Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4427
Revised: July 27, 2012
Accepted: August 3, 2012
Published online: August 28, 2012
AIM: To study the role of time-intensity curve (TIC) analysis parameters in a complex system of neural networks designed to classify liver tumors.
METHODS: We prospectively included 112 patients with hepatocellular carcinoma (HCC) (n = 41), hypervascular (n = 20) and hypovascular (n = 12) liver metastases, hepatic hemangiomas (n = 16) or focal fatty changes (n = 23) who underwent contrast-enhanced ultrasonography in the Research Center of Gastroenterology and Hepatology, Craiova, Romania. We recorded full length movies of all contrast uptake phases and post-processed them offline by selecting two areas of interest (one for the tumor and one for the healthy surrounding parenchyma) and consecutive TIC analysis. The difference in maximum intensities, the time to reaching them and the aspect of the late/portal phase, as quantified by the neural network and a ratio between median intensities of the central and peripheral areas were analyzed by a feed forward back propagation multi-layer neural network which was trained to classify data into five distinct classes, corresponding to each type of liver lesion.
RESULTS: The neural network had 94.45% training accuracy (95% CI: 89.31%-97.21%) and 87.12% testing accuracy (95% CI: 86.83%-93.17%). The automatic classification process registered 93.2% sensitivity, 89.7% specificity, 94.42% positive predictive value and 87.57% negative predictive value. The artificial neural networks (ANN) incorrectly classified as hemangyomas three HCC cases and two hypervascular metastases, while in turn misclassifying four liver hemangyomas as HCC (one case) and hypervascular metastases (three cases). Comparatively, human interpretation of TICs showed 94.1% sensitivity, 90.7% specificity, 95.11% positive predictive value and 88.89% negative predictive value. The accuracy and specificity of the ANN diagnosis system was similar to that of human interpretation of the TICs (P = 0.225 and P = 0.451, respectively). Hepatocellular carcinoma cases showed contrast uptake during the arterial phase followed by wash-out in the portal and first seconds of the late phases. For the hypovascular metastases did not show significant contrast uptake during the arterial phase, which resulted in negative differences between the maximum intensities. We registered wash-out in the late phase for most of the hypervascular metastases. Liver hemangiomas had contrast uptake in the arterial phase without agent wash-out in the portal-late phases. The focal fatty changes did not show any differences from surrounding liver parenchyma, resulting in similar TIC patterns and extracted parameters.
CONCLUSION: Neural network analysis of contrast-enhanced ultrasonography - obtained TICs seems a promising field of development for future techniques, providing fast and reliable diagnostic aid for the clinician.
- Citation: Streba CT, Ionescu M, Gheonea DI, Sandulescu L, Ciurea T, Saftoiu A, Vere CC, Rogoveanu I. Contrast-enhanced ultrasonography parameters in neural network diagnosis of liver tumors. World J Gastroenterol 2012; 18(32): 4427-4434
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4427
Rapid classification of liver masses is of utmost importance, as early diagnosis drastically improves survival chances of oncologic patients, determined by the appropriate curative therapy. Hepatocellular carcinoma (HCC) is the sixth cancer as incidence worldwide, being the third cause of cancer-related mortality [1,2].
Current guidelines include at least one dynamic imagistic method for establishing a positive HCC diagnosis[3,4], as with the widespread use in recent years of imaging techniques, the differential diagnosis of a newly discovered liver mass became less invasive for the patient.
Contrast-enhanced ultrasonography (CEUS) with second generation blood-pool contrast agents is one of the most cost-effective methods for determining tumor vascularization patterns[5-9]. Arterial contrast uptake followed by late portal wash-out is considered the radiological hallmark of HCC. Various other benign and malignant liver tumors show different uptake patterns, thus providing differential diagnosis. Since other tumors can sometimes mimic the filling patterns specific to HCC, different studies reported lower sensitivities for CEUS when compared to other dynamic imaging methods such as contrast-enhanced magnetic resonance imaging (CE-MRI) or 4-phase computed tomography (CT)[9,10]. Time intensity curves (TIC) are the graphical representation of contrast intensity represented in every moment of a CEUS investigation. Comparative TIC analysis between a tumoral region of interest (ROI) and a parenchymal equivalent ROI could enhance the diagnostic accuracy of CEUS, thus establishing its role in HCC diagnosis[11,12].
Artificial neural networks (ANN) emerged in recent years as potent diagnostic tools for malignant lesions, mainly because of their adaptability and excellent problem solving-oriented architecture. They have been employed in complex medical image analysis tasks with various degrees of success. Computer-aided diagnosis (CAD) systems have gained a reputation for providing integrative solutions for the diagnosis of several types of malignancies[13-15], with many applications in gastroenterology and tumor pathology associated with the digestive tract.
Our aim here was to establish the role of TIC analysis parameters in a complex system of neural networks designed to classify liver tumors.
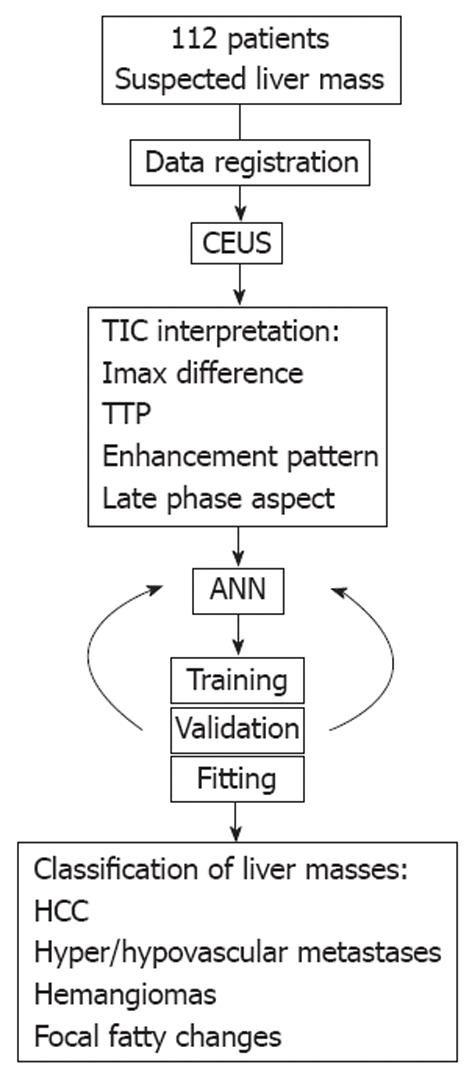
Between September 2008 and May 2011 we prospectively included 112 patients with hepatocellular carcinoma (n = 41), hypervascular (n = 20) and hypovascular (n = 12) liver metastases, hepatic hemangiomas (n = 16) or focal fatty changes (n = 23) who underwent CEUS in the Research Center of Gastroenterology and Hepatology, Craiova, Romania. Positive diagnosis was reached through a combination of other imagistic methods (CT and CE-MRI), liver biopsy in uncertain cases or follow-up for a minimum period of six months. The study was performed in accordance with the Declaration of Helsinki and received necessary approval of the Ethic Committee of the University of Medicine and Pharmacy of Craiova. All patients gave informed consents on all procedures and agreed in writing so that their anonymized CEUS investigations were to be used in the ANN model. An overview of the study protocol can be observed in Figure 1.
Full length CEUS recordings were retrieved in an uncompressed video for offline post-processing and subsequent TIC analysis. This ensured an optimal preservation of image features, color intensities and hues.
Digital files were transferred to a high-end graphical station where they were analyzed with in-house developed software (programmed by Ionescu M and Streba CT). At first, the team of gastroenterologists with extensive US and CEUS experience (Sandulescu L, Ciurea T, Saftoiu A, Vere CC and Rogoveanu I) supervised Streba CT in the selection of tumor and normal parenchyma ROIs at equal tissue depths, on each CEUS recording. The software recorded median color intensity values inside the ROIs for each frame and presented an accurate TIC for visual interpretation. The locations of the two ROIs were manually adjusted after a breathing motion, thus removing breathing artifacts. The team also visually analyzed the TICs and provided a diagnosis, blinded to any other patient details.
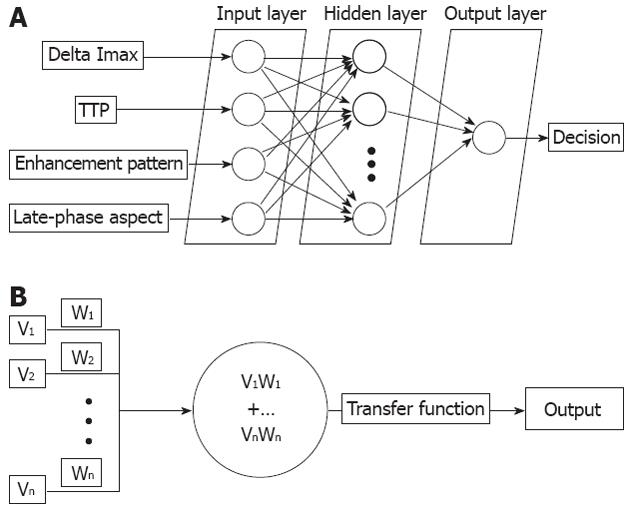
Raw intensity values were extracted as data sequences and analyzed by a feed forward back propagation multi-layer ANN which was trained to classify data into distinct classes, corresponding to each type of liver lesion. Based on previous studies which concluded that the best suited ANN layout for classification tasks should be as simple as possible, the network architecture of choice contained only one hidden layer, with an input layer and one layer dedicated for the output (Figure 2A)[14,16].
The first layer of the ANN consisted of several groups of input neurons (Figure 2B) corresponding to each imputed parameter, as follows: difference in maximum intensities, corresponding time to reaching peak intensities and the aspect of the late/portal phase, as quantified by the ANN on individual frame-by-frame differences between median intensities starting from the 45th second of the recording. As various tumors can be diagnosed by evaluating central versus peripheral enhancement, the software also calculated a ratio between median intensities of the central and peripheral areas, thus quantifying relative changes in contrast uptake.
Each neuron in an ANN was connected to every neuron of the consecutive layer hidden layer through connections called “synapses”, which are attributed different “weights”, based on strength of the connection[14,17,18]. These weights were calculated by the ANN in a hierarchical manner (basically, the system determined the importance of each parameter in the analysis).
A hidden 2nd layer contained neurons with associated transfer and processing functions which received weighted parameters as well as activity of the previous neurons. The sum of the products between synaptic weights and neuron activity decided an output category (in this case, any real number between 1 and 5, corresponding to the five distinctive tumor classes), similar to information processing within a human brain[17-20].
A final output layer was organized and provided five distinct values, one for each type of liver mass identified. This layer could have been varied in size depending on the total number of categories, if other types of tumors would have been present. This presented the user with a possible classification suggestion and probability score, calculated as a percentage from the total ideal score for the ideal value (i.e., if the total was 0.8, it would present the user with an 80% probability for the tumor to be HCC, as “1” was the assigned score for this diagnosis).
All 112 cases were randomly divided into training, validation and testing sets. The training phase represented the moment when the system learned from a selected data-set which varied in size and composition, thus determining respective weights for each synapses. The ANN was trained with the back propagation algorithm and 10-fold cross-validation was used to assess its performance, as previously described elsewhere[18,19]. This method minimized the risk of over-fitting, which is an increasingly rigid structure that could ultra-classify cases. Operators chose the learning rate, determining the epoch (that is, the number of iterations needed for going through the training phase), as previously experience showed[18,19]. Also, all cases were assessed by the team of experts according to the visual TIC representation, thus enabling a direct comparison with the diagnostic accuracy of TIC analysis as a stand-alone method.
The statistical analysis was performed using GraphPad Prism 5.0 (GraphPad Software Inc, LaJolla, CA). We calculated the respective sensitivities, specificities, positive (PPV) and negative (NPV) predictive values for both human visual and computerized ANN interpretations. We evaluated the differences between the sensitivity and specificity of the ANN-guided and human TIC evaluation-based diagnosis by using the McNemar’s test. A P value below 0.05 was considered significant. Moreover, we calculated the training and testing accuracy of the ANN and we performed a fitting analysis in order to better characterize the classification characteristics of the system.
The study lot consisted of 69 men and 43 women, their detailed characteristics being illustrated in Table 1.
| HCC | Hypervascular metastasis | Hypovascular metastasis | Hepatic hemangyoma | Fatty focal change | |
| Sex (n) | |||||
| Male | 30 | 9 | 5 | 4 | 16 |
| Female | 11 | 11 | 7 | 12 | 7 |
| Age (yr), median (range) | |||||
| Male | 64 (52-83) | 68 (45-89) | 66 (42-89) | 48 (32-64) | 43 (31-61) |
| Female | 66 (59-77) | 62 (38-72) | 62 (41-80) | 50 (41-62) | 44 (29-61) |
| Tumor size (cm), median (range) | |||||
| Male | 5.08 (2-10.7) | 5.09 (2-13.1) | 5.11 (2.1-12.7) | 3.1 (2-7) | 4 (2-6.5) |
| Female | 4.8 (2.3-11) | 5.7 (2-11.8) | 5.6 (2-13.01) | 3.2 (2-8.5) | 4.6 (2-6.1) |
| ANN classification | |||||
| HCC | 38 | 0 | 0 | 3 | 0 |
| Hypervascular metastasis | 0 | 18 | 0 | 2 | 0 |
| Hypovascular metastasis | 0 | 0 | 12 | 0 | 0 |
| Hepatic hemangyoma | 1 | 3 | 0 | 12 | 0 |
| Fatty focal change | 0 | 0 | 0 | 0 | 23 |
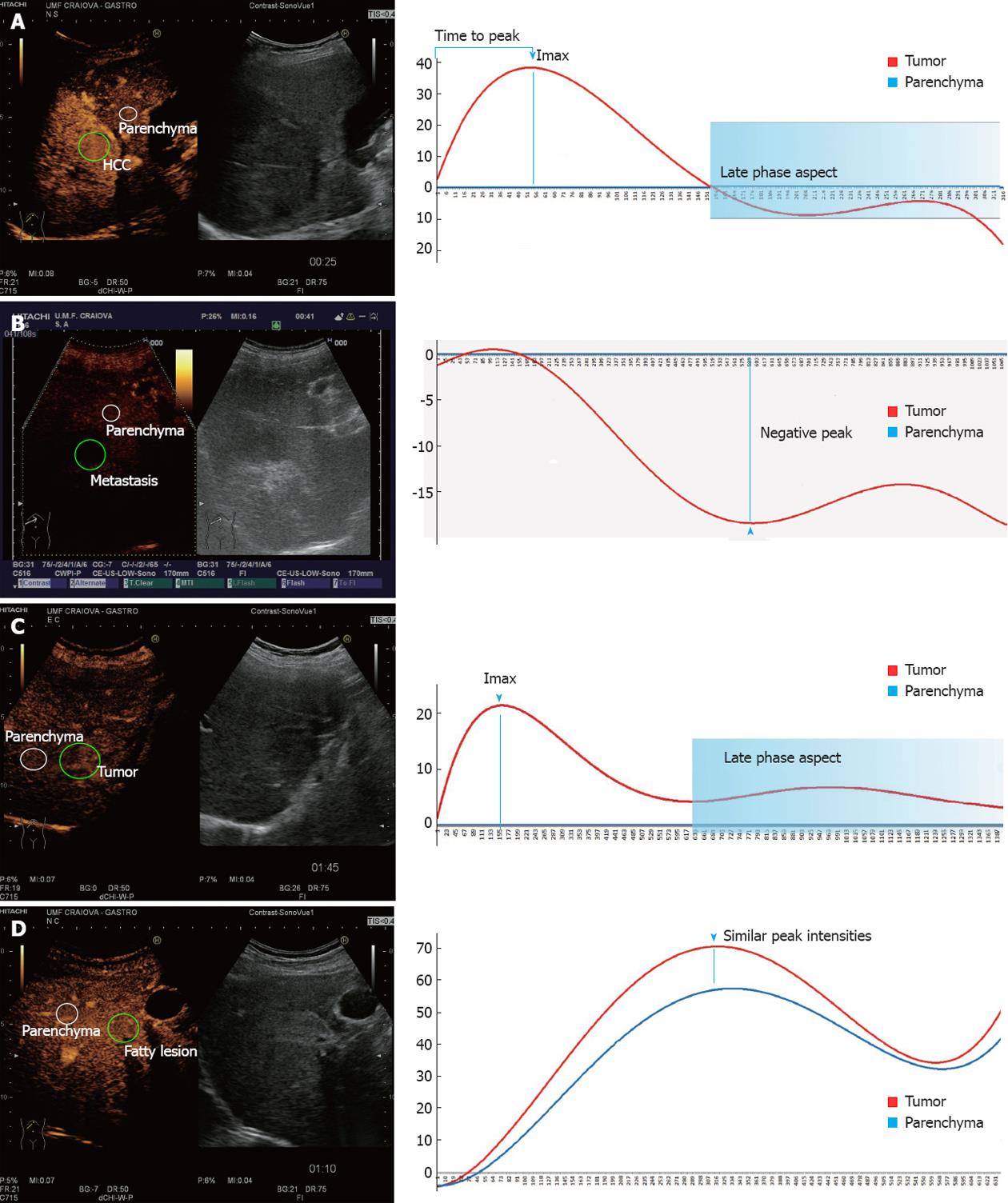
HCC cases showed contrast uptake during the arterial phase, followed by wash-out in the portal and first seconds of the late phases (Figure 3A). For the hypovascular metastases we recorded negative peak values which resulted in negative differences being imputed in the ANN (Figure 3B). For hypervascular metastases, wash-out in the late phase was registered in the majority of cases. Hemangiomas showed contrast uptake in the arterial phase, followed by the absence of wash-out during the portal-late phases (Figure 3C). Focal fatty changes registered similar TIC patterns to the surrounding parenchyma (Figure 3D).
The ANN containing TIC parameters showed similar diagnostic capabilities to human interpretation of TICs. The automatic classification process registered 93.2% sensitivity, 89.7% specificity, 94.42% PPV and 87.57% NPV. Comparatively, human visual interpretation of TICs showed 94.1% sensitivity, 90.7% specificity, 95.11% PPV and 88.89% NPV. The ANN incorrectly classified as hemangyomas three HCC cases and two hypervascular metastases, while in turn misclassifying four liver hemangyomas as HCC (one case) and hypervascular metastases (three cases) (Table 1). Overall, the ANN correctly identified 67/72 (93%) malignant lesions and 36/40 (90%) of all benign focal liver lesions. In turn, human evaluation based solely on TIC parameters misclassified four hypervascular metastases as hemangyomas and four hemangyomas as either HCC (two cases) or hypervascular metastases (two cases). The differences between the automatic ANN classification and human interpretation of TIC data were not statistically significant in terms of specificity (P = 0.225) and sensitivity (P = 0.451).
The neural network had 94.45% training accuracy (95% CI: 89.31%-97.21%) and 87.12% testing accuracy (95% CI: 86.83%-93.17%). Fitting coefficients demonstrated the overall classification capabilities of the ANN, with r = 0.989 (training); 0.991 (validation); 0.984 (testing) and 0.993 (overall).
We present here the first report on using an ANN-driven computer aided diagnostic system in conjunction with CEUS TIC analysis for the correct classification of liver masses. Our pilot study on an initial lot of 112 patients compared the diagnostic capability of such a system with visual TIC analysis by a team of gastroenterologists blinded to any other patient data. We obtained similar results, the ANN scoring 93.2% sensitivity and 89.7% specificity, compared to 94.1% sensitivity and 90.7% specificity for human visual interpretation, respectively.
Early correct diagnosis and appropriate staging of liver malignancies is of utmost importance for patient survival, as curative surgical interventions have narrow indications and are extremely specific to certain types of tumors[1-4]. HCC currently ranks third in terms of mortality worldwide and fifth in incidence with almost 750 000 new cases diagnosed each year, while being second in mortality among digestive cancers[5]. The diagnostic criteria for HCC, the most common primary liver malignancy worldwide, relay primarily on imagistic methods[3,4]. Current accepted guidelines worldwide are those proposed by the American Association for the Study of Liver Disease (revised in 2010)[3] and those of the association between the European Society for the Study of Liver and the European Organization for Research and Treatment of Cancer (EASL-EORTC, recently revised in April 2012)[4]. The introduction of new generations of contrast agents marked the adoption of a generally accepted radiological hallmark for positive HCC diagnosis, namely contrast up-take in the arterial phase followed by washout in the venous/late phase. The American Association for the Study of Liver Diseases guidelines stipulate that only one imaging technique with contrast uptake, either CT or MRI, showing the radiological hallmark, is sufficient for positive diagnosis of tumors between 1 and 2 cm in diameter (this being the optimum tumor size for curative surgery)[3,4]. Their European counterparts, however, are more cautious in applying a single imaging method, and recommend two coincidental techniques in suboptimal settings due to technical limitations[4].
The use of CEUS is regarded as controversial in the latest EASL guideline[4]. Second generation contrast agents use gas bubbles between 2 and 7 microns in diameter which resonate under the US probe, the amplified signal being registered by the US machine through the same probe[5,6]. While the radiological hallmark can be clearly identified in this technique, the contrast microbubbles are bound to the intravascular space, as opposed to iodinated contrast-CT or gadolinium-based MR imaging in which the contrast agents are rapidly cleared from the bloodstream into the surrounding parenchymal space[4,9,10]. According to some studies, intrahepatic cholangiocarcinoma or even some highly vascularized liver metastases can display uptake patterns similar to HCC during CEUS or gadolinium-based MRI, thus providing an important source of error[9]. Currently, efforts are being made to overcome the inherited issues with CEUS investigations and diminish the rate of false interpretations[11,12]. The use of TICs in the interpretation of CEUS movies seems a feasible method of increasing the specificity of this investigation[12,13]. The method relies on plotting and comparing on a time scale the median intensities of two user-defined areas, one within the suspected tumor and one in a parenchymal area with no major vessels, thus producing two curves which depict contrast uptake during CEUS[12]. The usual graphical representation show contrast uptake in the first 30-60 s, followed by tumor wash-out in portal and venous phases, when the maximum intensity are similar to those of the parenchymal-selected area of interest. While TIC quantification does add more precision to the investigation, several user-dependent and technique-dependent limitations have been identified, such as different depths of the analyzed tumor/parenchyma areas of interest, moving artifacts due to patient breathing or the interference of large blood vessels in the selected areas of interest[12]. Our system showed good capabilities in selecting the appropriate ROIs, as it allowed for breathing compensation to be applied during post processing.
The neural network developed and tested consisted of an input layer containing four classes of neurons corresponding to TIC-dependent parameters, one hidden layer for data processing and a variable output layer which classified the data into five categories. This model, the perceptron feed-forward multi-layered ANN with back-propagation algorithms is the preferred embodiment of a CAD system for medical diagnosis, as it provides rapid diagnosis with minimal over-fitting (the artificial increase of certain rules of inclusion, leading to incorrect classifications)[13-19]. Automated quantitative image analysis techniques have been introduced in medical practice for a number of years[21-25]. The use of ANNs or other adaptive, machine-based learning systems, can substantially improve the accuracy of any quantitative-based image analysis method[25,26]. Moreover, current image analysis methods employed with US or CEUS are heavily dependent on the expertise of the medical operator[22-26]. The neural network approach showed excellent training and validation characteristics, with fast and reliable cycles. The usage of ANNs proved adequate, having promising results compared to human interpretation of the data. Using an independent CAD system proved beneficial and can considerably reduce user-dependent bias from CEUS interpretation, perhaps improving its ability to correctly diagnose liver malignancy, further enhancing its role in current medical guidelines.
One possible limitation of our study is represented by the relatively low number of cases included in the analysis. This was resolved by applying specific training algorithms with a long history of success in small patient lots[14,18,19]. Patients presented some of the most masses most commonly encountered by the physician in daily practice, therefore the system showed good promise in providing an independent diagnostic aid based solely on accurate quantification of imaging data. The system has promising telemedicine application, as it can be accessed from small tertiary referral centers, with limited experience in diagnosing liver malignancies. It can provide an independent expert diagnosis and verification system for physicians who do not routinely encounter liver-related pathology. Another indication for the system would be in medical training, as an independent help for training gastroenterologists. The self-improving model specific to the architecture of any ANN will only benefit from an extended number of cases, a greater training set further improving its diagnosis performances. Future directions should include other intelligent artificial systems, such as support vector machines, genetic algorithms, Bayesian classifiers and so on, with perhaps even better results.
We previously presented preliminary results of introducing an ANN analysis of CEUS parameters in a cascade ANN system for the diagnosis of HCC and other liver malignancies, with good prospects in terms of accuracy and efficiency[27]. Therefore, we strongly believe that the merger between clinical data and imagistic parameters represent a necessary direction in future aiding clinicians in their therapeutic decisions.
In conclusion, neural network analysis of CEUS TICs seems a promising field of development for future techniques, providing fast and reliable diagnostic aid for the clinician. A multi-layer perceptron ANN proved sufficient for the classification of liver masses based on TIC analysis data, producing the most rapid and accurate results. Future studies are needed in order to validate the system in larger cohorts and perhaps improving the architecture of the proposed ANN. The system can ultimately become an independent objective quantifier of clinical and imagistic data which can improve the diagnostic accuracy of CEUS regarding liver malignancies.
An early diagnosis of liver malignancies represent a major concern, as the therapeutic options become increasingly limited as the disease progresses. The imagistic diagnosis based on contrast agents is the preferred method for hepatocellular carcinomas (HCC), the most important primary malignant tumor of the liver. The role of contrast-enhanced ultrasonography (CEUS) in the differential diagnosis of liver tumors is currently subject to international debate, while time-intensity curve (TIC) analysis provides a rapid method to quantify perfusion parameters. Several computer-aided diagnosis (CAD) systems are currently employed in the diagnosis of various malignancies, specifically artificial neural network (ANN) systems being employed in image-recognition tasks.
CEUS was found to provide limited data when differentiating hepatocellular carcinomas from other liver malignancies; however, the introduction of TIC analysis provides important parameters that can objectify the vascular particularities of liver masses. ANN systems designed for differential diagnosis of malignancies are employed in various areas of medicine, including gastroenterology, with various degrees of success.
This is, to the knowledge, the first report on an ANN-based diagnosis and classification system based on imagistic data, designed to differentiate between several types of liver tumors, both malignant and benign. The CAD system presented here relies on TIC parameters extracted from CEUS investigations which are imputed in a feed forward back propagation single-layer neural network trained to classify liver tumors.
This system can successfully be applied in telemedicine settings, where smaller referral centers or gastroenterologists with limited experience in CEUS-based diagnosis of liver tumors and especially HCC, may benefit from an independent, objective diagnosis suggestion. Another preferred application is in medical training of gastroenterologists who can fully benefit from the growing experience such a system can provide.
CEUS: Ultrasonography (US) using 2nd generation contrast agents containing micro bubbles which resonate under the US probe, the amplified signal being registered by the US machine; TIC analysis: Method to visualize the parallel dispersion of the contrast agent between the tumor and an area selected from the surrounding parenchyma; ANN: Decision making artificial intelligent systems designed to mimic the operating principles of the human central nervous system and their key components - neurons and synapses.
This is a high quality research in which authors analyze the role of TIC analysis parameters in a complex system of neural networks designed to classify liver tumors. The results are interesting and suggest that neural network analysis of CEUS-obtained TICs seems a promising field of development for future techniques, providing fast and reliable diagnostic aid for the clinician.
Peer reviewers: Dr. Orhan Sezgin, Professor, Department of Gastroenterology, School of Medicine, Mersin University, 33190 Mersin, Turkey; Zenichi Morise, MD, PhD, Professor and Chairman, Department of Surgery, Banbuntane Houtokukai Hospital, Fujita Health University School of Medicine, 3-6-10 Otobashi, Nakagawa-ku, Nagoya, Aichi 454-8509, Japan
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 1326] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6572] [Article Influence: 469.4] [Reference Citation Analysis (1)] |
| 4. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4519] [Article Influence: 347.6] [Reference Citation Analysis (2)] |
| 5. | Dietrich CF. Characterisation of focal liver lesions with contrast enhanced ultrasonography. Eur J Radiol. 2004;51 Suppl:S9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Rettenbacher T. Focal liver lesions: role of contrast-enhanced ultrasound. Eur J Radiol. 2007;64:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, Greiner L, Jäger K, Jong ND, Leen E. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Lencioni R, Piscaglia F, Bolondi L. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol. 2008;48:848-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 9. | Rimola J, Forner A, Reig M, Vilana R, de Lope CR, Ayuso C, Bruix J. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 10. | Bolondi L, Gaiani S, Celli N, Golfieri R, Grigioni WF, Leoni S, Venturi AM, Piscaglia F. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology. 2005;42:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Ignee A, Jedrejczyk M, Schuessler G, Jakubowski W, Dietrich CF. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-Reliability and potential sources of errors. Eur J Radiol. 2010;73:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Goertz RS, Bernatik T, Strobel D, Hahn EG, Haendl T. Software-based quantification of contrast-enhanced ultrasound in focal liver lesions--a feasibility study. Eur J Radiol. 2010;75:e22-e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Lisboa PJ, Taktak AF. The use of artificial neural networks in decision support in cancer: a systematic review. Neural Netw. 2006;19:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Grossi E, Mancini A, Buscema M. International experience on the use of artificial neural networks in gastroenterology. Dig Liver Dis. 2007;39:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Cucchetti A, Piscaglia F, Grigioni AD, Ravaioli M, Cescon M, Zanello M, Grazi GL, Golfieri R, Grigioni WF, Pinna AD. Preoperative prediction of hepatocellular carcinoma tumour grade and micro-vascular invasion by means of artificial neural network: a pilot study. J Hepatol. 2010;52:880-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Frize M, Ennett CM, Stevenson M, Trigg HC. Clinical decision support systems for intensive care units: using artificial neural networks. Med Eng Phys. 2001;23:217-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Jiang J, Trundle P, Ren J. Medical image analysis with artificial neural networks. Comput Med Imaging Graph. 2010;34:617-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Săftoiu A, Vilmann P, Gorunescu F, Gheonea DI, Gorunescu M, Ciurea T, Popescu GL, Iordache A, Hassan H, Iordache S. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Săftoiu A, Vilmann P, Gorunescu F, Janssen J, Hocke M, Larsen M, Iglesias-Garcia J, Arcidiacono P, Will U, Giovannini M. Efficacy of an artificial neural network-based approach to endoscopic ultrasound elastography in diagnosis of focal pancreatic masses. Clin Gastroenterol Hepatol. 2012;10:84-90.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 20. | Droste K, Bollschweiler E, Waschulzik T, Schütz T, Engelbrecht R, Maruyama K, Siewert JR. Prediction of lymph node metastasis in gastric cancer patients with neural networks. Cancer Lett. 1996;109:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Huang-Wei C, Bleuzen A, Bourlier P, Roumy J, Bouakaz A, Pourcelot L, Tranquart F. Differential diagnosis of focal nodular hyperplasia with quantitative parametric analysis in contrast-enhanced sonography. Invest Radiol. 2006;41:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Salvatore V, Borghi A, Sagrini E, Galassi M, Gianstefani A, Bolondi L, Piscaglia F. Quantification of enhancement of focal liver lesions during contrast-enhanced ultrasound (CEUS). Analysis of ten selected frames is more simple but as reliable as the analysis of the entire loop for most parameters. Eur J Radiol. 2012;81:709-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Zhang X, Kanematsu M, Fujita H, Zhou X, Hara T, Yokoyama R, Hoshi H. Application of an artificial neural network to the computer-aided differentiation of focal liver disease in MR imaging. Radiol Phys Technol. 2009;2:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Guo D, Qiu T, Bian J, Kang W, Zhang L. A computer-aided diagnostic system to discriminate SPIO-enhanced magnetic resonance hepatocellular carcinoma by a neural network classifier. Comput Med Imaging Graph. 2009;33:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Chiu JS, Wang YF, Su YC, Wei LH, Liao JG, Li YC. Artificial neural network to predict skeletal metastasis in patients with prostate cancer. J Med Syst. 2009;33:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Markaki VE, Asvestas PA, Matsopoulos GK. Application of Kohonen network for automatic point correspondence in 2D medical images. Comput Biol Med. 2009;39:630-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |