Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4308
Revised: August 16, 2012
Accepted: August 18, 2012
Published online: August 28, 2012
AIM: To elucidate the colonoscopic features of serrated lesions of the colorectum using magnifying colonoscopy.
METHODS: Broad division of serrated lesions of the colorectum into hyperplastic polyps (HPs), traditional serrated adenomas (TSAs), and sessile serrated adenomas/polyps (SSA/Ps) has been proposed on the basis of recent molecular biological studies. However, few reports have examined the colonoscopic features of these divisions, including magnified colonoscopic findings. This study examined 118 lesions excised in our hospital as suspected serrated lesions after magnified observation between January 2008 and September 2011. Patient characteristics (sex, age), conventional colonoscopic findings (location, size, morphology, color, mucin) and magnified colonoscopic findings (pit pattern diagnosis) were interpreted by five colonoscopists with experience in over 1000 colonoscopies, and were compared with histopathological diagnoses. The pit patterns were categorized according to Kudo’s classification, but a more detailed investigation was also performed using the subclassification [type II-Open (type II-O), type II-Long (type II-L), or type IV-Serrated (type IV-S)] proposed by Kimura T and Yamano H.
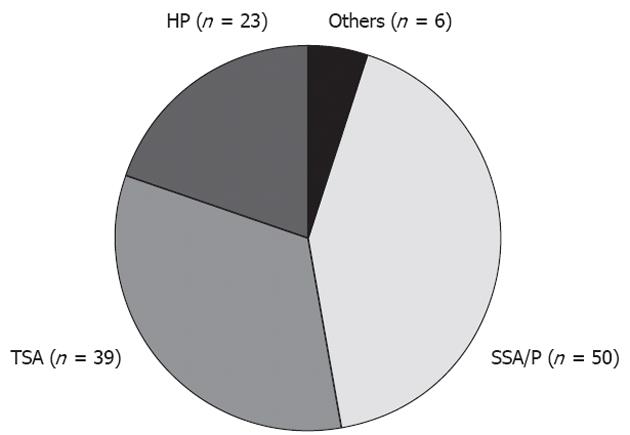
RESULTS: Lesions comprised 23 HPs (23/118: 19.5%), 39 TSAs (39/118: 33.1%: with cancer in one case), 50 SSA/Ps (50/118: 42.4%: complicated with cancer in three cases), and six others (6/118: 5.1%). We excluded six others, including three regular adenomas, one hamartoma, one inflammatory polyp, and one juvenile polyp for further analysis. Conventional colonoscopy showed that SSA/Ps were characterized as larger in diameter than TSAs and HPs (SSA/P vs HP, 13.62 ± 8.62 mm vs 7.74 ± 3.24 mm, P < 0.001; SSA/Ps vs TSA, 13.62 ± 8.62 mm vs 9.89 ± 5.73 mm, P < 0.01); common in the right side of the colon [HPs, 30.4% (7/23): TSAs, 20.5% (8/39): SSA/P, 84.0% (42/50), P < 0.001]; flat-elevated lesion [HPs, 30.4% (7/23): TSAs, 5.1% (2/39): SSA/Ps, 90.0% (45/50), P < 0.001]; normal-colored or pale imucosa [HPs, 34.8% (8/23): TSAs, 10.3% (4/39): SSA/Ps, 80% (40/50), P < 0.001]; and with large amounts of mucin [HPs, 21.7% (5/23): TSAs, 17.9% (7/39): SSA/Ps, 72.0% (36/50), P < 0.001]. In magnified colonoscopic findings, 17 lesions showed either type II pit pattern alone or partial type II pit pattern as the basic architecture, with 14 HPs (14/17, 70.0%) and 3 SSA/Ps. Magnified colonoscopy showed the type II-O pit pattern as characteristic of SSA/Ps [sensitivity 83.7% (41/49), specificity 85.7% (54/63)]. Cancer was also present in three lesions, in all of which a type VI pit pattern was also present within the same lesion. There were four HPs and four TSAs each. The type IV-S pit pattern was characteristic of TSAs [sensitivity 96.7% (30/31), specificity 89.9% (72/81)]. Cancer was present in one lesion, in which a type VI pit pattern was also present within the same lesion. In our study, serrated lesions of the colorectum also possessed the features described in previous reports of conventional colonoscopic findings. The pit pattern diagnosis using magnifying colonoscopy, particularly magnified colonoscopic findings using subclassifications of surface architecture, reflected the pathological characteristics of SSA/Ps and TSAs, and will be useful for colonoscopic diagnosis.
CONCLUSION: We suggest that this system could be a good diagnostic tool for SSA/Ps using magnifying colonoscopy.
- Citation: Ishigooka S, Nomoto M, Obinata N, Oishi Y, Sato Y, Nakatsu S, Suzuki M, Ikeda Y, Maehata T, Kimura T, Watanabe Y, Nakajima T, Yamano HO, Yasuda H, Itoh F. Evaluation of magnifying colonoscopy in the diagnosis of serrated polyps. World J Gastroenterol 2012; 18(32): 4308-4316
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4308.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4308
In addition to the previously known oncogenic pathways of adenoma-carcinoma sequence and de novo cancer[1], the so-called serrated neoplastic pathway has been proposed in recent years as a new oncogenic pathway from serrated lesions of the colorectum[2-6]. Sessile serrated adenomas/polyps (SSA/Ps) are a particular focus of attention as one type of precursor lesion for the microsatellite deficiency (MSI-H) cancers that comprise around 15% of sporadic colorectal cancers[7-11]. The low risk subtype of serrated adenocarcinomas is characterized by proximal location, BRAF-mutation, high CpG-island methylation with loss of MLH1 expression, and MSI-H phenotype[10,12-14]. Identifying serrated lesions of the colorectum during colorectal cancer surveillance is thus important, but few reports have described the colonoscopic characteristics of these lesions, particularly in terms of magnified colonoscopic findings, and a unified consensus has yet to be reached. We report herein our investigation of the colonoscopic characteristics of serrated lesions of the colorectum, using the surface architecture subclassification proposed by Kimura et al[15] in addition to conventional pit pattern diagnosis.
From among all suspected serrated lesions that were endoscopically resected in our hospital between January 2008 and September 2011, this study examined the 118 lesions for which magnified colonoscopic findings and histopathological specimens could be compared. The study was conducted in accordance with all rules and regulations of the St. Marianna University School of Medicine Institutional Review Board, and informed consent was obtained from each patient.
All lesions were sprayed with indigo carmine and observed under endoscopic guidance with a CF-H260AZI endoscope using the EVIS LUCERA system (Olympus, Inc., Tokyo, Japan).
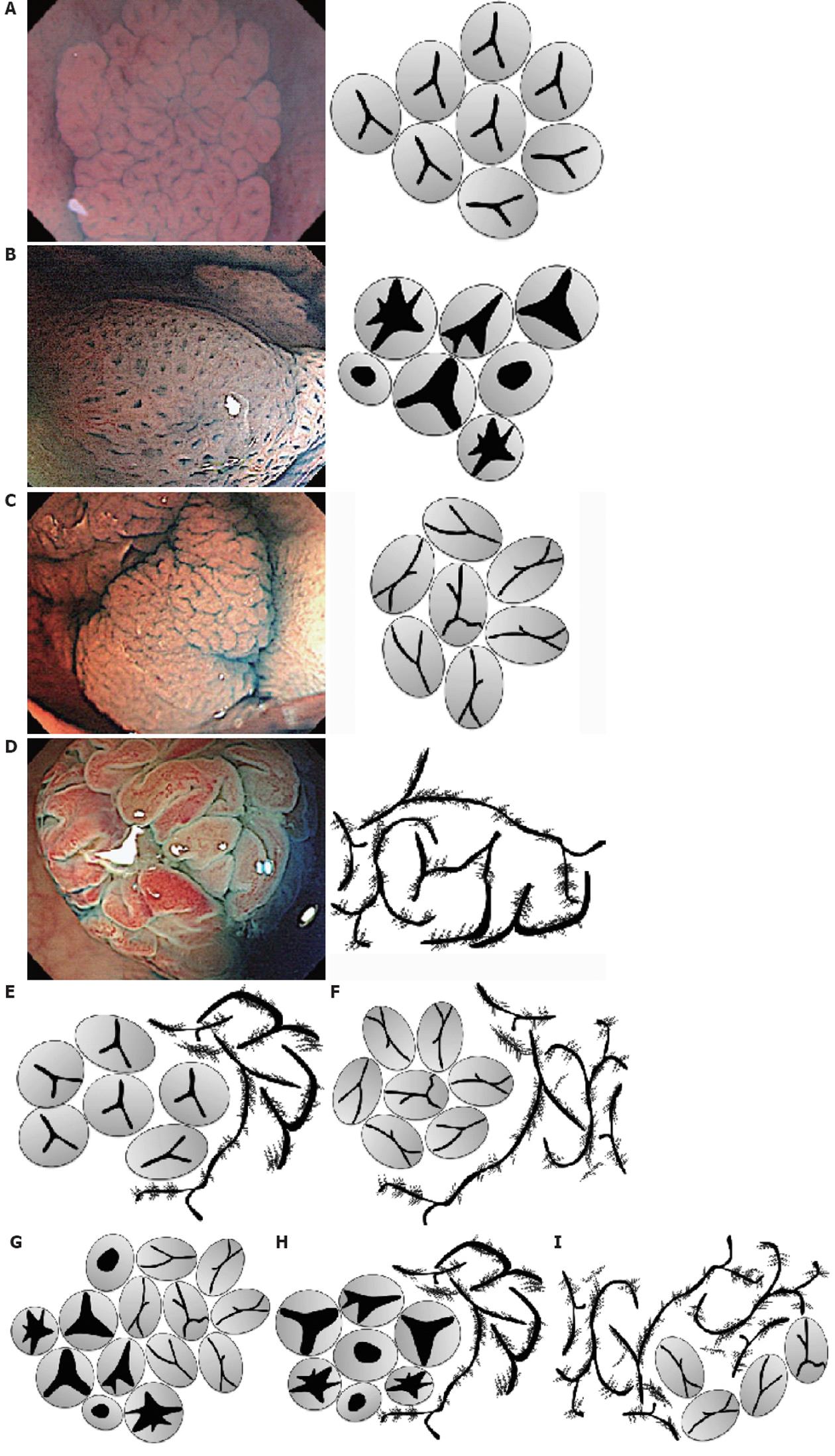
Five categories of conventional colonoscopic findings (location, size, shape, color, and presence or absence of mucin) and magnifying colonoscopic findings (pit pattern diagnosis) for these lesions were interpreted by five colonoscopists with experience in over 1000 colonoscopies, and compared with histopathological diagnoses. In addition to Kudo’s classification, the pit pattern was diagnosed in accordance with the subclassification proposed by Kimura et al[15] and Kudo et al[16] (Figure 1A-I). The endoscope was washed with an automatic washing machine and disinfectant (DISOPA Solution 0.55%, Johnson and Johnson, Langhorne, PA, United States) after each patient, according to the guidelines[17-19].
In addition to conventional type II pit pattern according to Kudo’s classification, surface architecture of the lesions was also broadly categorized as type II-Open (type II-O), type II-Long (type II-L), or type IV-Serrated (type IV-S) according to the provisional naming proposed by Kimura et al[15]. If several pit patterns were present in a single lesion, the pit pattern that occupied the greatest area was regarded as the basic architecture. We categorized into two groups as SSA/Ps or others on histopathological findings. Lesions that included a diagnosis other than that of serrated lesion of the colon are indicated as “others”, SSA/P, or SSA/P plus if adenoma was also present together with “SSA/Ps”.
Histopathological diagnosis followed conventional diagnostic criteria for regular adenomas, the diagnostic criteria of Torlakovic et al[20] for hyperplastic polyps (HPs) and traditional serrated adenomas (TSAs). For practical purposes, according to the 2010 WHO classification, the diagnostic criteria for SSA/Ps was established by the research project “Potential of Cancerization of Colorectal Serrated Lesions” led by the Japanese Society for Cancer of the Colon and Rectum (JSCCR)[20,21].
According to these criteria, SSA/Ps are composed of serrated cryptal epithelium with aberrant compartmentalization, essentially characterized by the architectural abnormalities listed below. If the serrated lesions had more than two findings [crypt dilation; irregularly branching crypts; horizontally arranged basal crypts (inverted T- and/or L- shaped crypts)], it can be diagnosed as SSA/Ps[21].
Statistical testing comprised t tests, χ2 tests, and Fisher’s exact test, with values of P < 0.05 regarded as significant. All statistical analysis were performed using PRISM software for Windows, Version 4 (GraphPad Prism, Inc., San Diego, CA).
Lesions comprised 23 HPs, 39 TSAs (complicated with cancer in one case), 50 SSA/Ps (complicated with cancer in three cases), and six others, including regular adenomas and juvenile polyps. We excluded these six other lesions for further analysis. Patient characteristics and conventional colonoscopic findings are shown in Table 1 and Figure 2. Mean age and sex (percentage of females) were 58.9 years/13% for HPs, 61.9 years/33.3% for TSAs, and 63.7 years/32% for SSA/Ps. No characteristic trends were evident in patient backgrounds (Table 1).
| HPs | TSAs | SSA/Ps | |
| Cases (n) | 23 | 39 | 50 |
| Age (yr), mean ± SD | 58.9 ± 12.8 | 61.9 ± 13.6 | 63.7 ± 10.6 |
| Location | |||
| Right side of colon | 7 (30.4) | 8 (20.5) | 42 (84.0) |
| Left side of colon | 16 (69.6) | 15 (79.5) | 8 (16.0) |
| Morphology | |||
| Flat type | 7 (30.4) | 2 (5.0) | 45 (90.0) |
| Protruded type | 16 (69.6) | 37 (95.0) | 5 (10.0) |
| Color | |||
| NC | 8 (34.8) | 4 (10.3) | 40 (80.0) |
| RC | 15 (65.2) | 35 (89.7) | 10 (20.0) |
| Mucin | |||
| With | 5 (21.7) | 7 (17.9) | 36 (72.0) |
| Without | 18 (78.3) | 32 (82.1) | 14 (28.0) |
| Size (mm), mean ± SD | 7.74 ± 3.24 | 9.89 ± 5.73 | 13.42 ± 8.62 |
We categorized the location of lesions as either right side of the colon (on the oral side of the transverse colon) or left side of the colon (on the anal side of the transverse colon). Results showed that HPs tended to locate on the left side of the colon 69.6% (16/23) rather than the right side 30.4% (7/23). TSAs were more frequently located on the left side 79.5% (31/39) rather than the right side 20.5% (8/39). 84.0% (42/50) of SSA/Ps were on the right and 16% (8/50) were on the left (Table 1). SSA/Ps were found significantly more often on the right, compared with TSAs and HPs (P < 0.001) (Table 1).
Macroscopic morphology was categorized as either flat type (includes 0-IIa, superficial flat/elevated tumors: LST, lateral spreading tumor) or protruded type (0-Ip, protruded/pedunculated: 0-Isp, protruded/mixed sessile and pedunculated) based on JSCCR Guidelines 2010 for the treatment of colorectal cancer[22]. Results were as follows: 30.4% (7/23) of HPs were flat type and 69.6% (16/23) were protruded type; 5.1% (2/39) of TSAs were flat type and 94.9% (37/39) were protruded type; and 90.0% (45/50) of SSA/Ps were flat type and 10.0% (5/50) were protruded type. Significantly more SSA/Ps were identified as flat surface lesions compared with TSAs and HPs (P < 0.001) (Table 1).
We categorized as either reddish change (RC) or normal-colored (NC) or pale mucosa). The results were as follows: 65.2% (15/23) of HPs were RC and 34.8% (8/23) were NC; 89.7% (35/39) of TSAs were RC and 10.3% (4/39) were NC; and 20.0% (10/50) of SSA/Ps were RC and 80.0% (40/50) were NC. Significantly more SSA/Ps were same to whitish change in color compared with TSAs and HPs (P < 0.001) (Table 1).
We categorized lesions as without mucin if they could be observed without washing or with only regular washing, or with mucin if they required several repeated washings before they could be observed. Results were as follows: 78.3% (18/23) of HPs were without mucin and 21.7% (5/23) were with mucin; 82.1% (32/39) of TSAs were without mucin and 17.9% (7/39) were with mucin; and 28% (14/50) of SSA/Ps were without mucin and 72% (36/50) were with mucin. Significantly more SSA/Ps showed abundant mucin compared with TSAs and HPs (P < 0.001) (Table 1).
According to the tumor size, mean lesion size was 7.74 ± 3.24 mm for HPs, 9.89 ± 5.73 mm for TSAs, and 13.62 ± 8.62 mm for SSA/Ps. SSA/Ps were significantly larger in mean size compared with TSAs and HPs (SSA/P vs HP, P < 0.001; SSA/P vs TSA, P < 0.01) (Table 1).
A comparison of magnified colonoscopic findings and histopathological diagnoses is given in Table 2. Seventeen lesions showed either type II pit pattern alone or partial type II pit pattern as the basic architecture, with 14 HPs (82.4%) and 3 SSA/Ps (Table 2).
| Pit pattern | n | HPs(n = 23) | TSAs(n = 38) | TSAs + cancer(n = 1) | SSA/Ps +α(n = 47) | SSA/Ps + cancer(n = 3) |
| Type II | 17 | 14 | 3 | |||
| Alone | 16 | 13 | 3 | |||
| With IV-S | 1 | 1 | ||||
| Type II-L | 15 | 4 | 5 | 6 | ||
| Alone | 10 | 4 | 1 | 5 | ||
| With IV-S | 5 | 4 | 1 | |||
| Type II-O | 49 | 4 | 4 | 38 | 3 | |
| Alone | 30 | 3 | 27 | |||
| With II-L | 9 | 1 | 8 | |||
| With IV-S | 7 | 1 | 3 | 3 | ||
| With VI | 3 | 3 | ||||
| Type IV-S | 31 | 1 | 29 | 1 | ||
| Alone | 28 | 1 | 27 | |||
| With II-L | 2 | 2 | ||||
| With VI | 1 | 1 |
Fifteen lesions showed either type II-L alone or partial type II-L as the basic architecture, of which 6 were SSA/Ps, 5 were TSAs, 4 were HPs. Forty-nine lesions showed either type II-O alone or partial type II-O as the basic architecture, with SSA/Ps histology evident in 41 (83.7%) (Figure 1, Table 2). Cancer was also present in 3 lesions, in all of which a type VI pit pattern was also present within the same lesion (Figure 3C and F). There were 4 HPs and 4 TSAs.
Thirty-one lesions showed either type IV-S alone or partial type IV-S as the basic architecture, including 30 TSAs (96.8%). Cancer was present in one lesion, in which a type VI pit pattern was also present within the same lesion.
Epithelial polyps, a serrated architecture without atypical cells, were previously called HPs. Such lesions were believed not to possess cancerous potential. In 1990, Longacre et al[23] proposed that lesions with a serrated architecture and atypicalities should be known as SAs. Torlakovic et al[20,24] subsequently suggested the existence of a subtype comprising atypia admixed within HPs, for which the name SSAs was proposed. Some, however, consider the term adenoma inappropriate, because these lesions cannot be recognized as tumorous. The latest 2010 WHO classification uses the term SSA/Ps, incorporating both adenoma and polyp[21,25]. The concept of serrated lesions, particularly SSA/Ps, is therefore comparatively new, with terminology and classifications that have only recently been standardized. Although this transition has yet to be fully adopted, the fact that it is widely recognized that there is no risk of cancer developing from HPs is one reason for the lack of detailed reports on the colonoscopic characteristics of these lesions.
Based on the WHO classification, serrated lesions of the colorectum can be broadly divided into HPs, TSAs, and SSA/Ps[25]. The present study investigated the characteristics of these three types from conventional colonoscopic findings and magnified colonoscopic findings (pit pattern diagnosis), which are routinely used in everyday colonoscopic evaluation[25]. To start with conventional colonoscopic findings, the characteristics of HPs are that they tend to occur in the left side of the colon, particularly in the rectum, are commonly less than 5 mm in size, and are NC or pale in color and flat[26]. In this study, HPs did not necessarily fit these characteristics. This may have been because the lesions investigated in the present study had been removed colonoscopically, and HPs that exhibited typical characteristics were not indicated for resection. HPs thus included many atypical lesions. The characteristics of TSAs are that they are Isp or Ip protruded lesions that tend to occur on the left side of the colon, vary in size from less than 5 mm to over 10 mm (although most are less than 10 mm)[23], and are often reddish in color. A further characteristic that was not included in this study is that some flat lesions show a surface architecture that can be described as highly protruding double elevation, pinecone-shaped, or coral-shaped[15,27,28]. In the present study, TSAs mostly satisfied these characteristics. SSA/Ps have been reported as a subclassification of HPs[20,24], and the colonoscopic findings are mostly similar. However, SSA/Ps tend to occur more often in the right colon compared with HPs; many are large, with a diameter exceeding 10 mm, and coloration may be yellowish due to mucin[29]. Our findings in the present study were similar.
In magnified colonoscopic findings, Rembacken et al[30] reported the value of SA diagnosis of lesions with serrated architecture with type IIIL tubiform pit pattern as type
IIIH pits, and type IV villiform pit pattern enlarged to resemble pine cones as type IVH pit pattern. These pits may be considered to correspond to the type IV-S pits of the present study. In this study, TSAs accounted for 76.9% (30/39) of lesions with a basic architecture of type IV-S pit pattern, and lesions without type IV-S pit pattern could be classified as non-TSAs with 96.7% sensitivity and 88.9% specificity. Lesions previously regarded as SAs are now considered to be TSAs, and type IV-S pit pattern can be considered a characteristic finding of TSAs.
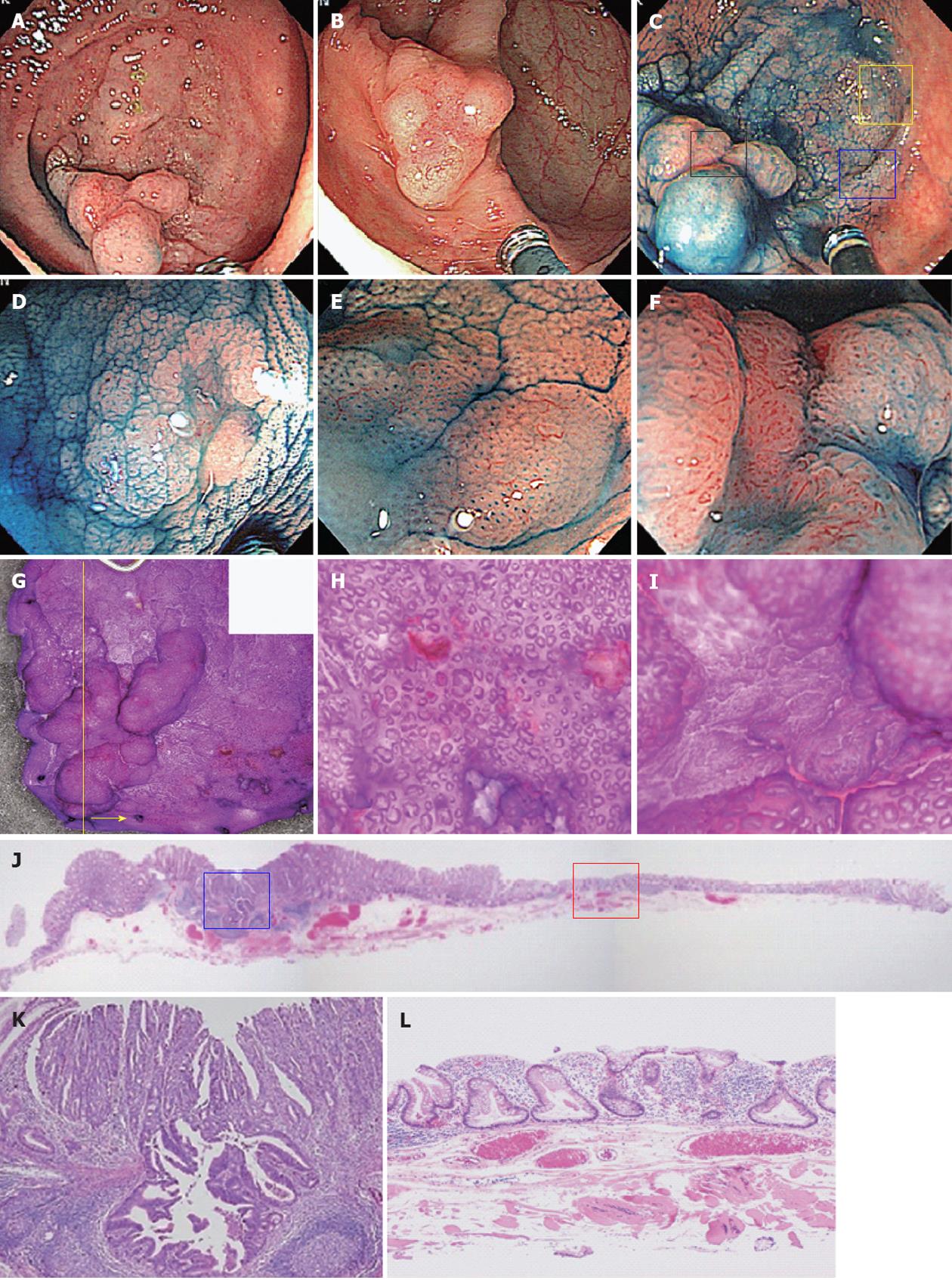
Pit patterns reflect the pathological characteristics of lesions, and if the characteristics differ, different pit patterns should also be apparent. HPs are characterized histologically by serrated crypts, with straight crypts evenly distributed throughout[31]. There should thus be no argument that the type II pit pattern of Kudo’s classification reflect this characteristic. Diagnostic criteria for SSA/Ps, however, have yet to be standardized, and the classifications of Higuchi et al[32,33] have mainly been used in Japan. The JSCCR diagnostic criteria used in the present study have extracted three categories from among those defined by Higuchi et al[32,33] that can be regarded as important expressions of the nature of SSA/Ps. Figure 3 shows a typical case of SSA/Ps complicated with cancer treated in our hospital. In terms of serrated crypt, as in HPs, “dilatation of crypts” can be named as an observable colonoscopic finding. This finding may be considered to correspond to the type II-O pit pattern proposed by Kimura et al[15]. The present study also found SSA/P histology in 41 of 50 lesions (82.0%) with type II-O pit pattern as the basic architecture. Lesions without type II-O pit pattern could be classified as non-SSA/Ps with 83.7% sensitivity and 85.7% specificity. We could not deny that we diagnosed as HPs some lesions that even had a tiny SSA/P component at the glandular base. Although it is therefore not possible to diagnose all SSA/Ps solely on the basis of type II-O pit pattern, at least this finding is useful for the diagnosis of SSA/Ps with dilatation of crypts. Even if not diagnosed as SSA/Ps according to current diagnostic criteria, lesions with type II-O pit pattern reportedly possess molecular biological commonalities and regarding these as a single population may be preferable[9,15].
TSAs show a greater number of characteristic findings compared with HPs and SSA/Ps, facilitating colonoscopic diagnosis. Magnified colonoscopic assessment of the histological characteristics of SSA/Ps is difficult when they do not exhibit dilatation of the crypts, and distinguishing them from HPs is difficult at this point. Our findings suggest, however, that type II-O pit pattern may be of assistance in the colonoscopic diagnosis of lesions with dilatation of crypts.
The concept of serrated lesions of the colorectum is relatively recent, and criteria for pathological diagnosis have yet to be standardized. As TSAs and SSA/Ps are undoubtedly precursor lesions of colon cancer, however, colonoscopists are faced with the task of establishing colonoscopic diagnoses and elucidating lesions for which treatment is indicated. Identifying serrated lesions of the colorectum during colorectal cancer surveillance is thus important. Rex et al recommend that all serrated lesions proximal to the sigmoid colon and all serrated lesions in the rectosigmoid > 5 mm in size, be completely removed[34-37]. However, many issues still remain, including whether all TSAs and SSA/Ps should be indicated for treatment as precursor lesions for colon cancer and the probability of progression to a cancerous state. We hope that the present findings will be useful as a reference for the accumulation of larger numbers of cases in further studies to elucidate the oncogenic pathways in serrated lesions of the colon and rectum.
Broad division of serrated lesions of the colorectum into hyperplastic polyps, traditional serrated adenomas (TSAs), and sessile serrated adenomas/polyps (SSA/Ps) has been proposed on the basis of recent molecular biological studies. However, few reports have examined the colonoscopic features of these divisions, including magnified colonoscopic findings. In this article, the authors verified the diagnostic potency for SSA/Ps using the pit pattern classifications by magnifying colonoscopy.
The pit patterns were categorized according to Kudo’s classification, but a more detailed investigation was also performed using the subclassification [type II-Open (type II-O), type II-Long (type II-L), or type IV-Serrated (type IV-S)] proposed by Kimura T and Yamano H. However, it has yet to be standardized. In this study, the authors performed herein the investigation of the colonoscopic characteristics of serrated lesions of the colorectum, using the surface architecture subclassification proposed by Kimura T and Yamano H in addition to conventional pit pattern diagnosis
The authors successfully confirmed that the pit pattern diagnosis using magnifying colonoscopy, particularly magnified colonoscopic findings using subclassifications of surface architecture, reflected the pathological characteristics of SSA/Ps and TSAs.
This study offers a better understanding of pathological characteristics of SSA/Ps and TSAs using subclassifications of surface architecture by magnified colonoscopy.
Pit patterns reflect the pathological characteristics of lesions, and if the characteristics differ, different pit patterns should also be apparent. Hyperplastic polyps are characterized histologically by serrated crypts, with straight crypts evenly distributed throughout. There should thus be no argument that the type II pit pattern of Kudo’s classification reflect this characteristic.
In magnified colonoscopic findings, lesions without type II-O pit pattern could be classified as non-SSA/Ps with high sensitivity and specificity. It is therefore not possible to diagnose all SSA/Ps solely on the basis of type II-O pit pattern, at least this finding is useful for the diagnosis of SSA/Ps with dilatation of crypts.
Peer reviewers: William Dickey, Altnagelvin Hospital, Londonderry BT47 6SB, Northern Ireland, United Kingdom; Navneet K Ahluwalia, MD, FRCP, PhD, AGAF, MBA, Stepping Hill Hospital, Poplar Grove, Stockport Sk2 7JE, United Kingdom
S- Editor Gou SX L- Editor Kerr C E- Editor Xiong L
| 1. | Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4616] [Cited by in RCA: 4464] [Article Influence: 120.6] [Reference Citation Analysis (0)] |
| 2. | Hawkins NJ, Bariol C, Ward RL. The serrated neoplasia pathway. Pathology. 2002;34:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Jass JR, Young J, Leggett BA. Hyperplastic polyps and DNA microsatellite unstable cancers of the colorectum. Histopathology. 2000;37:295-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138:2088-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 677] [Cited by in RCA: 725] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 6. | Shimoda T, Ikegami M, Fujisaki J, Matsui T, Aizawa S, Ishikawa E. Early colorectal carcinoma with special reference to its development de novo. Cancer. 1989;64:1138-1146. [PubMed] |
| 7. | Grady WM, Markowitz S. Genomic instability and colorectal cancer. Curr Opin Gastroenterol. 2000;16:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 788] [Cited by in RCA: 782] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 9. | Issa JP. Colon cancer: it's CIN or CIMP. Clin Cancer Res. 2008;14:5939-5940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Toyota M, Ho C, Ahuja N, Jair KW, Li Q, Ohe-Toyota M, Baylin SB, Issa JP. Identification of differentially methylated sequences in colorectal cancer by methylated CpG island amplification. Cancer Res. 1999;59:2307-2312. [PubMed] |
| 11. | Watanabe Y, Castoro RJ, Kim HS, North B, Oikawa R, Hiraishi T, Ahmed SS, Chung W, Cho MY, Toyota M. Frequent alteration of MLL3 frameshift mutations in microsatellite deficient colorectal cancer. PLoS One. 2011;6:e23320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Brennetot C, Duval A, Hamelin R, Pinto M, Oliveira C, Seruca R, Schwartz S. Frequent Ki-ras mutations in gastric tumors of the MSI phenotype. Gastroenterology. 2003;125:1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Carvalho B, Pinto M, Cirnes L, Oliveira C, Machado JC, Suriano G, Hamelin R, Carneiro F, Seruca R. Concurrent hypermethylation of gene promoters is associated with a MSI-H phenotype and diploidy in gastric carcinomas. Eur J Cancer. 2003;39:1222-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Pinto M, Oliveira C, Cirnes L, Carlos Machado J, Ramires M, Nogueira A, Carneiro F, Seruca R. Promoter methylation of TGFbeta receptor I and mutation of TGFbeta receptor II are frequent events in MSI sporadic gastric carcinomas. J Pathol. 2003;200:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Kimura T, Yamamoto E, Yamano HO, Suzuki H, Kamimae S, Nojima M, Sawada T, Ashida M, Yoshikawa K, Takagi R. A novel pit pattern identifies the precursor of colorectal cancer derived from sessile serrated adenoma. Am J Gastroenterol. 2012;107:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 16. | Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 707] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 17. | Allen JI. Quality colonoscopy. Preface. Gastrointest Endosc Clin N Am. 2010;20:xv-xvi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Allen JI. Quality assurance for gastrointestinal endoscopy. Curr Opin Gastroenterol. 2012;28:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Valdastri P, Simi M, Webster RJ. Advanced technologies for gastrointestinal endoscopy. Annu Rev Biomed Eng. 2012;14:397-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 21. | Fujimori Y, Fujimori T, Imura J, Sugai T, Yao T, Wada R, Ajioka Y, Ohkura Y. An assessment of the diagnostic criteria for sessile serrated adenoma/polyps: SSA/Ps using image processing software analysis for Ki67 immunohistochemistry. Diagn Pathol. 2012;7:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 23. | Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 418] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 24. | Torlakovic E, Snover DC. Serrated adenomatous polyposis in humans. Gastroenterology. 1996;110:748-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 239] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol. 2011;42:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 478] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 26. | Waye JD, Bilotta JJ. Rectal hyperplastic polyps: now you see them, now you don't--a differential point. Am J Gastroenterol. 1990;85:1557-1559. [PubMed] |
| 27. | Arao J, Sano Y, Fujii T, Kato S, Fu KI, Yoshino T, Ochiai A, Fujimori T, Yoshida S. Cyclooxygenase-2 is overexpressed in serrated adenoma of the colorectum. Dis Colon Rectum. 2001;44:1319-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Oka S, Tanaka S, Hiyama T, Ito M, Kitadai Y, Yoshihara M, Haruma K, Chayama K. Clinicopathologic and endoscopic features of colorectal serrated adenoma: differences between polypoid and superficial types. Gastrointest Endosc. 2004;59:213-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Langdon DE. Large hyperplastic polyps of the right colon. Gastrointest Endosc. 1998;48:659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Rembacken BJ, Trecca A, Fujii T. Serrated adenomas. Dig Liver Dis. 2001;33:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Jass JR, Whitehall VL, Young J, Leggett BA. Emerging concepts in colorectal neoplasia. Gastroenterology. 2002;123:862-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 323] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 32. | Higuchi T, Jass JR. My approach to serrated polyps of the colorectum. J Clin Pathol. 2004;57:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Higuchi T, Sugihara K, Jass JR. Demographic and pathological characteristics of serrated polyps of colorectum. Histopathology. 2005;47:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 34. | Kahi CJ, Li X, Eckert GJ, Rex DK. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc. 2012;75:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 35. | Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 362] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 36. | Lasisi F, Rex DK. Improving protection against proximal colon cancer by colonoscopy. Expert Rev Gastroenterol Hepatol. 2011;5:745-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, Goldblum JR, Guillem JG, Kahi CJ, Kalady MF. Serrated Lesions of the Colorectum: Review and Recommendations From an Expert Panel. Am J Gastroenterol. 2012;Jun 19; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 829] [Article Influence: 63.8] [Reference Citation Analysis (0)] |