Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4419
Revised: August 14, 2012
Accepted: August 18, 2012
Published online: August 28, 2012
AIM: To detect of colorectal cancer (CRC) circulating tumour cells (CTCs) surface antigens, we present an assay incorporating cadmium selenide quantum dots (QDs) in these paper.
METHODS: The principle of the assay is the immunomagnetic separation of CTCs from body fluids in conjunction with QDs, using specific antibody biomarkers: epithelial cell adhesion molecule antibody, and monoclonal cytokeratin 19 antibody. The detection signal was acquired from the fluorescence signal of QDs. For the evaluation of the performance, the method under study was used to isolate the human colon adenocarcinoma cell line (DLD-1) and CTCs from CRC patients’ peripheral blood.
RESULTS: The minimum detection limit of the assay was defined to 10 DLD-1 CRC cells/mL as fluorescence was measured with a spectrofluorometer. Fluorescence-activated cell sorting analysis and Real Time RT-PCR, they both have also been used to evaluate the performance of the described method. In conclusion, we developed a simple, sensitive, efficient and of lower cost (than the existing ones) method for the detection of CRC CTCs in human samples. We have accomplished these results by using magnetic bead isolation and subsequent QD fluorescence detection.
CONCLUSION: The method described here can be easily adjusted for any other protein target of either the CTC or the host.
- Citation: Gazouli M, Lyberopoulou A, Pericleous P, Rizos S, Aravantinos G, Nikiteas N, Anagnou NP, Efstathopoulos EP. Development of a quantum-dot-labelled magnetic immunoassay method for circulating colorectal cancer cell detection. World J Gastroenterol 2012; 18(32): 4419-4426
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4419.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4419
Colorectal cancer (CRC) is ranked as the second most common cause of cancer-related death worldwide[1]. Cancer-related death is most commonly caused by metastases derived from epithelial tumors like CRC[2]. The process of metastasis requires the potential and ability of cancer cells to enter into circulation, attach to the endothelium, invade the target organ and subsequently form metastases. The concept and existence of circulating tumour cells (CTCs) and the dissemination and settlement of these cells in secondary organs have been widely accepted[3]. Approximately 106 tumour cells per gram of primary tumour are daily released into the bloodstream[4]. However, shear forces in the physiological range can induce lethal damage in a high percentage of CTCs, thus only the 0.1% of those are viable and the 0.01% of the viable cells are responsible for the metastasis. A possible explanation why CTCs are still detectable in the blood months to years after complete removal of the primary tumour may therefore be the circulation and exchange of tumour cells between different metastatic sites and compartments[5].
The first report with regard to the identification of CTC specific biomarker and genes in CRC was published by Smirnov et al[6]. Many studies tried to identify biomarkers for CRC-derived CTCs, but further investigations are needed to evaluate the use of these biomarkers in an automated clinical practice[7,8]. Additionally, the potential prognostic significance of CTCs in CRC has been intensively and extensively reviewed[9]. Rahbari et al[10] have supported that the detection of CTCs in the peripheral blood is significantly associated with poor prognosis in CRC. Therefore, the identification of CTCs would be extremely useful to clinical practice, allowing for early cancer detection, as well as, early therapeutic intervention, monitoring and detection of disease recurrence.
Identifying CTCs in peripheral blood, however, has been proven to be more difficult than expected, due to the low concentration of CTCs in blood and lack of technology with sufficiently high sensitivity and specificity[11]. To date, the reverse transcriptase polymerase-chain reaction (RT-PCR) has been used for CTC detection in a variety of cancers. However, there are difficulties using RT-PCR for CTC detection. High RT-PCR sensitivity is associated with a susceptibility to false positive results in up to 5% of samples[12]. In addition, RT-PCR based methods are time-consuming, expensive and difficult to standardise[13]. Flow cytometry (FACS) has been used for CTC detection in several cancers[14]. CTC quantification is possible with FACS, and this might provide a more accurate measure of the risk of recurrence than current RT-PCR based techniques. Τhe introduction of immunomagnetic detection devices, such as the CellSearch® System (Veridex, Warren, NJ), has made possible the detection and at the same time the quantification of CTCs[15]. However, the equipment required for FACS and CellSearch® System is very bulky, expensive, and difficult to operate at the point-of care. Therefore, the development of alternative sensitive, speedy, specific and low cost methods for CTC detection is important for cancer prognosis.
Quantum dots (QDs) have been developed as a new class of high-sensitivity and high-specificity probes lacking the intrinsic limitations of organic dyes and fluorescent proteins[16,17]. In comparison with organic fluorophores, the QDs have unique optical and electronic properties, such as size- and composition-tunable fluorescence emission from visible to infrared wavelengths, large absorption coefficients across a wide spectral range and very high levels of brightness and photostability[16]. QDs have been applied in fluorescence labelling for cancer imaging in living animals, and cellular imaging[16,17].
The recent introduction of fluorescence detection technology using multifunctional magnetic beads and QDs has been reported[18-20]. In the present study, we demonstrate a sensitive assay that combines magnetic beads isolation and QDs fluorescence detection for the identification of CRC CTCs surface antigens. The principle of the assay is the separation of CTCs from body fluids using magnetic beads coupled with epithelial cell adhesion molecule (EpCAM) antibody, and monoclonal cytokeratin 19 (CK19) antibody. These complexes are then tagged with streptavidin-conjugated QDs which lead to the detection of a fluorescent signal. For the evaluation of the performance, the method under study was used to isolate human colon adenocarcinoma cell line (DLD-1) human CRC cell line from and CTCs from CRC patient’s peripheral blood. FACS analysis and Real Time RT-PCR have also been used to evaluate the performance of the described method. This method provides a simple, low cost and sensitive means of CTCs detection that can be easily adjusted for any other protein target, and can be directly applicable on clinical samples.
The following antibodies were incorporated in the assay under study: mouse anti-human EpCAM biotin conjugated monoclonal antibody (Acris Antibodies Inc. Acris GmbH, San Diego, CA, United States), mouse anti-human CK19 biotin conjugated monoclonal antibody (Novus Biologicals, Littleton, Colorado, United States), mouse anti-human IgG (Fc specific) biotin conjugated monoclonal antibody (Acris), IgG2b negative control antibody (Santa Cruz, CA, United States), and mouse anti-human CD45 Alexa Fluor 488 conjugated monoclonal antibody (Acris).
The DLD-1 cell line (American Type Culture Collection: CCL-221) was used in our experiments. The DLD-1 colorectal cancer cell line was cultured in RPMI-1640 (Invitrogen), supplemented with 10% foetal bovine serum (Invitrogen). Prior to testing, the cells were trypinized, washed and then resuspended in Ca2+ and Mg2+ free phosphate buffered saline (PBS). Peripheral blood samples were obtained from 9 CRC patients (Table 1) and 5 healthy donors that gave informed consent to be included in this study. Concerning CRC patints, peripheral venous blood was sampled immediately after patients were anaesthetised and prior surgery’s commencement. In all patients, an intravenous cannula was used to collect blood into 7-mL vacutainers containing sodium ethylenediaminetetraacetic acid (EDTA), discarding the first 7-mL aliquot of blood to reduce the risk of contamination of blood by skin epithelial cells. Three 30-mL samples were then collected at one-minute intervals (five 6-mL aliquots per 30-mL sample). Each 30-mL aliquot was thoroughly mixed and then divided into three 10-mL aliquots, one for analysis by RT-PCR, one by the proposed method and one by flow cytometry (FACS analysis). Human white blood cells were isolated from peripheral blood using Ficoll-Hypaque PLUS reagents (Amersham Bioscienses, Little Chalfont, NA, United Kingdom). Cells were counted manually using a Burker-Turk haemocytometer. Trypan blue (0.4%, Sigma, LΟ, United Kingdom) exclusion test was used to ensure cell viability was above 90% in experiments.
| Characteristics | Colorectal cancer patients (n) |
| Tumor location | |
| Rectum | 2 |
| Left colon | 5 |
| Right colon | 2 |
| Tumor size | |
| ≤ 4 cm | 5 |
| > 4 cm | 4 |
| Differentiation | |
| Well | 1 |
| Moderate | 7 |
| Poor | 1 |
| TNM stage | |
| I | 0 |
| II | 1 |
| III | 3 |
| IV | 3 |
Stretavidin coated magnetic beads (MBs) (Dynabeads M-280, Invitrogen) were functionalized with the biotinylated antibody EpCAM. For this purpose, 40 mg of antibody were added to 200 mL (10 g/L) streptavidin coated MBs and incubated at room temperature for 30 min. For the removal of unbound antibody, conjugated MBs were washed 5 times with PBS (1X) with the aid of a magnetic device (Dynal MPC-s, Invitrogen) and dissolved in 200 mL of PBS containing 0.1% bovine serum albumin (BSA). For negative control IgG2b antibody was used to replace EpCAM on MBs.
Cadmium selenide (CdSe) QDs (15-20 nm in size) with a maximum emission wavelength of 655 nm, shelled with ZnS and a polymer coating presenting carboxylic groups were purchased by Invitrogen. QDs were coated with streptavidin prior to testing according to the manufacturer’s instructions. Briefly, 50 mL of QDs were diluted in 400 mL of borate buffer 10 mmol/L (pH 7.4), 96 mL of streptavidin solution (10 g/L) (Invitrogen) and 11.4 mL of EDC (10 g/L) (Sigma-Aldrich, MO, United States) and incubated at room temperature for 90 min. Streptavidin coated QDs were washed 5 times with 500 mL of borate buffer 50 mmol/L (pH 8.3) on an Amicon Ultra-4 Filter (Millipore, MA, United States) and dissolved into 50 mmol/L borate buffer (pH 8.3) to a final volume of 500 mL.
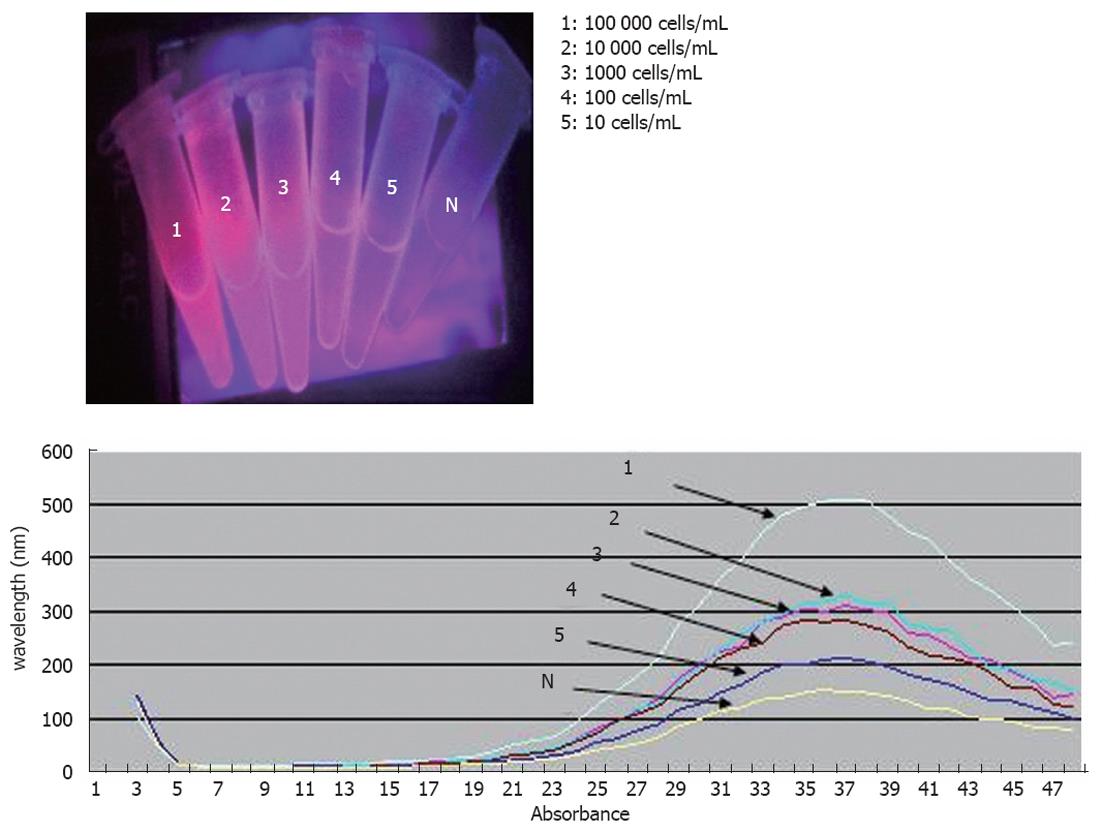
For the assessment of the limit of detection (LOD) of the method, our team had to add DLD-1 cells and duplicate solutions of DLD-1 cells in whole blood, both obtained from healthy control individuals, in order to test them. Two series of ten-fold dilutions ranging from 104 to 10 cells/mL were prepared. Two negative controls were used in our experiments. The first has consisted of white blood cells from healthy donors, and the second MBs were coupled with IgG2b antibody. The LOD was defined as the lowest concentration level that could be determined to be statistically different from the negative controls (Analytical detection limit guidance 1996).
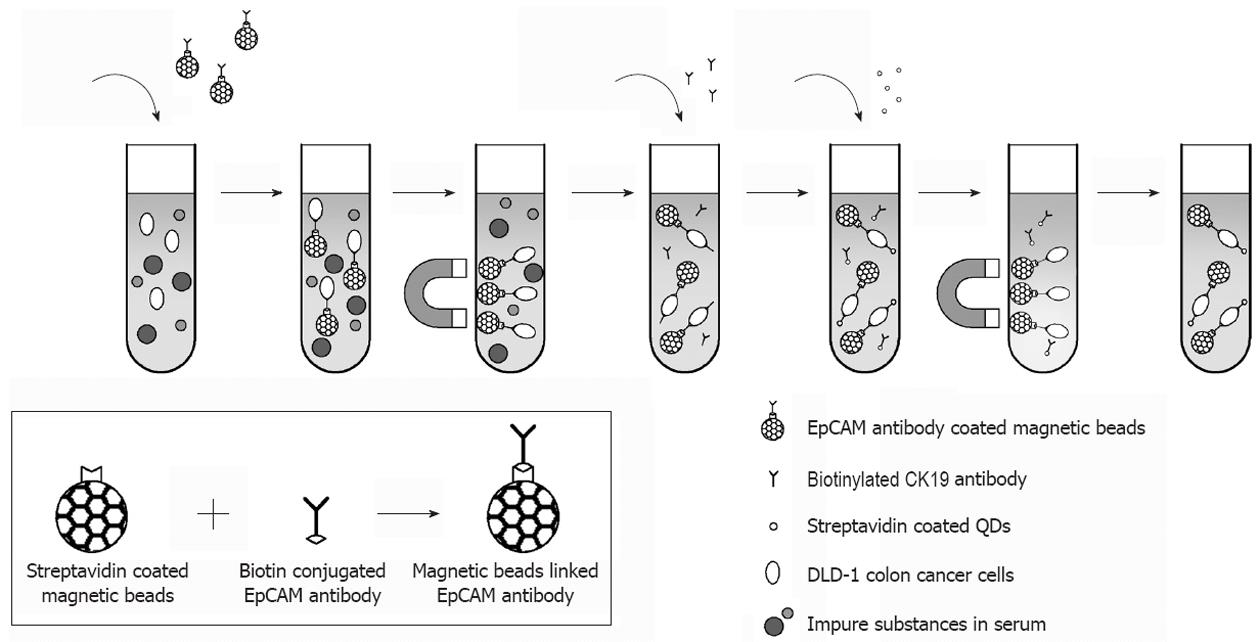
The principle of the assay is based upon the separation of cells using MBs coupled with anti-EpCAM (epithelial cell adhesion molecule). EpCAM is a carcinoma-associated antigen and is a member of a family that includes at least two type I membrane proteins and functions as a calcium-independent cell adhesion molecule. Like most other tumor-associated antigens, EpCAM is expressed on normal tissue. However, EpCAMs’ expression on a wide variety of carcinomas, mostly in gastrointestinal carcinomas, exceeds the expression and accessibility of the antigen compared with normal cells, thereby establishing a useful therapeutic and diagnostic window for a targeted antibody approach. These complexes are then tagged with anti-CK19 biotinylated antibody that specifically recognizes CTCs and finally streptavidin-conjugated QDs leading to the detection of a fluorescent signal (Figure 1). To this purpose, 500 μL of each dilution of the cells comprising positive and negative controls or an equal volume of PBS BSA 0.1% (blank) were coupled at first with 40 mL of EpCAM antibody conjugated MBs, and then with 10 mL (0.2 g/L) of CK19 biotinylated antibody, and finally 40 mL of streptavidin coated QDs. For each hybridization step the dilutions were incubated at 37 °C for 20 min with gentle agitation, separated by the matrix with the aid of a magnetic device (Dynal MPC-s, Invitrogen), washed once with PBS-BSA 0.1% and then re-suspended in 200 mL PBS 0.1% BSA. For the direct visual observation of fluorescence, the samples were transferred on a UV transilluminator with UV emission at 312 nm (Vilber Loumat, France). Fluorescence was also detected with a spectrofluorometer (excitation 480 nm emission 655 nm, PMT 450V), (Infinite M200, Tecan United States).
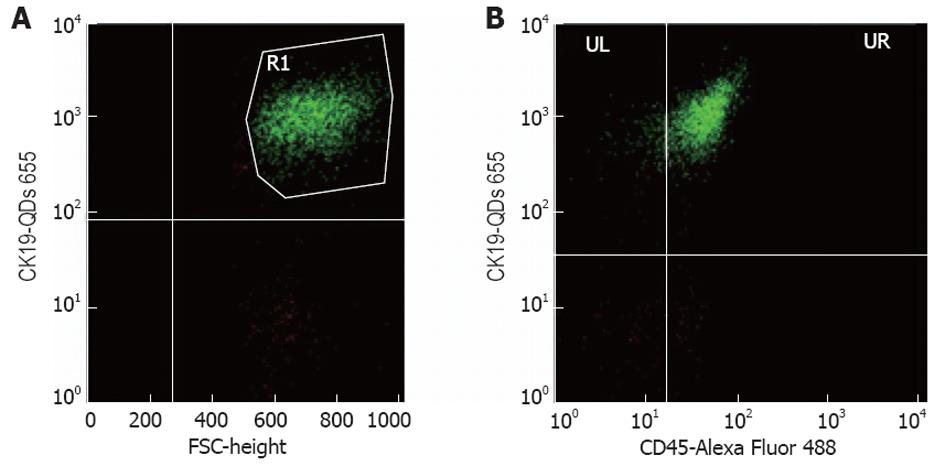
All samples were analyzed using a FACS Calibur BD Biosciences (United States) and the software BD FACStation™ System with the program Cell Quest, Mac. The antibodies used are described above and the staining of the isolated peripheral blood mononuclear cells samples was proceeded according to routine protocols. The anti-CK19 biotin conjugated monoclonal antibody with the QDs was used to evaluate the number of CTCs in the sample of interest and the CD45 Alexa Fluor 488 conjugated monoclonal antibody for the detection of non-specific interactions. Concerning the cell counting, for each sample 10 000 events were acquired. The parameter being used to describe individual samples was the % percentage of positive cells. In the first diagram (FSC/SSC) of FACS analysis the monocytes were gated, given that the CTCs are generally found in this population, in order to exclude most of the negative events. From the diagram of FSC/FL3 we have calculated the percentage of the cells that are fluorescent in FL3 (CK19+). In order to avoid non-specific binding, we have calculated at the same time the percentage of the cells that are fluorescent in FL3, but not in FL1 (CK19+/CD45-). In the FSC/FL3 diagram the cells that are fluorescent in FL3 (CK19+) were gated, and screened them in another diagram FL1/FL3. At last, the percentage of the cells that are fluorescent in FL3, but not in FL1 was calculated and the percentage in absolute cell number was converted. We deemed that if we found > 1 positive cell/mL in a sample, this would be a CTC, considering that the mean average of the healthy samples was 1000 cell/L (Figure 2).
Total RNA was isolated from DLD-1 cells, as well as RBC samples with the use of Trizol (Invitogen, ΤRI Reagent) according to the manufacturer’s instructions. The isolated RNA was dissolved in diethylpyrocarbonate-treated water and stored at -80 °C until used. Reverse transcription was performed by incubating 1 μg total RNA for 1 h at 42 °C in the presence of 500 mg/L of OligodT 12-18, 10 mmol/L deoxyribonucleotide triphosphates, 5 × first-strand buffer, 0.1 mol/L dithiothreitol, and 200 U/mL MMLV reverse transcriptase (Invitrogen). Assessment of the CK19 and EpCAM mRNA levels was performed by employing the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels as a reference gene. The following pair of primers were used: (1) GAPDH (Fw) 5’-GAAGGTGAAGGTCGGAGT-3’ and (Rv) 5’-GAAGATGGTGATGGGATTTC-3’ resulting in a 228-bp fragment; (2) CK19 (Fw) 5’-CCCGCGACTACAGCCACTA-3’ and (Rv) 5’-GCTCATGCGCAGAGCCTGTT-3’ resulting in a 193-bp fragment; and (3) EpCAM (Fw) 5’-GCCAAATAATAACGGGACCTA-3’ and (Rv) 5’-CCAGCTGAGAGACCAGGAGAA-3’ resulting in a 130-bp fragment. Real-time PCR was performed in an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, United States), as follows: initial denaturation for 2 min at 50 °C and for 10 min at 95 °C, followed by 40 cycles of PCR (95 °C for 15 s; 60 °C for 1 min). Reactions were performed in duplicate, using the SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer’s instructions. Data were analyzed using the comparative CT method for the relative quantitation of results[21]. Post-amplification denaturation curves showed that the primer pairs generated single products.
The consistency of the methods and comparison of total positive rate in the methods were analyzed using Cohen’s kappa statistic and χ2 test, respectively. All statistical analyses were carried out using GraphPad version 3.00 (GraphPad Software, San Diego, Calif). P < 0.05 was considered significant.
Our method for CTCs isolation and detection using EpCAM antibody coated MBs and CK19 antibody coated QDs is schematically illustrated in Figure 1. The assessment of the immobilization of antibodies on the surface of MBs was performed by using spectrophotometric measurement at 280 nm of the antibody solution before and after conjugation with MBs (data not shown).
For the evaluation of performance, the method under study was used to detect DLD-1 human CRC cells. The DLD-1 cells expressed EpCAM and CK19, as identified by RT-PCR. The direct visual observation of negative control did not reveal any fluorescent signal (Figure 3) as opposed to the DLD-1 cells reported above that reacted positively at or above the LOD concentration. The LOD of the methodology described in the present study was defined to 10 DLD-1 cells/mL of sample when results were assessed by visual observation of the test tubes.
In order to obtain an indication of the method’s performance with clinical samples, we applied the optimized assay to the detection of CTCs from human white blood cells that were isolated from peripheral blood of CRC patients, in comparison with real-time PCR and FACS analysis. Several studies used QDs-probes for FACS analysis[22-24]. The concordance of the positive results recorded by the proposed assay on CRC samples with those of real-time PCR and FACS was 78.57% (11 of 14) and 85.71% (12 of 14) respectively. The relevant percentage with regard to negative results was 100% in both cases (Table 2). Despite the fact that the number of clinical samples was limited, it was noted that the samples with tumor node metastasis (TNM) stage IV had increased number of CTCs compared to those with TNM stage II and III.
The repeatability of the method was defined as 100% since the results recorded for the samples included in this type of evaluation were identical for all assessments (n = 3).
The spreading of tumor cells is one of the primary causes of recrudescence at distant sites and of death from cancer. Thus, the detection of circulating metastatic cells is important to predict recurrence and improve survival. In the present study, we describe a newly developed technique for the detection of CTCs, incorporating CdSe QDs for the detection of CTCs specific surface antigens. In the aforementioned method for CTCs detection, we used EpCAM antibody coated MBs and CK19 antibody coated QDs. We used EpCAM since the anti-EpCAM-based immunomagnetic enrichment technology, have showed significant better recovery rates compared to other cytometric technologies in spiking experiments. In addition, EpCAM-based enrichment methods of CTCs in CRC patients have been successfully applied in several cases[25-27]. Regarding CK19, the later is a widely used biomarker to detect tumor cells which derive from epithelial tissues[27].
The developed methodology does not require sample processing for DNA isolation, which facilitates its incorporation at point-of-care. The use of QDs in the proposed approach bypasses the disadvantages of fluorescent dyes often incorporated into immuno-detection tests such as rapid photobleaching, narrow excitation spectrum and low signal intensity.
The accuracy of the QD detection system, as evaluated on clinical samples from CRC patients, compared to reverse transcriptase real-time PCR and FACS analysis applied to blood samples has ranged between 78.57% and 85.71%. Admittedly, PCR-based techniques as well as FACS analysis are among the most reliable and useful methodologies for the detection of CTCs in a variety of cancers. However, both techniques require trained personnel, dedicated space, and high-cost equipment.
It should be noted that while our method shows great promise in sensitive detection of CTCs, it is still far from achieving to detect a single CTC in the whole human circulating blood. At the present time, according to our knowledge, the most sensitive system in probably CellSearchTM, whose detection limit is claimed to be 1 CTC/7.5 mL[25]. However, this system is very expensive and partially subjective by distinguishing the CTC shapes from those of normal cells. Recently, the detection of CTC has also been achieved using surface enhanced Raman spectroscopy with a detection limit of 50 CTC/mL in whole blood[28-30]. The method we presented here, might provide a fast and low-cost alternative way for CTC cell detection. For reasons of applicability the method described here incorporates a combination of cancer and CTC antibodies that increases specificity and at the same time facilitates its adjustment for any other protein target, either of the tumor or the host. Evidently the method can be easily extended to the detection of any other tumor as the adaptation of the method would require only the incorporation of specific antibodies for the cancer or disease in question. Once fully developed, this method will be directly applicable on clinical samples in the context of the one reported above. This implies the potential use of the proposed methodology as a diagnostic technology platform.
The detection of circulating tumor cells (CTCs) is of great importance for the clinical management of patients with solid cancers like colorectal cancer (CRC), due to the fact that they have long been considered as a reflection of tumor aggressiveness. However, owing to the rarity of CTCs in peripheral blood, their detection requires methods combined with high sensitivity and specificity, which sets tremendous challenges for the implementation of these assays into clinical routine.
The concept and existence of CTCs as well as the dissemination and settlement of these cells in secondary organs have been widely accepted. The development of alternative sensitive, speedy, specific and low cost methods for CTC detection is important for cancer prognosis. In the present study, authors demonstrate a sensitive assay that combines magnetic beads isolation and quantum dots (QDs) fluorescence detection for the identification of CRC CTCs surface antigens.
The developed methodology does not require sample processing for DNA isolation, which facilitates its incorporation at point-of-care. The use of QDs in the proposed approach bypasses the disadvantages of fluorescent dyes often incorporated into immuno-detection tests such as rapid photobleaching, narrow excitation spectrum and low signal intensity.
This method can be easily extended to the detection of any other tumor as the adaptation of the method would require only the incorporation of specific antibodies for the cancer or disease in question.
The topic is of significant clinical importance as the detection of circulating cancer cells may used for early diagnosis of the recurrence of the disease.
Peer reviewers: Ferenc Sipos, MD, PhD, Cell Analysis Laboratory, 2nd Department of Internal Medicine, Semmelweis University, Szentkirályi u. 46., 1088 Budapest, Hungary; Fabio Grizzi, PhD, Laboratories of Quantitative Medicine, Istituto Clinico Humanitas IRCCS, Via Manzoni 56, 20089 Rozzano, Milan, Italy
S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1579] [Cited by in RCA: 1612] [Article Influence: 107.5] [Reference Citation Analysis (0)] |
| 2. | Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer--a review. Eur J Surg Oncol. 2011;37:459-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 3. | Rahbari NN, Bork U, Kircher A, Nimitz T, Schölch S, Kahlert C, Schmidt T, Steinert G, Ulrich AB, Reissfelder C. Compartmental differences of circulating tumor cells in colorectal cancer. Ann Surg Oncol. 2012;19:2195-2202. [PubMed] |
| 4. | Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci USA. 2000;97:14608-14613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 459] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 5. | Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat Rev Cancer. 2008;8:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 883] [Cited by in RCA: 868] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 6. | Smirnov DA, Zweitzig DR, Foulk BW, Miller MC, Doyle GV, Pienta KJ, Meropol NJ, Weiner LM, Cohen SJ, Moreno JG. Global gene expression profiling of circulating tumor cells. Cancer Res. 2005;65:4993-4997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Findeisen P, Röckel M, Nees M, Röder C, Kienle P, Von Knebel Doeberitz M, Kalthoff H, Neumaier M. Systematic identification and validation of candidate genes for detection of circulating tumor cells in peripheral blood specimens of colorectal cancer patients. Int J Oncol. 2008;33:1001-1010. [PubMed] |
| 8. | Gazzaniga P, Gradilone A, Petracca A, Nicolazzo C, Raimondi C, Iacovelli R, Naso G, Cortesi E. Molecular markers in circulating tumour cells from metastatic colorectal cancer patients. J Cell Mol Med. 2010;14:2073-2077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Büchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138:1714-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Rahbari NN, Bork U, Motschall E, Thorlund K, Büchler MW, Koch M, Weitz J. Molecular detection of tumor cells in regional lymph nodes is associated with disease recurrence and poor survival in node-negative colorectal cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Losanoff JE, Zhu W, Qin W, Mannello F, Sauter ER. Can mitochondrial DNA mutations in circulating white blood cells and serum be used to detect breast cancer? Breast. 2008;17:540-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Wharton RQ, Patel H, Jonas SK, Glover C, Weston M, Allen-Mersh TG. Venesection needle coring increases positive results with RT-PCR for detection of circulating cells expressing CEA mRNA. Clin Exp Metastasis. 2000;18:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (1)] |
| 13. | Keilholz U, Willhauck M, Rimoldi D, Brasseur F, Dummer W, Rass K, de Vries T, Blaheta J, Voit C, Lethé B. Reliability of reverse transcription-polymerase chain reaction (RT-PCR)-based assays for the detection of circulating tumour cells: a quality-assurance initiative of the EORTC Melanoma Cooperative Group. Eur J Cancer. 1998;34:750-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Tsavellas G, Huang A, McCullough T, Patel H, Araia R, Allen-Mersh TG. Flow cytometry correlates with RT-PCR for detection of spiked but not circulating colorectal cancer cells. Clin Exp Metastasis. 2002;19:495-502. [PubMed] |
| 15. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1951] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 16. | Shao L, Gao Y, Yan F. Semiconductor quantum dots for biomedicial applications. Sensors (Basel). 2011;11:11736-11751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 17. | Pericleous P, Gazouli M, Lyberopoulou A, Rizos S, Nikiteas N, Efstathopoulos EP. Quantum dots hold promise for early cancer imaging and detection. Int J Cancer. 2012;131:519-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Hsieh YH, Lai LJ, Liu SJ, Liang KS. Rapid and sensitive detection of cancer cells by coupling with quantum dots and immunomagnetic separation at low concentrations. Biosens Bioelectron. 2011;26:4249-4252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Eastman PS, Ruan W, Doctolero M, Nuttall R, de Feo G, Park JS, Chu JS, Cooke P, Gray JW, Li S. Qdot nanobarcodes for multiplexed gene expression analysis. Nano Lett. 2006;6:1059-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 20. | Liandris E, Gazouli M, Andreadou M, Sechi LA, Rosu V, Ikonomopoulos J. Detection of pathogenic mycobacteria based on functionalized quantum dots coupled with immunomagnetic separation. PLoS One. 2011;6:e20026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24144] [Cited by in RCA: 25955] [Article Influence: 1081.5] [Reference Citation Analysis (0)] |
| 22. | Zheng H, Chen G, DeLouise LA, Lou Z. Detection of the cancer marker CD146 expression in melanoma cells with semiconductor quantum dot label. J Biomed Nanotechnol. 2010;6:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Abrams B, Dubrovsky T. Quantum dots in flow cytometry. Methods Mol Biol. 2007;374:185-203. [PubMed] |
| 24. | Smith RA, Giorgio TD. Quantitative measurement of multifunctional quantum dot binding to cellular targets using flow cytometry. Cytometry A. 2009;75:465-474. [PubMed] |
| 25. | Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213-3221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1354] [Cited by in RCA: 1409] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 26. | Tol J, Koopman M, Miller MC, Tibbe A, Cats A, Creemers GJ, Vos AH, Nagtegaal ID, Terstappen LW, Punt CJ. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol. 2010;21:1006-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 27. | Königsberg R, Gneist M, Jahn-Kuch D, Pfeiler G, Hager G, Hudec M, Dittrich C, Zeillinger R. Circulating tumor cells in metastatic colorectal cancer: efficacy and feasibility of different enrichment methods. Cancer Lett. 2010;293:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Shimada R, Iinuma H, Akahane T, Horiuchi A, Watanabe T. Prognostic significance of CTCs and CSCs of tumor drainage vein blood in Dukes' stage B and C colorectal cancer patients. Oncol Rep. 2012;27:947-953. [PubMed] |
| 29. | Sha MY, Xu H, Natan MJ, Cromer R. Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J Am Chem Soc. 2008;130:17214-17215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 244] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 30. | Zhang H, Harpster MH, Park HJ, Johnson PA, Wilson WC. Surface-enhanced Raman scattering detection of DNA derived from the west nile virus genome using magnetic capture of Raman-active gold nanoparticles. Anal Chem. 2011;83:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |