Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4335
Revised: July 30, 2012
Accepted: August 3, 2012
Published online: August 28, 2012
AIM: To examine the relative prevalence and temporal variation of dysphagia etiologies in patients undergoing upper endoscopy (EGD) over the past decade.
METHODS: EGDs with the indication of dysphagia at an urban, university medical center in 1999, 2004 and 2009 were retrospectively identified from the electronic medical record. The entire patient chart, including EGD, pathology, manometry, radiographic and clinician reports, was reviewed for demographic and clinical data and to determine the etiology of dysphagia. The number of EGDs in which an esophageal biopsy was performed was also noted. Gastroesophageal reflux disease (GERD) as a cause of dysphagia independent of peptic stricture was defined by symptoms with erosive esophagitis or symptom response to proton pump inhibition (PPI). Cases of eosinophilic esophagitis (EoE) were defined by an appropriate clinical history and histological criteria of ≥ 15 eosinophils per high powered field. PPI-responsive esophageal eosinophilia was not routinely reported prior to 2008. Statistical analysis was performed using one-way analysis of variance to analyze for trends between 1999, 2004 and 2009 and a post-hoc Tukey analysis was performed following a significant main effect.
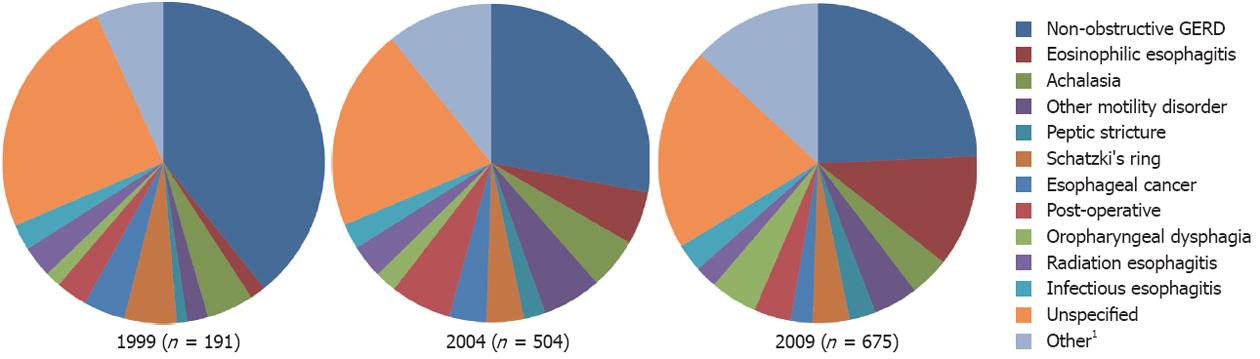
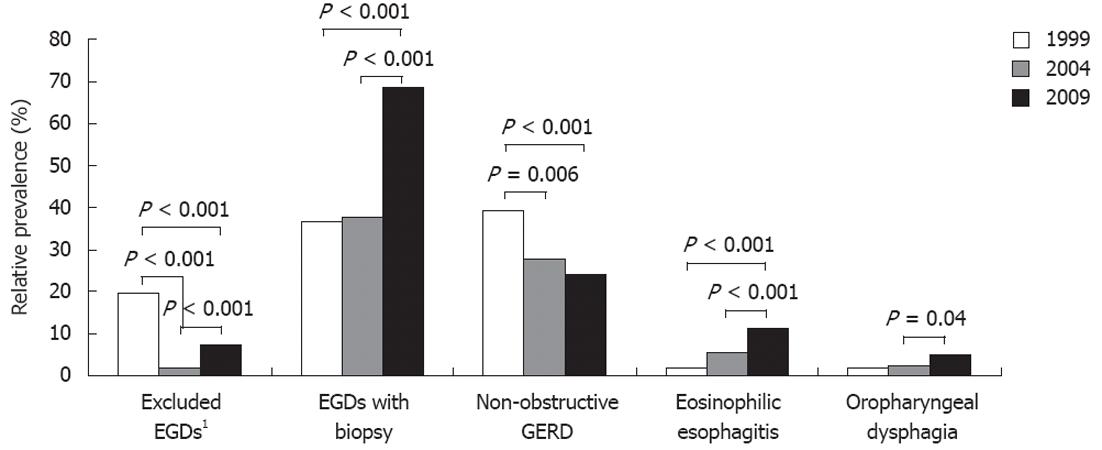
RESULTS: A total of 1371 cases (mean age 54 years, 43% male) met pre-specified inclusion criteria with 191, 504 and 675 cases in 1999, 2004 and 2009, respectively. Patients were older in 2004 compared to 2009 (mean ± SD, 54.0 ± 15.7 years vs 52.3 ± 16.8 years, P = 0.02) and there were more males in 1999 compared to 2004 (57.5% vs 40.8%, P = 0.005). Overall, GERD (27.6%) and EoE (7.7%) were the most common identifiable causes of dysphagia. An unspecified diagnosis accounted for 21% of overall cases. There were no significant differences in the relative prevalence of achalasia or other motility disorders, peptic stricture, Schatzki’s ring, esophageal cancer or unspecified diagnoses over the 10-year time period. There was, however, a decrease in the relative prevalence of GERD (39.3% vs 24.1%, P < 0.001) and increases in the relative prevalence of EoE (1.6% vs 11.2%, P < 0.001) and oropharyngeal disorders (1.6% vs 4.2%, P = 0.02) from 1999 to 2009. Post-hoc analyses determined that the increase in relative prevalence of EoE was significant between 1999 and 2009 as well as 2004 and 2009 (5.4% vs 11.6%, P < 0.001), but not between 1999 and 2004 (1.6% P 5.4%, P = 0.21). On the other hand, the decrease in relative prevalence of GERD was significant between 1999 and 2009 and 1999 and 2004 (39.3% vs 27.7%, P = 0.006), but not between 2004 and 2009 (27.7% vs 24.1%, P = 0.36). There were also significantly more EGDs in which a biopsy was obtained in 1999 compared to 2009 (36.7% vs 68.7%, P < 0.001) as well as between 2004 and 2009 (37.5% vs 68.7%, P < 0.001). While total EGD volume did increase over the 10-year time period, the percentage of EGDs for the indication of dysphagia remained stable making increasing upper endoscopy an unlikely reason for the observed increased prevalence of EoE.
CONCLUSION: EoE has emerged as a dominant cause of dysphagia in adults. Whether this was due to a rise in disease incidence or increased recognition is unclear.
- Citation: Kidambi T, Toto E, Ho N, Taft T, Hirano I. Temporal trends in the relative prevalence of dysphagia etiologies from 1999-2009. World J Gastroenterol 2012; 18(32): 4335-4341
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4335
Dysphagia is a common indication for referral to a gastroenterology specialist, and is classified based on location as either oropharyngeal or esophageal[1,2]. Common causes of oropharyngeal dysphagia include cerebrovascular accidents (CVA), radiation injury, and Parkinson’s disease[2]. Frequently identified etiologies of esophageal dysphagia include structural lesions such as peptic strictures, Schatzki’s rings, and neoplasm as well as non-obstructive disorders such as gastroesophageal reflux disease (GERD) and esophageal motility disorders[1,2]. Upper endoscopy (EGD) has largely supplanted upper gastrointestinal radiologic examination for the initial evaluation of dysphagia[1]. Little published data exists reporting the frequency of the various dysphagia etiologies for patients undergoing EGD.
Recent cross-sectional studies have demonstrated that eosinophilic esophagitis (EoE) is an important and emerging cause of dysphagia[3-6]. These studies, examining patients undergoing an EGD in the evaluation of dysphagia, report an EoE prevalence between 6.5% and 15%[3-5]. The studies did not report the frequency of additional etiologies of dysphagia nor whether the prevalence of EoE has changed over time. A histologic analysis of 296 esophageal biopsies from children in Australia demonstrated an 18 fold increase in the prevalence of EoE from 1995 to 2004, but did not include analysis of clinical or endoscopic data[7]. A population based study from Olten County, Switzerland recently reported a 12 fold increase in the prevalence of EoE from 1989 to 2009[8].
The aims of this study were to examine the prevalence of various etiologies of dysphagia amongst patients undergoing EGD and to determine the relative prevalence of EoE over the past decade.
We performed a retrospective review of patients undergoing EGD for the indication of dysphagia at a single, urban, academic medical center, with the goal of determining the relative prevalence of dysphagia-related diagnoses. Endoscopy at this medical center is performed by both academic and private practice gastroenterologists (approximately two-thirds academic faculty and one-third private practice).
Patients were identified by a query of the electronic medical record (EMR) for all adult inpatients and outpatients who had an EGD ordered with an associated ICD-9 code for dysphagia (787.2) from 1999 through 2010. For ease of data analysis, our search was narrowed to the years of 1999, 2004 and 2009. The total number of EGDs performed for all indications was extracted to assess endoscopy volume. Clinician office and EGD reports were reviewed to confirm that dysphagia was the actual indication for the EGD and that an EGD was, in fact, performed. Cases were excluded if the indication for the EGD was found not to be dysphagia, if a scheduled EGD was never performed, or if the EGD report was absent from the EMR. If a particular patient had multiple EGDs during this time period, that patient was included in the earliest year but final diagnosis was still determined based on the entirety of their medical record. The medical center completed its transition to an EMR in 2003 and medical records prior to this date were uploaded retrospectively to the database, leading to numerous absences of reports prior to 2003.
Pertinent demographic data were collected for the patients who met inclusion criteria. EGD, pathology, manometry, radiographic, and clinician consultation notes were reviewed to determine the etiology of dysphagia. When necessary and available, records from subsequent years were reviewed to determine the final diagnosis. The number of EGDs in which an esophageal biopsy was performed was also noted.
Non-obstructive dysphagia related to GERD was diagnosed by the exclusion of esophageal stricture and response of dysphagia to treatment of acid reflux with proton pump inhibition (PPI)[9]. All-GERD related dysphagia was the sum of non-obstructive GERD and peptic strictures. Non-obstructive dysphagia related to GERD was diagnosed by the exclusion of esophageal stricture and response of dysphagia to treatment of acid reflux. Cases of EoE were defined by an appropriate clinical history (i.e., dysphagia) and ≥ 15 eosinophils per high powered field (EOS/HPF) on histological review. PPI-responsive esophageal eosinophilia was not routinely reported prior to 2008 and was therefore not analyzed separately from GERD as recommended by the 2007 and 2011 consensus statements on EoE[10,11]. Achalasia was defined by characteristic manometric features as well as supportive endoscopic and radiologic abnormalities. Additional abnormal esophageal dysmotility assessed by manometry, such as nutcracker esophagus, diffuse esophageal spasm, or aperistalsis, was grouped as “other motility disorder” and considered the etiology of dysphagia only if subsequent clinician notes specifically attributed dysphagia to esophageal dysmotility. Schatzki’s rings were considered the etiology of dysphagia only if the EGD report explicitly stated that they contributed to the dysphagia; in cases where the EGD or clinician report specifically stated that the Schatzki’s ring was widely open, non-obstructing, or not likely to be contributing to the dysphagia, an alternative diagnosis was considered. Post-operative and iatrogenic etiologies included anastomotic strictures, marginal ulcers, and symptomatic type III paraesophageal hernias. Oropharyngeal disorders included CVA, traumatic brain injury, and neurologic disorders such as amyotrophic lateral sclerosis or multiple sclerosis in which patients had an unremarkable endoscopic exam along with either an abnormal radiologic swallow study and, or an abnormal swallow evaluation by a speech and language pathologist. Functional dysphagia was deemed the final diagnosis after all other organic causes were ruled out based on negative EGD, pathology reports, and motility studies, and if the clinician’s reports indicated that the patient’s dysphagia was functional. Given its low prevalence, it was included in the “other” category. In cases where review of the chart was unable to determine an etiology, the diagnosis was considered “unspecified”.
Data were analyzed using statistical software, SPSS version 20 (IBM SPSS Inc, Chicago, IL). The number of excluded EGDs was expressed as a percentage of the total number of EGDs for dysphagia in that year. The total number of included EGDs for dysphagia was the difference between the total number of EGDs for dysphagia and the excluded EGDs for dysphagia. The number of EGDs in which an esophageal biopsy was performed and the relative prevalence of the various etiologies for dysphagia were expressed as a percentage of the included EGDs for dysphagia in the given year.
One-way analysis of variance was used to analyze for trends between 1999, 2004 and 2009. Following the significant main effect, a Tukey post-hoc analysis was performed. A P value of less than 0.05 was considered statistically significant. The study was approved by the Northwestern University Institutional Review Board.
A total of 1478 patients were identified who had an EGD ordered for dysphagia, with 237, 513 and 728 cases in 1999, 2004 and 2009, respectively. A total of 1371 cases met inclusion criteria with 191, 504 and 675 cases in 1999, 2004 and 2009, respectively (Table 1). In 1999, 46 cases (19.4%) were excluded because EGD reports were unavailable in the EMR (44 cases) or the indication for EGD was found to be upper gastrointestinal bleeding. In 2004, 9 cases (1.8%) were excluded because review of the EMR revealed the indication for EGD was either an upper gastrointestinal bleed or iron-deficiency anemia. In 2009, 53 cases (7.3%) were excluded because an EGD was not performed (46 cases) or because the indication was found not be dysphagia on review of the chart. There was a significant difference in the number of excluded EGDs between 1999, 2004, and 2009 (P < 0.001).
| 1999 | 2004 | 2009 | P value | |
| Total EGDs performed | 2456 | 5944 | 9071 | -- |
| Total included EGDs for dysphagia | 191 | 504 | 675 | -- |
| Excluded EGDs for dysphagia | 46 (19.4) | 9 (1.8) | 53 (7.3) | < 0.001 |
| Age (yr), mean (SD) | 55.5 (16.1) | 54.0 (15.7) | 52.3 (16.8) | NS |
| Male sex (%) | 57.5 | 40.8 | 40.7 | 0.005 |
| EGD with biopsy performed | 70 (36.7) | 189 (37.5) | 464 (68.7) | < 0.001 |
| Diagnosis | ||||
| Non-obstructive GERD | 75 (39.3) | 140 (27.7) | 163 (24.1) | < 0.001 |
| Eosinophilic esophagitis | 3 (1.6) | 27 (5.4) | 76 (11.2) | < 0.001 |
| Achalasia | 9 (4.7) | 26 (5.1) | 27 (4.0) | NS |
| Other motility disorder | 4 (2.1) | 30 (5.8) | 30 (4.4) | NS |
| Peptic stricture | 2 (1.0) | 11 (2.1) | 18 (2.6) | NS |
| Schatzki's ring | 10 (5.2) | 19 (3.7) | 25 (3.7) | NS |
| Esophageal cancer | 8 (4.2) | 19 (3.7) | 15 (2.2) | NS |
| Post-operative | 6 (3.1) | 31 (6.1) | 25 (3.7) | NS |
| Oropharyngeal dysphagia | 3 (1.6) | 11 (2.1) | 32 (4.7) | 0.02 |
| Radiation esophagitis | 6 (3.1) | 17 (3.4) | 15 (2.2) | NS |
| Infectious esophagitis | 5 (2.6) | 13 (2.5) | 19 (2.8) | NS |
| Unspecified | 47 (24.6) | 103 (20.4) | 138 (20.4) | NS |
| Other1 | 13 (6.8) | 54 (10.7) | 88 (13.0) | NS |
The mean age of the patients included in the study was 53.5 years (SD = 16.3 years) with 43% of the patients being men (n = 591). Patients were older in 2004 compared to 2009 (P = 0.04) and there were more men in 1999 compared to 2004 (P = 0.003).
Overall, non-obstructive GERD (27.6%) and EoE (7.7%) were the most common identifiable causes of dysphagia over the ten year period. An unspecified diagnosis accounted for 21.0% of cases. Other motility disorders (4.7%), achalasia (4.5%) and dysphagia secondary to a post-operative/miscellaneous etiology (4.5%) were other important identifiable causes.
The relative prevalence of the various etiologies for dysphagia over the ten year time period is shown in Table 1 and Figure 1. When analyzing for temporal trends, there was a decrease in the relative prevalence of non-obstructive GERD (39.3% in 1999 and 24.1% in 2009; P < 0.001), and increases in the relative prevalence of EoE (1.6% in 1999 and 11.2% in 2009, P < 0.001) and oropharyngeal disorders (1.6% in 1999 and 4.7% in 2009; P = 0.02). There was no significant difference in the relative prevalence of all-GERD related dysphagia (non-obstructive GERD and peptic strictures) over the 10-year time period (P = 0.07).
Select post-hoc Tukey analyses are shown graphically in Figure 2. There was a decrease in the relative prevalence of non-obstructive GERD from 1999 to 2004 (P = 0.006) as well as from 1999 to 2009 (P < 0.001). The increase in the relative prevalence of EoE between 1999 and 2009 was significant (P < 0.001) as was the increase between 2004 and 2009 (P < 0.001). Of the three patients in 1999 ultimately diagnosed with EoE who had an EGD for dysphagia, one patient was actually diagnosed with EoE in 2003 and another patient was diagnosed in 2005. As specified in the methods section, these patients were included in the 1999 cohort. The increase in relative prevalence of oropharyngeal disorders was significant between 2004 and 2009 (P = 0.04). There was no significant difference in the relative prevalence of achalasia, other esophageal motility disorders, peptic strictures, Schatzki’s rings, esophageal cancer, or unspecified diagnosis over the ten year time period (Table 1 and Figure 1).
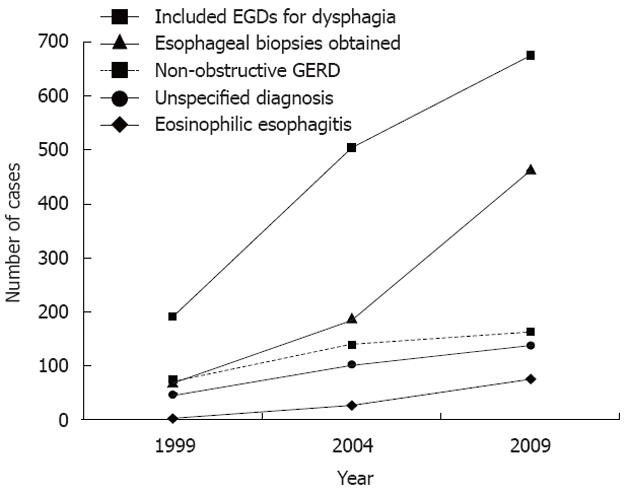
The percentage of EGDs in which an esophageal biopsy was obtained increased over the time period (P < 0.001). As shown in Figure 2, this increase was significant between 1999 and 2009 (P < 0.001) as well as between 2004 and 2009 (P < 0.001); there was no significant difference in the proportion of EGDs in which a biopsy was performed in 1999 compared to 2004 (P = 0.98). Total EGD volume at the medical center also increased in the ten year time period with 2456 EGDs performed in 1999, 5944 in 2004 and 9071 in 2009. The percentage of EGDs performed for the indication of dysphagia remained stable at 8%-9%.
This is the first study to describe the relative prevalence of distinct etiologies of dysphagia in an adult patient population undergoing EGD. In our cohort, non-obstructive GERD was the most common, identifiable cause of dysphagia but its relative prevalence decreased over the past decade while EoE was the second most common identifiable cause and its relative prevalence increased from 1.6% in 1999 to 11.2% in 2009. Interestingly, the relative prevalence of all-GERD related dysphagia remained constant. The number of EGDs performed for the indication of dysphagia as a percentage of total EGD volume remained stable at 8%-9%. However, the proportion of EGDs with esophageal biopsies obtained did increase from 36.7% in 1999 to 68.7% in 2009.
The emergence of EoE as a leading cause of dysphagia is of interest and supports earlier studies. The relative prevalence of EoE in 2009 of 11.2% is consistent with the results of recent prospective, short-term, cross-sectional studies[3-5] that reported an EoE prevalence ranging from 10%-15% in patients with dysphagia undergoing EGD. While these studies reported EoE prevalence, they did not report the other etiologies of dysphagia in the non-EoE patients. In these studies, EoE was defined by histological criterion of ≥ 20 EOS/HPF, which was more stringent than our definition of ≥ 15 EOS/HPF. In two of the studies[4,5], all patients underwent esophageal biopsies regardless of endoscopic findings while 59% of the patients in the other study[3] underwent esophageal biopsy. In all of the studies, a specific protocol for obtaining multiple biopsies along the length of the esophagus (midesophagus vs proximal and distal) was employed; in our retrospective study, a standardized biopsy strategy could not be used. Regardless of the differences in study designs, it is interesting to note that the high prevalence of EoE in our study was similar to that reported in other prospective studies of patients with dysphagia undergoing EGD.
The rise in the relative prevalence of EoE over the past decade is another interesting finding of this study. In a retrospective epidemiological study of Olmsted County, Prasad et al[6] reviewed pathology specimens that mentioned “eosinophils” and/or “esophagitis” from 1976 to 2005 and found that there was an increase in the incidence of EoE over time (P < 0.001). The histological criterion used in that study was ≥ 15 EOS/HPF and esophageal symptoms were assessed by review of the medical record. However, the authors noted a parallel increase in endoscopy utilization over the three decades and raised the possibility that recognition bias from increased endoscopy volume contributed to the rise in EoE diagnoses. In contrast to our cohort, the absolute number of just 78 EoE cases in the Olmsted County population was quite small. Additionally, the population in the study was not limited to patients with dysphagia.
The largest EoE prevalence study to date utilized a national pathology database to identify 363 cases of EoE from biopsy specimens taken between January 2002 and May 2006 with “eosinophilic” in the diagnosis and/or comment text[12]. In their subset analyses of pathology specimens from 12 465 patients who underwent an EGD for the indication of dysphagia, they found an increasing prevalence of EoE from 2002 to 2005 (P < 0.001), with a prevalence of 0.1% in 2002 and 1.9% in 2005. This study had many important differences from ours. Cases were identified from a pathology database and the timeframe studied was shorter. Additionally, dysphagia as the indication for EGD was not confirmed by review of the medical chart, which, as seen in our study, would have potentially excluded a number of cases. Lastly, only EGDs for dysphagia in which a biopsy was obtained were included in their study, which is only a subset of the total number of EGDs performed for dysphagia although it is surprising that the reported prevalence was much lower than that of the present study.
It is difficult to ascertain whether the increase in relative prevalence of EoE seen in our study is due to a true rise in population prevalence or secondary to heightened recognition from increased numbers of biopsies or increased clinician awareness of the disorder. Interestingly, the prevalence of an “unspecified” cause of dysphagia remained constant at 20%-24% over the measured time period implying that EoE was not simply misclassified as “unknown” a decade ago. It is possible that EoE was initially misclassified as non-obstructive GERD and then properly classified as EoE with increased awareness of the disease, although each patient’s entire EMR was reviewed so that if a subsequent diagnosis of EoE was made at our medical center it would have been detected. However, another plausible explanation for the decrease in prevalence of non-obstructive GERD is the increasing utilization of PPIs by both patients (over-the-counter) and primary care physicians so that only a smaller percentage of PPI-refractory patients are undergoing EGDs.
Increased recognition of EoE may explain the rise in prevalence given the increase in esophageal biopsies obtained, as shown in Figure 3. Lending credibility to this theory is the fact that as the number of biopsies increased between 1999 and 2009 as well as between 2004 and 2009, the prevalence of EoE also increased (Figure 2). On the other hand, when there was not a significant increase in biopsies, such as between 1999 and 2004, the relative prevalence of EoE also did not significantly increase. In contrast to the study by Prasad et al[6] which suggested that increased endoscopy volume may have contributed to increased recognition of EoE, our study provides more specific insight into the issue of increased recognition by identifying trends in the number of biopsies taken.
The stable and relatively low overall prevalence of esophageal cancer (3.1%) was surprising given the rise in esophageal adenocarcinoma seen in the past five decades[13,14]. One possible explanation for the low prevalence of esophageal cancer seen in our study is that our medical center is a tertiary care center and patients with esophageal cancer causing dysphagia were being diagnosed in the community and referred directly to oncology and surgery.
The mechanism for non-obstructive dysphagia in GERD is not clear. Several studies have demonstrated that non-obstructive dysphagia in GERD is common and improves with therapy directed at acid reflux[15,16]. Manometric abnormalities are commonly cited as explanations for this entity[17], but the role of altered visceral sensation as well as diminished esophageal wall compliance owing to inflammation have not been adequately examined. In a study by Triadafilopoulos[18], dysphagia was more commonly associated with severity of acid reflux on pH monitoring or erosive esophagitis with only a minority having abnormal motility.
The primary mechanism for dysphagia in EoE is esophageal remodeling secondary to subepithelial fibrosis that is identified in over 90% of patients[11]. Additionally, structural alterations of the esophageal luminal diameter in the form of focal strictures, esophageal rings or narrow caliber esophagus can be identified in most adults with EoE. In fact, the impact of these structural alterations in EoE have been verified and shown to decrease esophageal mural compliance and lead to significantly reduced esophageal distensibility in EoE patients compared to normal controls[19]. Furthermore, there is indirect evidence of the role of fibrostenotic complications in the pathogenesis of dysphagia in EoE given the effectiveness of esophageal dilation in the treatment of dysphagia in EoE[20,21].
There were several important limitations to this study. The large number of excluded cases in 1999 was due to unavailable data in the EMR (given the transition from paper charting in 2003) while the excluded cases in 2009 were because the EGDs were not performed; both of these pitfalls were largely attributable to the retrospective nature of this study. The large number of excluded cases and the difference in age of the patients in 2004 compared to 2009 raise the question of whether the groups being analyzed were subsets of the same larger population. The retrospective nature of the study did not allow for a standardized protocol for esophageal biopsies, which may have led to confounding by indication since biopsies were more likely to be obtained when the EGD showed the classic EoE features[3,11] of rings, linear furrows, and exudates. The setting of the study was a single, urban, tertiary care center with active esophageal motility and EoE research, so there was the potential for loss to follow-up outside of the medical center in addition to referral bias that limits the generalizability of the results to other practice settings. The utilization of EGD as the primary modality for the evaluation of dysphagia may affect the generalizability of the results to populations where dysphagia may be assessed with barium esophagrams. In addition, many referred patients may have had a diagnostic endoscopy for dysphagia performed at an outside facility that already established the etiology. When undergoing a follow up EGD at our medical center, such patients may have had listed indications for EGD such as “GERD” or “unspecified esophagitis” and would have been potentially excluded in our analysis.
Another potential problem with this study was how EoE was defined. It is likely that PPI-responsive esophageal eosinophilia was included in the EoE cohort[11]. Our definition of EoE did not require the exclusion of GERD with a trial of PPI therapy or a normal pH monitoring study as suggested by the 2007 consensus definition[10] and its 2011 update[11], since this criterion was not applied widely prior to 2008. The understanding of interactions between GERD and EoE has become increasingly complex and remains controversial[22,23]. However, significant esophageal eosinophilia (≥ 15 EOS/HPF) is uncommon in GERD, being demonstrated in less than 2% of patients in one large study[24]. While a significant proportion of patients with suspected EoE respond to PPI therapy, this response may not be specific for acid reflux[25] and may occur with an allergic pattern of inflammation[26].
In summary, the relative prevalence of EoE in patients undergoing EGD for dysphagia increased from 1.6% to 11.2% over the past decade. EoE has emerged as one of the dominant, identifiable causes of dysphagia in adults, second only to GERD. Prospective, long-term studies are needed to discern whether this is due to a true increase in disease prevalence or increased recognition.
Dysphagia is a commonly encountered clinical problem and limited data exist regarding the prevalence of dysphagia etiologies. Recently, cross-sectional studies have demonstrated that eosinophilic esophagitis (EoE) is an important cause of dysphagia.
Upper endoscopy (EGD) is critical in the evaluation and management of patients with dysphagia. To the knowledge, this is the first study to integrate clinical, pathological, EGD, manometry, and imaging reports in order to systematically report the relative prevalence of all dysphagia-related diagnoses in a large series of patients undergoing EGD, which provides an evidence-based differential diagnosis for the practicing gastroenterologist. Cross-sectional studies have reported on the prevalence of EoE in patients with dysphagia, however these studies have not looked at EoE prevalence over time and with relation to other dysphagia diagnoses. This study demonstrated that between 1999 and 2009, the relative prevalence of gastroesophageal reflux disease (GERD) decreased while the relative prevalence of EoE increased.
Recent studies have reported an EoE relative prevalence between 10%-15% amongst patients undergoing EGD for dysphagia. This study shows that the relative prevalence of EoE has risen significantly from 1.6% to 11.2% over the past decade making it the second most common identifiable cause of dysphagia. Furthermore, the authors’post-hoc analysis show that in contrast to previous studies, the proportion of EGDs for dysphagia remained stable over the measured time, but the percentage of EGDs in which a biopsy was performed did increase significantly, providing a plausible mechanism for increased recognition of EoE.
By providing an evidence based differential diagnosis, this study informs clinicians’ decision making in the evaluation of dysphagia. Given the possible inclusion of proton pump inhibition (PPI)-responsive esophageal eosinophilia in the EoE cohort and the increasing proportion of EGDs with biopsies, prospective, long-term studies would be beneficial to discern whether the findings were due to a true increase in EoE prevalence or increased recognition.
EoE is a chronic, immune/antigen driven inflammatory disease of the esophagus defined by clinical symptoms of esophageal dysfunction as well as pathological criteria of ≥ 15 eosinophils per high powered field. Current guideline recommendations require exclusion of other causes of esophageal eosinophilia, such as GERD, to make the diagnosis of EoE. By acknowledging the accumulating body of evidence showing that PPIs have effects beyond acid suppression alone, a new disease entity termed “PPI-responsive esophageal eosinophilia”, which is distinct from GERD, has been recognized.
The authors examined the relative prevalence and temporal variation of dysphagia etiologies in patients undergoing EGD over the past decade and found a decrease in the prevalence of GERD and increases in the prevalence of EoE and oropharyngeal disorders. This is an interesting research.
Peer reviewer: Mauro Bortolotti, MD, Professor, Department of Internal Medicine and Gastroenterology, University of Bologna, Via Massarenti 48, 40138 Bologna, Italy
S- Editor Gou SX L- Editor A E- Editor Xiong L
| 1. | Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s Principles of Internal Medicine. 18th ed. New York: McGraw-Hill 2011; Chapter 38-Dysphagia. |
| 2. | Clouse RE. Approach to the patient with dysphagia or odynophagia. Textbook of Gastroenterology. 4th edition. Philadelphia, PA: Lippincott Williams and Wilkins 2003; 678-691. |
| 3. | Prasad GA, Talley NJ, Romero Y, Arora AS, Kryzer LA, Smyrk TC, Alexander JA. Prevalence and predictive factors of eosinophilic esophagitis in patients presenting with dysphagia: a prospective study. Am J Gastroenterol. 2007;102:2627-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 205] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Mackenzie SH, Go M, Chadwick B, Thomas K, Fang J, Kuwada S, Lamphier S, Hilden K, Peterson K. Eosinophilic oesophagitis in patients presenting with dysphagia--a prospective analysis. Aliment Pharmacol Ther. 2008;28:1140-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Veerappan GR, Perry JL, Duncan TJ, Baker TP, Maydonovitch C, Lake JM, Wong RK, Osgard EM. Prevalence of eosinophilic esophagitis in an adult population undergoing upper endoscopy: a prospective study. Clin Gastroenterol Hepatol. 2009;7:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Prasad GA, Alexander JA, Schleck CD, Zinsmeister AR, Smyrk TC, Elias RM, Locke GR, Talley NJ. Epidemiology of eosinophilic esophagitis over three decades in Olmsted County, Minnesota. Clin Gastroenterol Hepatol. 2009;7:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 378] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 7. | Cherian S, Smith NM, Forbes DA. Rapidly increasing prevalence of eosinophilic oesophagitis in Western Australia. Arch Dis Child. 2006;91:1000-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Hruz P, Straumann A, Bussmann C, Heer P, Simon HU, Zwahlen M, Beglinger C, Schoepfer AM. Escalating incidence of eosinophilic esophagitis: a 20-year prospective, population-based study in Olten County, Switzerland. J Allergy Clin Immunol. 2011;128:1349-1350.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 9. | DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 526] [Article Influence: 26.3] [Reference Citation Analysis (2)] |
| 10. | Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, Bonis P, Hassall E, Straumann A, Rothenberg ME. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1253] [Cited by in RCA: 1155] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 11. | Liacouras CA, Furuta GT, Hirano I, Atkins D, Attwood SE, Bonis PA, Burks AW, Chehade M, Collins MH, Dellon ES. Eosinophilic esophagitis: updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128:3-20.e6; quiz 21-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1605] [Cited by in RCA: 1486] [Article Influence: 106.1] [Reference Citation Analysis (1)] |
| 12. | Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA. Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology. 2008;134:1316-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 13. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [PubMed] |
| 14. | Abrams JA, Sharaiha RZ, Gonsalves L, Lightdale CJ, Neugut AI. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940-2007. Cancer Epidemiol Biomarkers Prev. 2011;20:183-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Vakil NB, Traxler B, Levine D. Dysphagia in patients with erosive esophagitis: prevalence, severity, and response to proton pump inhibitor treatment. Clin Gastroenterol Hepatol. 2004;2:665-668. [PubMed] |
| 16. | Grande L, Lacima G, Ros E, Pujol A, Garcia-Valdecasas JC, Fuster J, Visa J, Pera C. Dysphagia and esophageal motor dysfunction in gastroesophageal reflux are corrected by fundoplication. J Clin Gastroenterol. 1991;13:11-16. [PubMed] |
| 17. | Kahrilas PJ, Dodds WJ, Hogan WJ, Kern M, Arndorfer RC, Reece A. Esophageal peristaltic dysfunction in peptic esophagitis. Gastroenterology. 1986;91:897-904. [PubMed] |
| 18. | Triadafilopoulos G. Nonobstructive dysphagia in reflux esophagitis. Am J Gastroenterol. 1989;84:614-618. [PubMed] |
| 19. | Kwiatek MA, Hirano I, Kahrilas PJ, Rothe J, Luger D, Pandolfino JE. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 286] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 20. | Schoepfer AM, Gonsalves N, Bussmann C, Conus S, Simon HU, Straumann A, Hirano I. Esophageal dilation in eosinophilic esophagitis: effectiveness, safety, and impact on the underlying inflammation. Am J Gastroenterol. 2010;105:1062-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 233] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 21. | Jung KW, Gundersen N, Kopacova J, Arora AS, Romero Y, Katzka D, Francis D, Schreiber J, Dierkhising RA, Talley NJ. Occurrence of and risk factors for complications after endoscopic dilation in eosinophilic esophagitis. Gastrointest Endosc. 2011;73:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Spechler SJ, Genta RM, Souza RF. Thoughts on the complex relationship between gastroesophageal reflux disease and eosinophilic esophagitis. Am J Gastroenterol. 2007;102:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 23. | Hirano I. Eosinophilic esophagitis and gastroesophageal reflux disease: there and back again. Clin Gastroenterol Hepatol. 2011;9:99-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Fiocca R, Mastracci L, Engström C, Attwood S, Ell C, Galmiche JP, Hatlebakk J, Junghard O, Lind T, Lundell L. Long-term outcome of microscopic esophagitis in chronic GERD patients treated with esomeprazole or laparoscopic antireflux surgery in the LOTUS trial. Am J Gastroenterol. 2010;105:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312-2317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 252] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 26. | Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, Spechler SJ, Souza RF. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2012;May 12; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 261] [Article Influence: 21.8] [Reference Citation Analysis (0)] |