Published online Sep 7, 2012. doi: 10.3748/wjg.v18.i33.4481
Revised: August 13, 2012
Accepted: August 16, 2012
Published online: September 7, 2012
Several receptors have been identified as implicated on viral entry into the hepatocyte; and, this interaction between the virus and potential receptors could modulate infection, spontaneous viral clearance, persistence of the infection and the widespread of the virus as outbreak. Nevertheless, the playing role of each of them remains controversial. The Niemann-Pick type C1 like 1 gene (NPC1L1) receptor has been recently implicated on hepatitis C virus (HCV) entry into the cell and ezetimibe, an anti-cholesterol drug seems to block that, emerging the idea to control hepatitis C outbreak modulating lipid-related receptors. Hepatitis C infection seems to modulate lipid metabolism according to host genetic background. Indeed, it circulates like a lipoviroparticle. The main aim of this field of vision would be to discuss the role of hepatocyte receptors implicated on virus entry, especially NPC1L1 and the therapeutic options derived from the better knowledge about HCV-lipids- receptors interaction.
- Citation: Del Campo JA, Rojas &, Romero-Gómez M. Entry of hepatitis C virus into the cell: A therapeutic target. World J Gastroenterol 2012; 18(33): 4481-4485
- URL: https://www.wjgnet.com/1007-9327/full/v18/i33/4481.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i33.4481
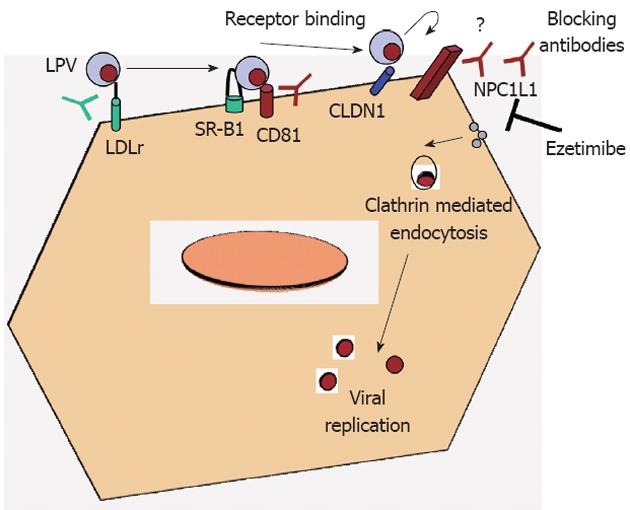
Viral entry is the first step of virus-host cell interactions and, is a highly orchestrated process involving several viral and host cell factors. Indeed, the virus is able to escape from neutralizing antibodies and promote direct cell-cell transmission. Understanding the mechanisms of viral entry and escape is a prerequisite to define the viral and cellular targets that will give broad protection against hepatitis C virus (HCV) infection. HCV is an enveloped single-strand RNA virus that mainly targets hepatocytes. Due to the difficulty to grow HCV in vitro and the species specificity of this virus, surrogate model systems have been developed to study HCV entry into hepatocytes: recombinant envelope glycoproteins[1], HCV-like particles[2], HCV pseudo-particles[3,4] and recombinant infectious HCV[5-7] have been used to study the interactions of the viral envelope with human hepatoma cells or primary human hepatocytes. Four cellular factors have been described as essential for HCV entry (Figure 1): the tetraspanin molecule CD81, the scavenger receptor class B member I and the tight junction proteins claudin-1 and occludin[8]. Interestingly, neutralizing antibodies against CD81 are able to block HCV entry in vitro and also in immunodeficient mice transplanted with human hepatocytes, the best currently available small animal model of HCV infection[9]. Neutralizing antibodies against the HCV envelope proteins E1 and E2 could also represent a promising approach to avoid HCV infection, but the main challenge here is the enormous genetic variability of the virus. The requirement for sequential interactions between the viral envelope and key host receptors/co-receptors may provide new drug targets that could be exploited by small-molecule inhibitors. Recently, Syder et al[10] discovered and optimized a series of 1,3,5-triazine compounds that are potent, selective and non-cytotoxic inhibitors of HCV entry. Representative compounds fully suppress both cell-free virus and cell-to-cell spread of HCV in vitro. To date, only one oral HCV entry inhibitor with a defined mechanism of action, ITX-5061, has entered clinical testing (phase 2a)[11]. ITX-5061 binds directly to SR-B1 and blocks a key post-binding step in the viral entry process. In chronically infected patients we cannot find a single isolate of HCV but rather a population of related yet different viral variants (quasispecies), containing a vast repertoire of preformed variants that allow rapid escape from selective pressures such as neutralizing antibodies or anti-viral drugs. Recently, two well-known molecules have been shown to inhibit HCV entry: the green tea catechin epigallocatechin-3-gallate (EGCG) and the tyrosine kinase inhibitor erlotinib. Erlotinib blocks HCV entry by inhibition of the activity of the epidermal grow factor -receptor which is required for formation of CD81-claudin-1 co-receptor associations[12]. EGCG inhibits viral attachment to the target cell as well as cell-to-cell transmission between adjacent cells.
Sainz et al[13] have recently discovered a novel surface receptor involved in HCV entry, the Niemann-Pick C1-like 1 cholesterol absorption receptor (NPC1L1), a 13transmembrane-domain cell surface cholesterol-sensing receptor, expressed on the apical surface of intestinal enterocytes and human hepatocytes, including Huh7 cells, is responsible for cellular cholesterol absorption and whole-body cholesterol homeostasis. NPC1L1 was first identified as a homolog of Niemann-Pick C1 protein[14], the deficiency of which causes Niemann-Pick disease type C1, a genetic disorder characterized by intracellular accumulation of unesterified cholesterol in the endosomal/lysosomal system of neurons that causes neurodegeneration and premature death[15,16]. Human NPC1L1 gene maps to chromosome 7p13, spans 29 kb, encodes a 5 kb mRNA and predominantly produces a protein of 1332 amino acids[17]. NPC1L1 locates to the brush border membrane of the enterocyte and the canalicular membrane of the hepatocyte. Biliary cholesterol, which is secreted into bile by hepatocytes, accounts for more than two thirds of the total amount of cholesterol in the gut lumen. Since the cholesterol is water-insoluble, it is delivered to the brush border membranes by bile salt micelles.
These authors have shown that using NPC1L1-specific antibody, HCV infection was reduced in a similar way that CD-81 specific antibodies did. Ezetimibe is a 2 azetidinone-class drug that has been approved by the Food and Drug Administration as a cholesterol-lowering medication[18]. Sainz et al[13] also shown the role of NPC1L1 receptor on HCV infection in vivo, using immunodeficiency mice repopulated with human hepatocytes and treated them via oral gavage with ezetimibe. They have demonstrated that ezetimibe treatment delayed the establishment of HCV infection in mice pretreated for 2 wk, confirming the ability of this drug to inhibit HCV infection in vivo. However, ezetimibe concentration in those experiments was high (30 μmol/L). That means for a 70 kg weight adult to ingest 84 ezetimibe 10 mg tablets per day, when the usual doses for ezetimibe is one 10 mg tablet per day. So far, an ezetimibe based therapy for HCV does not seem suitable. Probably the use of antibodies against NPC1L1 would be a better alternative.
HCV infection is tightly associated with alterations in lipid metabolism and lipids have been shown to play important roles during the viral replication cycle[19,20]. Indeed, recent studies based on transcriptome and proteomic analyses have demonstrated that expression of host genes involved in the biosynthesis, degradation and transport of intracellular lipids is profoundly altered upon infection. The expression of sterol regulatory element binding proteins, which control transcription of genes required for cholesterol biosynthesis, is stimulated by HCV infection. In agreement with this, the expression of fatty acid synthase (FASN) and other genes related to the synthesis and transport of fatty acids is upregulated in infected cells[21,22]. Moreover, the inhibition of FASN activity blocks HCV RNA replication and production of infectious virus particles[23]. Finally, expression of genes regulating geranylgeranylation of cellular proteins important for HCV replication is also upregulated in HCV infected cells[21] .
HCV from infected patients (sera) can be precipitated with antibodies against lipoproteins (LP), indicating that HCV circulates associated to LP. Removal of LP by apheresis reduced HCV RNA level by 77%, suggesting that most of the viral particles are tightly associated with lipoproteins[24]. Two different studies[25] suggest that infectious HCV particles are highly associated with LP. Lipoproteins are easily endocytosed, supporting the hyphotesis that HCV can use this association to LP to adhere the cell and subsequently enter into the host cell by endocytosis rather than direct fusion to the membrane[26].
Lipids droplets are necessary for the lipoviroparticle formation. HCV is hypothesized to initiate assembly in close association with lipid droplets by coating lipid droplets with the core protein and bringing together nonstructural (NS) and structural proteins in a NS2-dependent manner[27-29]. Following capsid assembly, nascent virions bud into the lumen of the endoplasmic reticulum (ER) where the glycoproteins E1/E2 reside in addition to the very low density lipoprotein (VLDL) secretion machinery. HCV is infectious upon envelopment at the ER, and it is thought that apolipoprotein E (ApoE) is acquired early during assembly because knockdown of ApoE reduces intracellular and extracellular virus; also NS5A interacts with ApoE[30,31]. Core proteins disturb microsomal triglyceride transfer protein (MTP) activity in the hepatocyte[32] and it has been described that NS5A could be interfered with MTP function. MTP is an essential chaperone for the assembly of VLDL, which transfers triglyceride, phospholipids, and cholesterol from the hepatocytes. Reduced activity of MTP results in decreased secretion of VLDL, leading to lipid accumulation. This fact could explain development of steatosis.
The low-density lipoprotein receptor (LDLr) was proposed as a potential entry factor for HCV[33], however, its implication in virus entry remains unclear. Moreover, by using HCV particles isolated from patients, a correlation has been shown between the accumulation of HCV RNA into primary hepatocytes, expression of LDLr messenger RNA, and LDL entry[34]. The potential involvement of the LDLr in HCV entry has also been reported in the HCVcc system[35]. Albecka et al[36] have shown that HCV particles can interact with the LDLr. However, this interaction does not necessarily lead to a productive infection. Furthermore, those data indicate a role for the LDLr as a lipid-providing receptor, which modulates viral RNA replication.
Quercetin, an abundant flavonoid found in fruits and vegetable, has been implicated in lowering the risk of cardiovascular disease that is often associated with high plasma levels of LDL cholesterol. Quercetin was found to inhibit NS3 activity in a specific dose-dependent manner in an in vitro catalysis assay, also inhibiting HCV RNA replication in the subgenomic HCV RNA replicon system and virus production in the HCV infectious cell culture system[37]. Gonzalez et al[38] have shown the marked reduction in viral production imparted by heat shock proteins synthesis inhibitor Quercetin. The low toxicity and pharmacokinetics of Quercetin are well known, and it has been approved for other uses in clinical trials. In fact, a phase I study evaluating the safety and tolerability of Quercetin in hepatitis C patients who have contraindications to standard antiviral treatment started last year (http://www.clinicaltrials.gov).
Administration of the exogenous interferons (IFNs) alpha, beta, and gamma in the setting of treatment for chronic HCV infection and other conditions has been shown to lower LDL cholesterol and raise triglyceride levels in VLDL, concomitant with suppression of lipoprotein lipase[39,40]. Li et al[41] have shown an association between rs12979860 genotype and host serum lipid levels, suggesting a relationship between endogenous IFN response and lipids. They hypothesize that the IFN-lambda rs12979860 CC responder genotype, which was associated with both increased likelihood of treatment response and higher LDL cholesterol levels in the studied cohort, is associated with lower IFN-lambda activity or lower intrahepatic IFN signaling gene expression. Our results indicate that LDL and total cholesterol levels were higher in patients infected with HCV genotype 1 harbouring the favourable genotype for interleukin 28B gene (Del Campo et al[29] unpublished data). These results suggest that observed associations are directly related to HCV-host interactions instead of a direct effect of this locus on lipid metabolism. At least in part, this host factor could select virus infection and promote chronic infection or spontaneous clearance according to viral genotype and lipid metabolism interplay.
HCV entry is a highly orchestrated process involving several viral and host cell factors, affecting infection, spontaneous viral clearance, persistent infection and widespread, and thereby offering multiple novel targets for antiviral therapy. A recently discovered novel surface receptor involved in HCV entry, NPC1L1, is responsible for cellular cholesterol absorption. This receptor arose as a new therapeutic target for HCV infection, since specific antibodies can block HCV entry. Ezetimibe treatment delayed the establishment of HCV infection in an animal model, emphasizing the relevance of the interaction between the host lipid metabolism and the establishment of a persistent infection, although utilized doses could not be translate to clinical practice. Host and virus genetic variation together with the interaction with hepatocyte receptors were assumed to explain the heterogeneity in HCV outcomes across individuals.
Peer reviewer: Ferruccio Bonino, MD, PhD, Professor of Gas-troenterology, Director of General Medicine 2 Unit, Di-rector of Liver and Digestive Disease Division, Department of Internal Medicine, University Hospital of Pisa, University of Pisa, Via Roma 67, 56124 Pisa, Italy
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Rosa D, Campagnoli S, Moretto C, Guenzi E, Cousens L, Chin M, Dong C, Weiner AJ, Lau JY, Choo QL. A quantitative test to estimate neutralizing antibodies to the hepatitis C virus: cytofluorimetric assessment of envelope glycoprotein 2 binding to target cells. Proc Natl Acad Sci USA. 1996;93:1759-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 259] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 2. | Baumert TF, Ito S, Wong DT, Liang TJ. Hepatitis C virus structural proteins assemble into viruslike particles in insect cells. J Virol. 1998;72:3827-3836. [PubMed] |
| 3. | Bartosch B, Dubuisson J, Cosset FL. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J Exp Med. 2003;197:633-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 885] [Cited by in RCA: 879] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 4. | Hsu M, Zhang J, Flint M, Logvinoff C, Cheng-Mayer C, Rice CM, McKeating JA. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA. 2003;100:7271-7276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 644] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 5. | Wakita T, Pietschmann T, Kato T, Date T, Miyamoto M, Zhao Z, Murthy K, Habermann A, Kräusslich HG, Mizokami M. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat Med. 2005;11:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2275] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 6. | Lindenbach BD, Evans MJ, Syder AJ, Wölk B, Tellinghuisen TL, Liu CC, Maruyama T, Hynes RO, Burton DR, McKeating JA. Complete replication of hepatitis C virus in cell culture. Science. 2005;309:623-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1849] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 7. | Zhong J, Gastaminza P, Cheng G, Kapadia S, Kato T, Burton DR, Wieland SF, Uprichard SL, Wakita T, Chisari FV. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294-9299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1475] [Cited by in RCA: 1466] [Article Influence: 73.3] [Reference Citation Analysis (0)] |
| 8. | von Hahn T, Steinmann E, Ciesek S, Pietschmann T. Know your enemy: translating insights about the molecular biology of hepatitis C virus into novel therapeutic approaches. Expert Rev Gastroenterol Hepatol. 2010;4:63-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Coburn GA, Fisch DN, Moorji SM, de Muys JM, Murga JD, Paul D, Provoncha KP, Rotshteyn Y, Han AQ, Qian D. Novel small-molecule inhibitors of hepatitis C virus entry block viral spread and promote viral clearance in cell culture. PLoS One. 2012;7:e35351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Syder AJ, Lee H, Zeisel MB, Grove J, Soulier E, Macdonald J, Chow S, Chang J, Baumert TF, McKeating JA. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J Hepatol. 2011;54:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Lupberger J, Zeisel MB, Xiao F, Thumann C, Fofana I, Zona L, Davis C, Mee CJ, Turek M, Gorke S. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat Med. 2011;17:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 569] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 12. | Ciesek S, von Hahn T, Colpitts CC, Schang LM, Friesland M, Steinmann J, Manns MP, Ott M, Wedemeyer H, Meuleman P. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology. 2011;54:1947-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 13. | Sainz B, Barretto N, Martin DN, Hiraga N, Imamura M, Hussain S, Marsh KA, Yu X, Chayama K, Alrefai WA. Identification of the Niemann-Pick C1-like 1 cholesterol absorption receptor as a new hepatitis C virus entry factor. Nat Med. 2012;18:281-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 349] [Cited by in RCA: 353] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 14. | Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1132] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 16. | Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA. Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science. 1997;277:232-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 634] [Cited by in RCA: 645] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 17. | Altmann SW, Davis HR, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1297] [Cited by in RCA: 1295] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 18. | Bays HE, Neff D, Tomassini JE, Tershakovec AM. Ezetimibe: cholesterol lowering and beyond. Expert Rev Cardiovasc Ther. 2008;6:447-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Negro F. Correction of insulin resistance in chronic hepatitis C patients not responding to the standard of care: more questions than answers. J Hepatol. 2009;50:1271-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561-2566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 406] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 21. | Blackham S, Baillie A, Al-Hababi F, Remlinger K, You S, Hamatake R, McGarvey MJ. Gene expression profiling indicates the roles of host oxidative stress, apoptosis, lipid metabolism, and intracellular transport genes in the replication of hepatitis C virus. J Virol. 2010;84:5404-5414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Woodhouse SD, Narayan R, Latham S, Lee S, Antrobus R, Gangadharan B, Luo S, Schroth GP, Klenerman P, Zitzmann N. Transcriptome sequencing, microarray, and proteomic analyses reveal cellular and metabolic impact of hepatitis C virus infection in vitro. Hepatology. 2010;52:443-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Yang W, Hood BL, Chadwick SL, Liu S, Watkins SC, Luo G, Conrads TP, Wang T. Fatty acid synthase is up-regulated during hepatitis C virus infection and regulates hepatitis C virus entry and production. Hepatology. 2008;48:1396-1403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Schettler V, Monazahian M, Wieland E, Ramadori G, Grunewald RW, Thomssen R, Müller GA. Reduction of hepatitis C virus load by H.E.L.P.-LDL apheresis. Eur J Clin Invest. 2001;31:154-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Bradley D, McCaustland K, Krawczynski K, Spelbring J, Humphrey C, Cook EH. Hepatitis C virus: buoyant density of the factor VIII-derived isolate in sucrose. J Med Virol. 1991;34:206-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | André P, Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, Pol S, Bréchot C, Paranhos-Baccalà G, Lotteau V. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919-6928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 495] [Cited by in RCA: 517] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 27. | Jirasko V, Montserret R, Lee JY, Gouttenoire J, Moradpour D, Penin F, Bartenschlager R. Structural and functional studies of nonstructural protein 2 of the hepatitis C virus reveal its key role as organizer of virion assembly. PLoS Pathog. 2010;6:e1001233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 28. | Ma Y, Anantpadma M, Timpe JM, Shanmugam S, Singh SM, Lemon SM, Yi M. Hepatitis C virus NS2 protein serves as a scaffold for virus assembly by interacting with both structural and nonstructural proteins. J Virol. 2011;85:86-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Del Campo JA, Romero-Gómez M. Steatosis and insulin resistance in hepatitis C: a way out for the virus? World J Gastroenterol. 2009;15:5014-5019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Benga WJ, Krieger SE, Dimitrova M, Zeisel MB, Parnot M, Lupberger J, Hildt E, Luo G, McLauchlan J, Baumert TF. Apolipoprotein E interacts with hepatitis C virus nonstructural protein 5A and determines assembly of infectious particles. Hepatology. 2010;51:43-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Cun W, Jiang J, Luo G. The C-terminal alpha-helix domain of apolipoprotein E is required for interaction with nonstructural protein 5A and assembly of hepatitis C virus. J Virol. 2010;84:11532-11541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Domitrovich AM, Felmlee DJ, Siddiqui A. Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J Biol Chem. 2005;280:39802-39808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Agnello V, Abel G, Elfahal M, Knight GB, Zhang QX. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc Natl Acad Sci USA. 1999;96:12766-12771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 705] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 34. | Molina S, Castet V, Fournier-Wirth C, Pichard-Garcia L, Avner R, Harats D, Roitelman J, Barbaras R, Graber P, Ghersa P. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J Hepatol. 2007;46:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 35. | Owen DM, Huang H, Ye J, Gale M. Apolipoprotein E on hepatitis C virion facilitates infection through interaction with low-density lipoprotein receptor. Virology. 2009;394:99-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 174] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Albecka A, Belouzard S, Op de Beeck A, Descamps V, Goueslain L, Bertrand-Michel J, Tercé F, Duverlie G, Rouillé Y, Dubuisson J. Role of low-density lipoprotein receptor in the hepatitis C virus life cycle. Hepatology. 2012;55:998-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 37. | Bachmetov L, Gal-Tanamy M, Shapira A, Vorobeychik M, Giterman-Galam T, Sathiyamoorthy P, Golan-Goldhirsh A, Benhar I, Tur-Kaspa R, Zemel R. Suppression of hepatitis C virus by the flavonoid quercetin is mediated by inhibition of NS3 protease activity. J Viral Hepat. 2012;19:e81-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Gonzalez O, Fontanes V, Raychaudhuri S, Loo R, Loo J, Arumugaswami V, Sun R, Dasgupta A, French SW. The heat shock protein inhibitor Quercetin attenuates hepatitis C virus production. Hepatology. 2009;50:1756-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 39. | Shinohara E, Yamashita S, Kihara S, Hirano K, Ishigami M, Arai T, Nozaki S, Kameda-Takemura K, Kawata S, Matsuzawa Y. Interferon alpha induces disorder of lipid metabolism by lowering postheparin lipases and cholesteryl ester transfer protein activities in patients with chronic hepatitis C. Hepatology. 1997;25:1502-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Andrade RJ, García-Escaño MD, Valdivielso P, Alcántara R, Sánchez-Chaparro MA, González-Santos P. Effects of interferon-beta on plasma lipid and lipoprotein composition and post-heparin lipase activities in patients with chronic hepatitis C. Aliment Pharmacol Ther. 2000;14:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Li JH, Lao XQ, Tillmann HL, Rowell J, Patel K, Thompson A, Suchindran S, Muir AJ, Guyton JR, Gardner SD. Interferon-lambda genotype and low serum low-density lipoprotein cholesterol levels in patients with chronic hepatitis C infection. Hepatology. 2010;51:1904-1911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |