Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4435
Revised: August 16, 2012
Accepted: August 18, 2012
Published online: August 28, 2012
AIM: To investigate the long-term outcomes of endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) with a fully covered self-expandable metallic stent (FCSEMS).
METHODS: From April 2009 to August 2010, 15 patients with distal malignant biliary obstructions who were candidates for alternative techniques for biliary decompression due to a failed endoscopic retrograde cholangiopancreatography (ERCP) were included. These 15 patients consisted of 8 men and 7 women and had a median age of 61 years (range: 30-91 years). The underlying causes of the distal malignant biliary obstruction were pancreatic cancer (n = 9), ampulla of Vater cancer (n = 2), renal cell carcinoma (n = 1), advanced gastric cancer (n = 1), lymphoma (n = 1), and duodenal cancer (n = 1).
RESULTS: The technical success rate of EUS-CDS with an FCSEMS was 86.7% (13/15), and functional success was achieved in 100% (13/13) of those cases. In two patients, the EUS-CDS failed because an FCSEMS with a delivery device could not be passed into the common bile duct. The mean duration of stent patency was 264 d. Early adverse events developed in three patients (3/13, 23.1%), including self-limited pneumoperitoneum in two patients and cholangitis requiring stent reposition in one patient. During the follow-up period (median: 186 d, range: 52-388 d), distal stent migration occurred in four patients (4/13, 30.8%). In 3 patients, the FCSEMS could be reinserted through the existing choledochoduodenal fistula tract.
CONCLUSION: EUS-CDS with an FCSEMS is technically feasible and can lead to effective palliation of distal malignant biliary obstructions after failed ERCP.
- Citation: Song TJ, Hyun YS, Lee SS, Park DH, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol 2012; 18(32): 4435-4440
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4435.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4435
A self-expandable metallic stent insertion is a well-established palliative treatment that has been verified in numerous studies for patients with inoperable malignant biliary obstructions[1-4]. Although a self-expandable metallic stent can be inserted via the transpapillary route in most cases using endoscopic retrograde cholangiopancreatography (ERCP), a metallic stent insertion may be impossible due to duodenal obstruction, altered anatomy due to a previous operation (e.g., Roux-en-Y anastomosis), or tumor invasion of the major duodenal papilla[5,6]. Until recently, percutaneous transhepatic biliary drainage (PTBD) has mainly been used in these cases. PTBD, however, is often accompanied by procedure-related adverse events and by many issues related to external drainage, such as pain, catheter dislodgement, and cosmetic problems. Thus, PTBD may cause a serious decline in the quality of life[7,8].
Since the endoscopic ultrasound (EUS)-guided bile duct puncture was first reported in 1996, sporadic case reports on EUS-guided biliary drainage (EUS-BD) suggested that it was a feasible and effective alternative in patients with failed conventional ERCP stenting[9-13]. Currently, three types of EUS-BD have been described, depending on the route of intervention [e.g., EUS-guided choledochoduodenostomy (EUS-CDS), EUS-guided hepaticogastrostomy, and EUS-guided gallbladder drainage][5,14,15]. Plastic stents usually have been used in the EUS-BD procedure, but several studies suggested that EUS-BD with a fully covered self-expandable metallic stent (FCSEMS) might be a feasible and useful alternative to PTBD[6,16]. Although EUS-CDS with an FCSEMS is expected to show longer patency duration and fewer adverse events in patients with malignant biliary obstructions, to the best of our knowledge there have been no studies that address the long-term follow-up results of such a procedure. Therefore, we studied the long-term outcomes of EUS-CDS with an FCSEMS after failed conventional ERCP.
From April 2009 to August 2010, a total of 2844 ERCPs were performed in a 2680-bed tertiary referral hospital; of these, 926 ERCPs were performed to relieve biliary obstruction. Endoscopic transpapillary biliary drainage failed in 115 (12.4%) patients, with 69 patients undergoing PTBD and 46 patients undergoing EUS-BD. EUS-CDS with an FCSEMS was attempted in 15 patients.
These 15 patients included 8 men and 7 women and had a median age of 61 years (range: 30-91 years). The causes of distal malignant biliary obstruction were 9 pancreatic cancers, 2 ampulla of Vater cancers, 1 renal cell carcinoma, 1 advanced gastric cancer, 1 lymphoma, and 1 duodenal cancer. The inclusion criteria were the presence of a distal malignant biliary obstruction and failed conventional ERCP stenting, and the exclusion criteria were an inability to sedate the patient due to advanced heart or pulmonary diseases and a lack of informed consent. Finally, we excluded any patients who had been included in previous publications[6].
Five experienced endoscopists (Lee SS, Park DH, Seo DW, Lee SK and Kim MH) performed the ERCP procedures, and two of them (Lee SS and Park DH) performed the EUS-CDS. These two endosonographers perform more than 500 EUS procedures for pancreaticobiliary diseases annually. This study was approved by the Institutional Review Board of our center. All patients provided written informed consent.
We administered broad-spectrum, prophylactic antibiotics directed against gram-positive and gram-negative organisms before the procedure to minimize the risk of infection. Initially, ERCP was performed using a therapeutic duodenoscope (TJF-260, Olympus Optical, Tokyo, Japan). When the ERCP was unsuccessful, the EUS-CDS was performed using a linear-array echoendoscope (GF-UCT 240-AL 5) during the same endoscopy session or 1-2 d later.
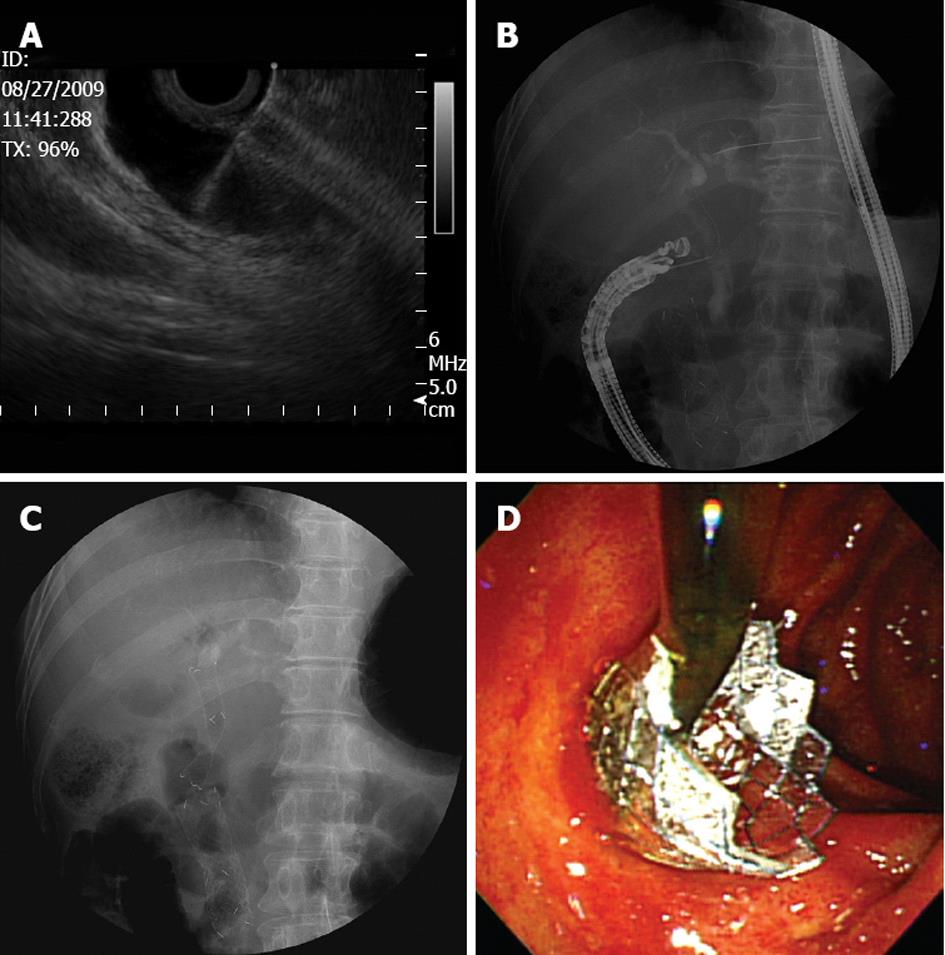
The dilated extrahepatic duct was usually accessed with the echoendoscope placed at the duodenal bulb. The initial puncture was performed under real-time ultrasound and color Doppler guidance with a 19-gauge aspiration needle (EUSN-19-T, Cook Endoscopy, Winston-Salem, NC). After the puncture, the aspiration of bile and cholangiography was performed to confirm that there was an adequate puncture. Next, a 0.0889 cm guidewire was inserted through the needle and coiled in the bile duct lumen. The needle was exchanged for a 6F and a 7F tapered biliary dilator catheter (catheter tip, 4F; Cook Endoscopy, Winston-Salem, NC) to dilate the tract. If there was resistance to the advance of the dilator catheter, a triple-lumen needle-knife (Microtome, Boston Scientific, Natick, MA) with a 7F shaft diameter was gently inserted over the guidewire to dilate the tract using a brief burst of pure cutting current. Finally, an FCSEMS with an 8F deployment system (nitinol stent, 8 to 10 mm in diameter, 4 to 6 cm in length, and flared at both ends to prevent distal or proximal migration; BONASTENT, Standard Sci Tech, Seoul, South Korea) was inserted under echoendoscopic and fluoroscopic guidance (Figure 1).
Technical success was defined as the passage of a metallic stent across the duodenum, along with the flow of contrast medium and/or bile through the stent. Functional success was defined as a decrease in serum total bilirubin to < 75% of the pretreatment value within 4 wk. An early adverse event was defined as any stent-related adverse event within 4 wk, including pneumoperitoneum, bleeding, biloma, bile peritonitis, and stent migration. A late adverse event was defined as any stent-related adverse event occurring > 4 wk after the stent placement, such as stent migration or stent occlusion. Biliary re-intervention was defined as any type of endoscopic, percutaneous, or surgical procedure that was required to improve biliary drainage after the stent placement. The duration of stent patency was defined as the time between functionally successful EUS-CDS and the occurrence of stent occlusion, stent revision, or patient death.
Follow-up data were prospectively collected after the procedure until September 2011. Biochemical parameters and a simple abdominal X-ray were assessed on the day following the procedure, 1 wk after stent placement, and every month thereafter. The follow-up results of the patients were based on the findings from outpatient examinations.
The cumulative patency duration of the EUS-CD with an FCSEMS was estimated using the Kaplan-Meier technique. All statistical analyses were performed using SPSS software (version 12.0; SPSS Inc., Chicago, IL).
EUS-CDS with an FCSEMS was performed in 15 patients with a technical success rate of 86.7% (13/15). In two patients, the FCSEMS with a delivery device could not be passed into the CBD through the guidewire even after dilatation of the fistula tract with a needle knife and use of a 4-mm dilatation balloon because of the acute angulation of the scope. A 7F plastic stent, which is more flexible than an FCSEMS, was successfully inserted in one of these two patients, and PTBD was performed in the other patient as a rescue method.
The functional success rate of EUS-CDS with an FCSEMS was 100% (13/13). The baseline demographic characteristics of the 13 patients who underwent successful EUS-CDS with an FCSEMS are shown in Table 1.
| No. | Age/sex | Diagnosis | Reason for failed ERCP | Diameter/length of FCSEMS (mm/mm) | Early adverse events | Late adverse events | Re-intervention |
| 1 | 74/M | Pancreatic cancer | Periampullary tumor infiltration | 8/40 | None | None | None |
| 2 | 63/F | Pancreatic cancer | Duodenal obstruction | 10/50 | None | Distal migration | Reinsertion of FCSEMS |
| 3 | 56/M | Pancreatic cancer | Periampullary tumor infiltration | 8/50 | Pneumoperitoneum | None | None |
| 4 | 78/F | Pancreatic cancer | Periampullary tumor infiltration | 8/60 | Pneumoperitoneum | None | None |
| 5 | 75/F | Pancreatic cancer | Periampullary tumor infiltration | 8/60 | None | Distal migration | Reinsertion of FCSEMS |
| 6 | 46/M | DLBL | Duodenal obstruction | 8/50 | None | None | None |
| 7 | 91/M | AOV cancer | Periampullary tumor infiltration | 8/60 | None | Distal migration | Reinsertion of FCSEMS |
| 8 | 59/F | RCC | Duodenal obstruction | 8/60 | None | Distal migration | None |
| 9 | 30/M | Pancreatic cancer | Duodenal obstruction | 8/50 | None | None | None |
| 10 | 68/M | AOV cancer | Periampullary tumor infiltration | 8/50 | None | None | None |
| 11 | 54/F | Pancreatic cancer | Periampullary tumor infiltration | 8/60 | None | None | None |
| 12 | 45/F | AGC | Periampullary tumor infiltration | 8/60 | Cholangitis | None | Repositioning of stent |
| 13 | 58/M | Duodenal cancer | Duodenal obstruction | 8/60 | None | None | None |
Early adverse events developed in 3 patients (3/13, 23.1%), including 2 cases of self-limited pneumoperitoneum and 1 case of cholangitis. In 1 patient, the proximal tip of the stent was placed in the left intrahepatic duct, impairing drainage of the right intrahepatic duct and leading to cholangitis of the right intrahepatic duct. The cholangitis improved after the tip of the stent was repositioned below the hilar region.
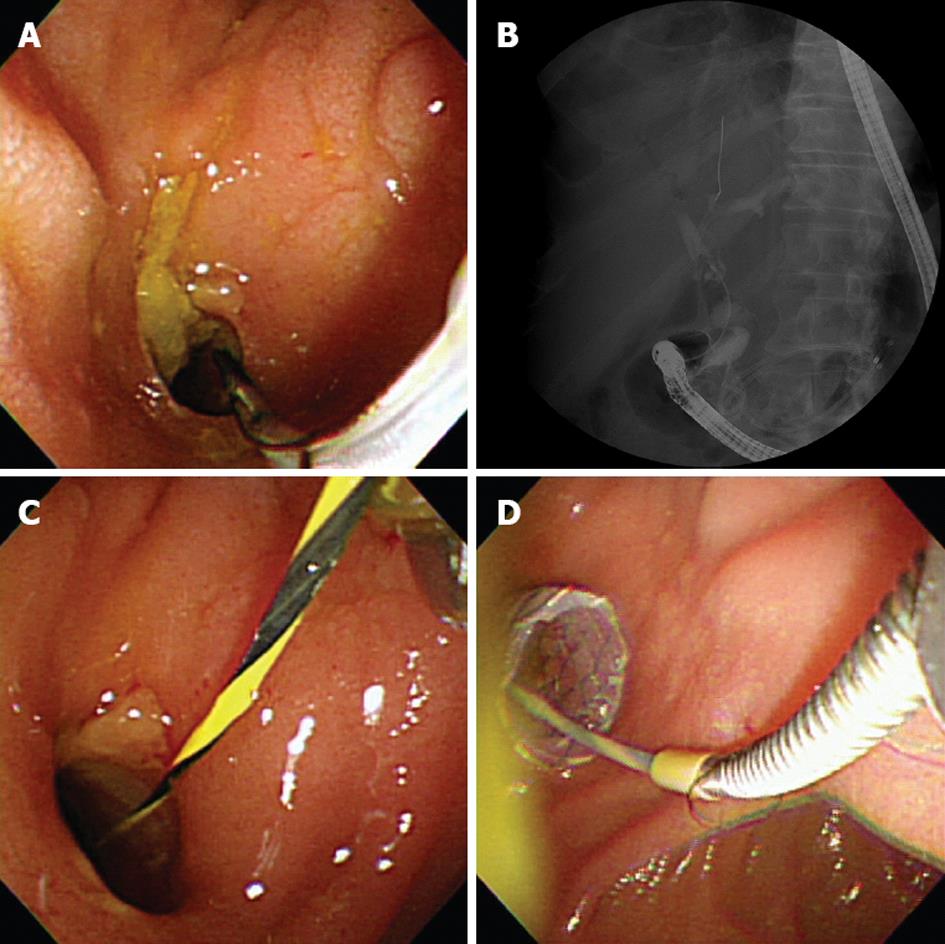
During the follow-up period, distal stent migration occurred in 4 patients (4/13, 30.8%) as a late adverse event. Among them, 2 patients presented with cholangitis, and 1 patient presented with jaundice. In these patients, an FCSEMS could be reinserted through the existing choledochoduodenal fistula tract (Figure 2). In another patient, distal stent migration was observed during a routine, follow-up X-ray without the patient experiencing adverse symptoms: additional stent insertion was not considered because his life expectancy was less than a month and bile was draining through his maturated fistula tract.
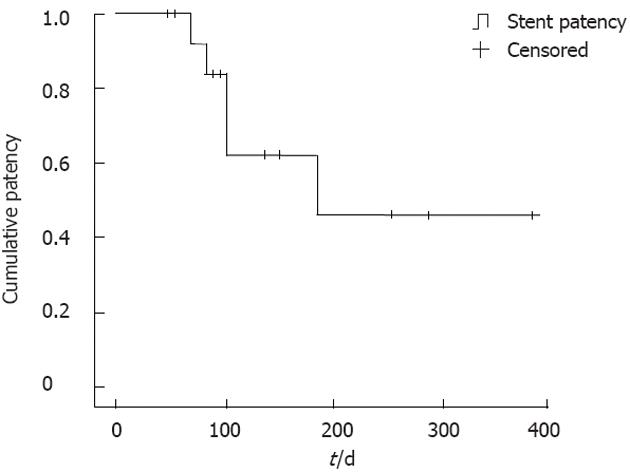
All patients were followed up until their time of death, with a median follow-up period of 186 d (range: 52-388 d). Stent patency was maintained until death in 9 patients, while distal stent migration occurred in 4 patients during the follow-up period (range: 68-185 d). The mean patency duration of the stents was 264 d (Figure 3).
Previous studies on EUS-CDS have shown favorable technical success rates, with resolution of obstructive jaundice observed in all patients after stent placement. To date, EUS-CDS with a metallic stent insertion has been reported in 11 patients, including covered metallic stents in 6 patients, partially covered metallic stents in 3, and uncovered metallic stents in 2[6,17-20]. However, there have not yet been any long-term, follow-up results of stent patency in patients who have undergone EUS-CDS with a metallic stent, making this study the first long-term follow-up evaluation of EUS-CDS with an FCSEMS.
Until recently, there has been only one study to our knowledge that has reported on the long-term results of EUS-CDS with plastic stents. Yamao et al[11] reported that the mean duration of stent patency of EUS-CDS with plastic stents was 211.8 d. Usually, the diameter of metallic stents is 8-10 mm, which is larger than that of plastic stents (7-10F). Thus, theoretically, metallic stents have an advantage in terms of stent patency over plastic stents. In transpapillary drainage through the ERCP, metallic stents have been shown to have a longer patency duration compared with plastic stents, and the mean patency duration of a metallic stent inserted via the transpapillary route was 234-506 d[4,21]. In our study, the mean duration of stent patency was 264 d, which is comparable with that observed in transpapillary drainage with an FCSEMS.
We found that the technical success rate of EUS-CDS with an FCSEMS was 86.7% (13/15). Although this rate is acceptable, it also indicates that EUS-CDS may not be simple to perform and that careful consideration and adequate experience in EUS-related intervention are mandatory. In this study, EUS-CDS with an FCSEMS was unsuccessful in 2 patients. Because of the acute angulation of the echoendoscope in the duodenal bulb in these patients, the force exerted on the FCSEMS resulted in the echoendoscope being pushed away from the duodenal wall, thereby leading to technical failure. This finding indicates that despite the creation of a fistula with an adequate diameter for FCSEMS insertion, stent insertion may fail if the echoendoscope has an acute angulation because an undeployed metallic stent is rigid.
In previous studies, early adverse events of EUS-CDS included bile peritonitis, pneumoperitoneum, and hemobilia, all of which are self-limiting[10,14,17,22,23]. In EUS-CDS, the puncture site is selected to bypass the tumor around the distal bile duct, and thus, the puncture may be located close to the hepatic hilum. If the puncture is made near the hepatic hilum, then the proximal end of the stent can be inserted into the right or left intrahepatic duct. In this case, a fully covered metallic stent may block the opposite duct because its diameter is larger than that of a plastic stent. With uncovered metallic stents, although the drainage of the opposite duct is possible through the open mesh, its use is restricted because the risk of a bile leak through the fistula tract from the duodenum into the bile duct exists[24]. In our study, 1 case showed cholangitis caused by the obstruction of the right intrahepatic duct, which resulted from the insertion of the proximal end of the stent into the left intrahepatic duct. Therefore, the puncture should be made while avoiding the hepatic hilum if possible, and the insertion of a plastic stent is suggested to be better than a metallic stent in cases where the puncture site is close to the hepatic hilum. In addition, the development of a partially covered metallic stent-in which the proximal end is specially designed to lack a covered membrane-is required to overcome these disadvantages.
In this study, the stent patency of 9 patients was maintained until their death, but distal stent migration occurred in 4 patients during the follow-up period. These results indicate that the most important factor in maintaining stent patency in patients undergoing EUS-CDS with an FCSEMS may be the prevention of stent migration. Because the FCSEMS has a large bore diameter, it may theoretically have advantages over the conventional plastic stents in terms of stent revision. In patients with an FCSEMS insertion, stent revision was relatively easy because the opening of the fistula tract was large enough to be able to easily find, even after stent migration. In an FCSEMS inserted via the transpapillary method, tumor ingrowth due to a crack in the covered membrane or tumor overgrowth can be observed[25,26]. EUS-CDS, however, has advantages in that it reduces the risk of tumor ingrowth or tumor overgrowth because it bypasses the tumor instead of directly passing through the tumor. Stent obstruction by tumor ingrowth or overgrowth was not found in this study.
This study has several limitations. First, the study population was not sufficiently large to allow for a decisive conclusion regarding our results. Second, standard techniques and devices for the EUS-guided drainage procedure have not yet been established. Each endosonographer used a slightly different technique, which may have affected the results of the study. A prospective multicenter evaluation may be valuable to overcome this limitation. Third, this study is an observational study. Thus, a prospective randomized study comparing EUS-CDS with an FCSEMS and other alternative drainage methods, such as percutaneous metallic stent insertion, is necessary.
In conclusion, we have prospectively assessed the long-term outcomes of EUS-CDS with an FCSEMS. EUS-CDS with an FCSEMS was a safe and effective method in patients with distal malignant biliary obstructions and had a comparatively long patency duration. Nevertheless, the significant rate of distal stent migration cannot be ignored, suggesting the need for a newly designed metallic stent for EUS-CDS.
Endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS) may be a feasible and useful alternative in patients with distal malignant biliary obstructions after failed endoscopic retrograde cholangiopancreatography (ERCP). Little is known, however, about the long-term outcomes of EUS-CDS with a fully covered self-expandable metallic stent (FCSEMS).
This is the first study that addresses the long-term follow-up results of patients who underwent EUS-CDS with an FCSEMS.
The technical and functional success rates of EUS-CDS with an FCSEMS were 13 (86.7%) and 13 (100%), respectively. EUS-CDS with an FCSEMS is technically feasible and can lead to effective palliation of distal malignant biliary obstructions after failed ERCP. EUS-CDS with an FCSEMS showed a comparatively long patency duration (264 d).
Although EUS-CDS with an FCSEMS showed a high success rate and comparatively long patency duration, the significant rate (30.8%) of distal stent migration suggests the need for a newly designed metallic stent for EUS-CDS.
EUS-CDS is a new technique for biliary drainage using EUS-guided puncture of the common bile duct from the duodenal bulb and is usually performed as a rescue drainage method when endoscopic transpapillary stenting fails.
The authors examined the usefulness and long-term outcomes of EUS-CDS with an FCSEMS. It revealed that EUS-CDS with an FCSEMS is technically feasible and can lead to effective palliation of distal malignant biliary obstructions after failed ERCP. However, it also showed a significant rate of distal stent migration. Therefore, a prospective randomized study on EUS-CDS with newly developed stents is needed.
Peer reviewer: Tamir Miloh, MD, Associate Professor, Director of Pediatric Liver/Liver Transplantation Program, Division of Gastroenterology, Phoenix Children’s Hospital, 1919 E Thomas Rd, Main Building, Second Floor, Phoenix, AZ 85016, United States
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Lai EC, Mok FP, Tan ES, Lo CM, Fan ST, You KT, Wong J. Endoscopic biliary drainage for severe acute cholangitis. N Engl J Med. 1992;326:1582-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 328] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 2. | Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 554] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 3. | Park do H, Kim MH, Choi JS, Lee SS, Seo DW, Kim JH, Han J, Kim JC, Choi EK, Lee SK. Covered versus uncovered wallstent for malignant extrahepatic biliary obstruction: a cohort comparative analysis. Clin Gastroenterol Hepatol. 2006;4:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Yoon WJ, Ryu JK, Yang KY, Paik WH, Lee JK, Woo SM, Park JK, Kim YT, Yoon YB. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effectiveness in a country with a low ERCP cost. Gastrointest Endosc. 2009;70:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 480] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 6. | Park do H, Koo JE, Oh J, Lee YH, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: a prospective feasibility study. Am J Gastroenterol. 2009;104:2168-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Oh HC, Lee SK, Lee TY, Kwon S, Lee SS, Seo DW, Kim MH. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol. 2001;4:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Wiersema MJ, Sandusky D, Carr R, Wiersema LM, Erdel WC, Frederick PK. Endosonography-guided cholangiopancreatography. Gastrointest Endosc. 1996;43:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 178] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Itoi T, Yamao K. EUS 2008 Working Group document: evaluation of EUS-guided choledochoduodenostomy (with video). Gastrointest Endosc. 2009;69:S8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Yamao K, Bhatia V, Mizuno N, Sawaki A, Ishikawa H, Tajika M, Hoki N, Shimizu Y, Ashida R, Fukami N. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of long-term follow-up. Endoscopy. 2008;40:340-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Püspök A, Lomoschitz F, Dejaco C, Hejna M, Sautner T, Gangl A. Endoscopic ultrasound guided therapy of benign and malignant biliary obstruction: a case series. Am J Gastroenterol. 2005;100:1743-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Tarantino I, Barresi L, Repici A, Traina M. EUS-guided biliary drainage: a case series. Endoscopy. 2008;40:336-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Burmester E, Niehaus J, Leineweber T, Huetteroth T. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 196] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Lee SS, Park do H, Hwang CY, Ahn CS, Lee TY, Seo DW, Lee SK, Kim MW. EUS-guided transmural cholecystostomy as rescue management for acute cholecystitis in elderly or high-risk patients: a prospective feasibility study. Gastrointest Endosc. 2007;66:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Park do H, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010;71:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 18. | Belletrutti PJ, Gerdes H, Schattner MA. Successful endoscopic ultrasound-guided transduodenal biliary drainage through a pre-existing duodenal stent. JOP. 2010;11:234-236. [PubMed] |
| 19. | Belletrutti PJ, DiMaio CJ, Gerdes H, Schattner MA. Endoscopic ultrasound guided biliary drainage in patients with unapproachable ampullae due to malignant duodenal obstruction. J Gastrointest Cancer. 2011;42:137-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Artifon EL, Takada J, Okawa L, Moura EG, Sakai P. EUS-guided choledochoduodenostomy for biliary drainage in unresectable pancreatic cancer: a case series. JOP. 2010;11:597-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Isayama H, Nakai Y, Kawakubo K, Kogure H, Togawa O, Hamada T, Ito Y, Sasaki T, Yamamoto N, Sasahira N. Covered metallic stenting for malignant distal biliary obstruction: clinical results according to stent type. J Hepatobiliary Pancreat Sci. 2011;18:673-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Hara K, Yamao K, Niwa Y, Sawaki A, Mizuno N, Hijioka S, Tajika M, Kawai H, Kondo S, Kobayashi Y. Prospective clinical study of EUS-guided choledochoduodenostomy for malignant lower biliary tract obstruction. Am J Gastroenterol. 2011;106:1239-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Ang TL, Teo EK, Fock KM. EUS-guided transduodenal biliary drainage in unresectable pancreatic cancer with obstructive jaundice. JOP. 2007;8:438-443. [PubMed] |
| 24. | Itoi T, Isayama H, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tanaka R. Stent selection and tips on placement technique of EUS-guided biliary drainage: transduodenal and transgastric stenting. J Hepatobiliary Pancreat Sci. 2011;18:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 25. | Song TJ, Lee SS, Yun SC, Park do H, Seo DW, Lee SK, Kim MH. Paclitaxel-eluting covered metal stents versus covered metal stents for distal malignant biliary obstruction: a prospective comparative pilot study. Gastrointest Endosc. 2011;73:727-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Kim JH, Song HY, Shin JH, Jung HY, Kim SB, Kim JH, Park SI. Membrane degradation of covered stents in the upper gastrointestinal tract: frequency and clinical significance. J Vasc Interv Radiol. 2008;19:220-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |