J, Schalm SW. Ten-year neonatal hepatitis B vaccination program, The Netherlands, 1982-1992: protective efficacy and long-term immunogenicity. Vaccine. 1997;15:1624-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Lin HH, Chang MH, Chen DS, Sung JL, Hong KH, Young YC, Yang KH, Lee TY. Early predictor of the efficacy of immunoprophylaxis against perinatal hepatitis B transmission: analysis of prophylaxis failure. Vaccine. 1991;9:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Boot HJ, Hahné S, Cremer J, Wong A, Boland G, van Loon AM. Persistent and transient hepatitis B virus (HBV) infections in children born to HBV-infected mothers despite active and passive vaccination. J Viral Hepat. 2010;17:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Wiseman E, Fraser MA, Holden S, Glass A, Kidson BL, Heron LG, Maley MW, Ayres A, Locarnini SA, Levy MT. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489-492. [PubMed] |
| 40. | Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, Liu ZH, Wang FS. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Shin JI, Namgung R, Park MS, Park KI, Lee C. Immunoprophylaxis failure against vertical transmission of hepatitis B virus: what is the mechanism and do other factors also play a role? Eur J Pediatr. 2008;167:489-490. [PubMed] |
| 42. | Singh AE, Plitt SS, Osiowy C, Surynicz K, Kouadjo E, Preiksaitis J, Lee B. Factors associated with vaccine failure and vertical transmission of hepatitis B among a cohort of Canadian mothers and infants. J Viral Hepat. 2011;18:468-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418-1423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 144] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | del Canho R, Grosheide PM, Schalm SW, de Vries RR, Heijtink RA. Failure of neonatal hepatitis B vaccination: the role of HBV-DNA levels in hepatitis B carrier mothers and HLA antigens in neonates. J Hepatol. 1994;20:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Abstracts of the 59th Annual Meeting of the American Association for the Study of Liver Diseases, 2008. October 31-November 4, 2008. San Francisco, California, USA. Hepatology. 2008;48 Suppl 1:309A-1187A. [PubMed] |
| 46. | Buchanan C, Tran TT. Management of chronic hepatitis B in pregnancy. Clin Liver Dis. 2010;14:495-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Chen LZ, Zhou WQ, Zhao SS, Liu ZY, Wen SW. A nested case-control study of maternal-neonatal transmission of hepatitis B virus in a Chinese population. World J Gastroenterol. 2011;17:3640-3644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Han GR, Cao MK, Zhao W, Jiang HX, Wang CM, Bai SF, Yue X, Wang GJ, Tang X, Fang ZX. A prospective and open-label study for the efficacy and safety of telbivudine in pregnancy for the prevention of perinatal transmission of hepatitis B virus infection. J Hepatol. 2011;55:1215-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 274] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 49. | Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok AS. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 50. | Antiretroviral Pregnancy Registry Steering Committee . Antiretroviral Pregnancy Registry International interim report for 1 January 1989-31 July 2010. Antiviral Pregnancy Registry, July. 2010; Available from: http: //www.apregistry.com/forms/interim_report.pdf. |
| 51. | van Zonneveld M, van Nunen AB, Niesters HG, de Man RA, Schalm SW, Janssen HL. Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2003;10:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 218] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 52. | Xu WM, Cui YT, Wang L, Yang H, Liang ZQ, Li XM, Zhang SL, Qiao FY, Campbell F, Chang CN. Lamivudine in late pregnancy to prevent perinatal transmission of hepatitis B virus infection: a multicentre, randomized, double-blind, placebo-controlled study. J Viral Hepat. 2009;16:94-103. [PubMed] |
| 53. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1154] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 54. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2169] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 55. | Chotiyaputta W, Lok AS. Role of antiviral therapy in the prevention of perinatal transmission of hepatitis B virus infection. J Viral Hepat. 2009;16:91-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Boland GJ, Veldhuijzen IK, Janssen HL, van der Eijk AA, Wouters MG, Boot HJ. [Management and treatment of pregnant women with hepatitis B]. Ned Tijdschr Geneeskd. 2009;153:A905. [PubMed] |
| 57. | Petersen J. HBV treatment and pregnancy. J Hepatol. 2011;55:1171-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 58. | Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, Crowther L, Condreay LD, Woessner M, Rubin M. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1007] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 59. | Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, Moyer LA, Bell BP, Alter MJ. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1-31. [PubMed] |