Published online Aug 28, 2012. doi: 10.3748/wjg.v18.i32.4278
Revised: July 30, 2012
Accepted: August 3, 2012
Published online: August 28, 2012
AIM: To investigate whether butyrate or glutamine enemas could diminish inflammation in experimental diversion colitis.
METHODS: Wistar specific pathogen-free rats were submitted to a Hartmann’s end colostomy and treated with enemas containing glutamine, butyrate, or saline. Enemas were administered twice a week in the excluded segment of the colon from 4 to 12 wk after the surgical procedure. Follow-up colonoscopy was performed every 4 wk for 12 wk. The effect of treatment was evaluated using video-endoscopic and histologic scores and measuring interleukin-1β, tumor necrosis factor-alpha, and transforming growth factor beta production in organ cultures by enzyme linked immunosorbent assay.
RESULTS: Colonoscopies of the diverted segment showed mucosa with hyperemia, increased number of vessels, bleeding and mucus discharge. Treatment with either glutamine or butyrate induced significant reductions in both colonoscopic (P < 0.02) and histological scores (P < 0.01) and restored the densities of collagen fibers in tissue (P = 0.015; P = 0.001), the number of goblet cells (P = 0.021; P = 0.029), and the rate of apoptosis within the epithelium (P = 0.043; P = 0.011) to normal values. The high levels of cytokines in colon explants from rats with diversion colitis significantly decreased to normal values after treatment with butyrate or glutamine.
CONCLUSION: The improvement of experimental diversion colitis following glutamine or butyrate enemas highlights the importance of specific luminal nutrients in the homeostasis of the colonic mucosa and supports their utilization for the treatment of human diversion colitis.
- Citation: Pacheco RG, Esposito CC, Müller LC, Castelo-Branco MT, Quintella LP, Chagas VLA, de Souza HSP, Schanaider A. Use of butyrate or glutamine in enema solution reduces inflammation and fibrosis in experimental diversion colitis. World J Gastroenterol 2012; 18(32): 4278-4287
- URL: https://www.wjgnet.com/1007-9327/full/v18/i32/4278.htm
- DOI: https://dx.doi.org/10.3748/wjg.v18.i32.4278
Diversion colitis is a complex, nonspecific inflammatory disease that occurs in an excluded colonic segment in almost all patients submitted to a fecal diversion, such as loop colostomy or Hartmann’s procedure (end colostomy with closure of the distal colon’s segment)[1,2]. Clinical manifestations typically include tenesmus with abundant rectal discharge of mucus or blood and abdominal pain. The inflammatory process and the severity of symptoms are highly variable, and occasionally, areas of erosions and ulcerations spread throughout the segment and may cause severe colonic hemorrhage[3,4]. In some cases, it may be difficult to distinguish diversion colitis from other diseases that mimic not only the clinical manifestations but also the colonoscopic appearance. It is essential that diversion colitis not be mistaken for other diseases, especially ulcerative colitis or Crohn’s disease, for this may delay the initiation of appropriate treatment. Reestablishment of the fecal stream is the ideal therapy for diversion colitis, but the best outcomes are directly related to the correct diagnosis[5]. However, many patients need to remain with the colostomy for long periods, and some will never attain the reconstruction of intestinal continuity. As a consequence, it is expected that diversion colitis will impair the quality of life in a significant number of patients.
The diagnosis and follow-up of diversion colitis usually require endoscopic and histologic analyses, but none of these are specific[6]. The endoscopic appearance ranges from absent to severe inflammatory features, including mucus discharge, luminal narrowing, friability, erythema, ulceration and a distorted mucosal vascular pattern[7,8]. Mucosal biopsy of the diverted segment is an important auxiliary tool for the clinical or surgical management. Histological findings usually include mucosal edema, inflammatory infiltration with follicular lymphoid hyperplasia, vascular congestion, decreased number and depth of crypts, expansion of cellular elements and edema of the lamina propria[9,10].
Bacterial proliferation[11,12], overproduction of free oxygen radicals[13], impairment of butyrate oxidation[14,15], defective transport of short-chain fatty acids (SCFAs)[16], and immunological factors[17] are some of the pathogenic mechanisms proposed for explaining the intestinal inflammation after a surgical fecal diversion. Nevertheless, the exact etiopathogenesis of diversion colitis remains unclear. Although an imbalance in the mucosal production of proinflammatory and immunoregulatory cytokines has been implicated in the etiopathogenesis of inflammatory bowel disease (IBD)[18,19], this mechanism has not been well characterized in diversion colitis, and its potential pathogenic role is yet to be determined.
Some new data suggest that the lack of luminal nutrients may be crucial in the development of diversion colitis. SCFAs, in particular butyrate, are considered the major energy source for colonocytes and have been associated with a mucosal trophic effect[16,20,21]. Therefore, it seems that dietetic supplementation with SCFAs or glutamine increases the function and repair of the intestinal mucosa under several conditions, such as radiotherapy, chemotherapy, inflammation, trauma and sepsis[22-24]. In animal models, butyrate and glutamine administered through the oral route or enemas reduce inflammatory processes of the intestinal colonic mucosa[25-29]. However, the available data concerning the treatment of human diversion colitis are still not clear.
Hence, the aim of this study was to investigate whether administering essential nutrients for colonocytes, such as butyrate and glutamine, could alleviate mucosal inflammation and minimize scarring lesions in a model of diversion colitis.
The present study was carried out at the Center of Experimental Surgery of the School of Medicine of the Federal University of Rio de Janeiro (UFRJ). The care and use of animals and the procedures reported in this study were approved by the Ethical Committee for Laboratory Animals of the UFRJ and were in accordance with the guidelines of the International Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals.
Sixty adults Wistar rats (Rattus norvegicus) of both sexes, specific pathogen-free and five months old, with mean body weight 250 g, were maintained on a 12 h/12 h light/dark cycle in a temperature-controlled room (24 °C). Animals were fed standard rat chow and submitted to fasting for 24 h prior to the surgical procedure. Water was offered ad libitum during the whole experiment.
Animals were randomly assigned to four groups of 12 animals each: the control group consisted of rats without any intervention (no surgery) but subjected to a colonoscopy and to the extraction of the distal segment of the colon. The treatment groups were submitted to a Hartmann’s colostomy (standardized surgical procedure) followed by treatment with enemas containing saline, butyrate, or glutamine.
The anesthetic procedure consisted of the intraperitoneal administration of ketamine (1 mg/kg) associated with xylazine (0.1 mg/kg). Immediately after anesthetic administration, animals were immobilized in the dorsal decubitus position, and the skin was cleaned with an antiseptic surgical scrub solution after shaving the surgical site.
After a midline abdominal incision, animals were subjected to a Hartmann’s colostomy. With the exception of the control group, all animals had the left colon transected transversally and the proximal segment brought out as an end colostomy, while the distal segment was sewn over and left within the abdomen as a blind rectal pouch. After the surgical manipulation, the laparotomy was closed by two layers of continuous suture (Figure 1).
In the first two postoperative days, animals received analgesia with dipyrone (1 g/L) in the drinking water. A caloric supplement with glucose was also provided because they had been deprived of solid food for the 24 h before surgery.
A standard volume of 1 mL containing saline (placebo), butyrate (40 mmol) or glutamine (50 g/L) was instilled into the distal excluded segment of the colon through a rubber cannula (4 cm long), under light anesthesia. Animals were maintained in the Trendelenburg position for 2 min after the procedure. Enemas were administered twice a week beginning at week 4 after surgery and going through week 12.
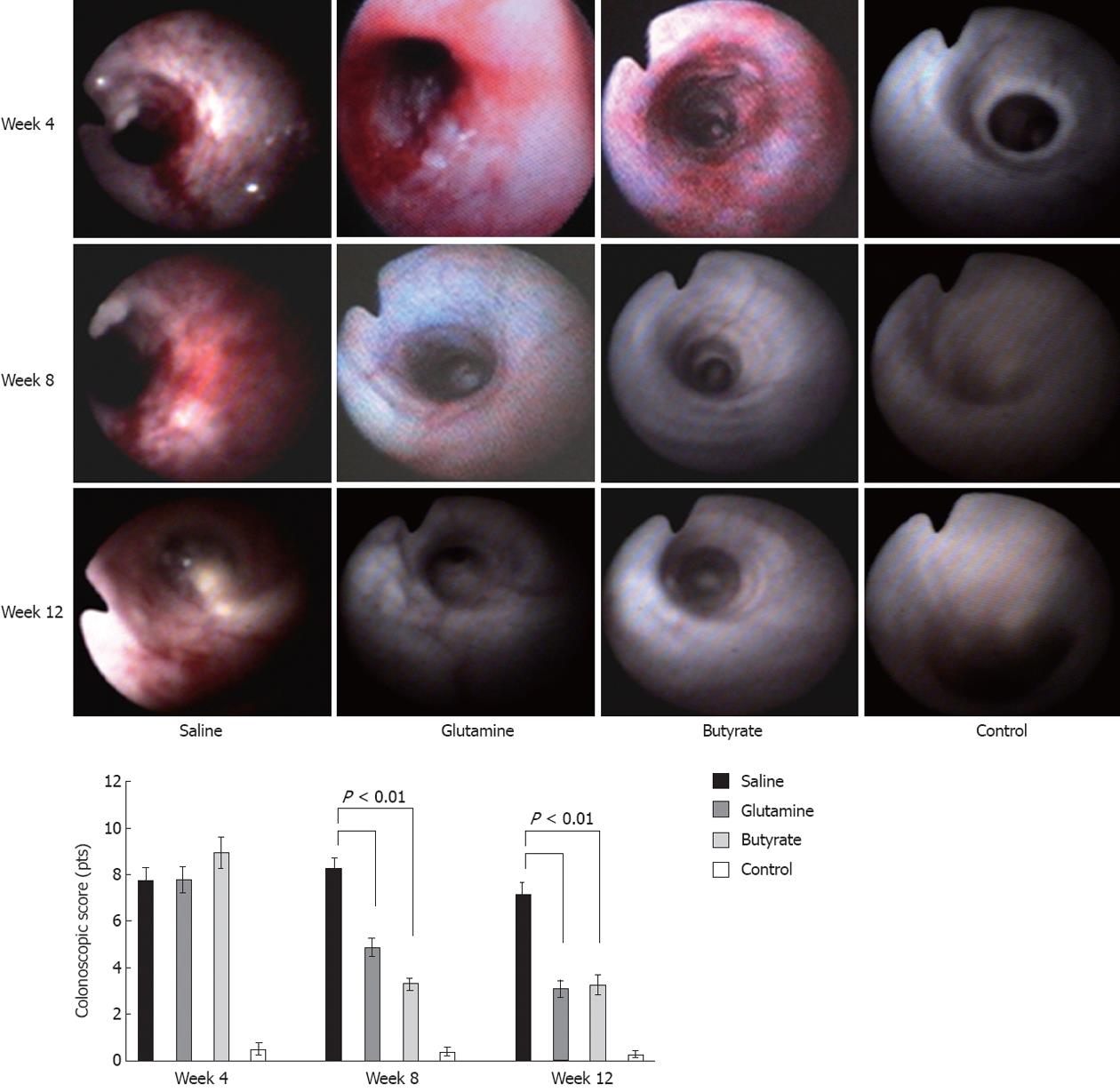
Colonoscopies were performed under light anesthesia before the surgical procedure and thereafter every 4 wk, starting at 4 wk and going through the total follow-up of 12 wk. In addition to the real-time evaluation of mucosal appearance, video-endoscopic recorded movies were also used to confirm the results before and after the different treatments. Criteria for the endoscopic analysis were based on four parameters: hyperemia, number and size of vessels, bleeding, and mucous secretion, as previously described[7]. Each parameter was assigned 0 to 4 points, and the sum of all parameters was used to generate a final endoscopic score.
After the 8th-week colonoscopic procedure, half of the animals from each group (n = 6) were euthanized by anesthetic overdose, without any pain or suffering. Three samples were excised from the colon for histological assessment and for organ cultures. The other animals from all groups (n = 6) where euthanized after the last colonoscopic procedure.
Specimens were fixed in 40 g/L formaldehyde saline, embedded in paraffin, cut into serial sections of 5 μm, and submitted to the different staining procedures. Histomorphological analysis were performed under light microscopy by two independent observers who were unaware of the experimental data and who examined all tissue sections and captured images (Leica Microsystems Ltd, Switzerland).
The following histological parameters involving inflammatory and trophic alterations were studied in hematoxylin-eosin-stained slides: mucosal edema, lymphoplasmacytic inflammatory infiltrate, lymphoid follicular hyperplasia, vascular congestion, number and depth of crypts, and the density of cellular elements in the lamina propria. Each parameter was assigned 0 to 2 points, and a total score was obtained by the sum of all parameters, as previously described[30].
The phosphomolybdic acid-picro-sirius red dye was used to stain collagen fibers in the tissue of serial paraffin sections obtained as described above. At least 10 different areas per tissue section were analyzed under light microscopy at ×100 magnification. The density of collagen fibers was calculated as the area positively stained for collagen in relation to total intestinal tissue using an imaging analysis system (Leica QWin Plus V 3.5.1, Leica Microsystems Ltd, Switzerland).
The periodic acid of Schiff (PAS) was used to stain goblet cells within the intestinal epithelium. The density of goblet cells was defined as the percentage of PAS-positive cells within at least 500 epithelial cells in the crypts and in the surface epithelium of longitudinally sectioned colonic pits.
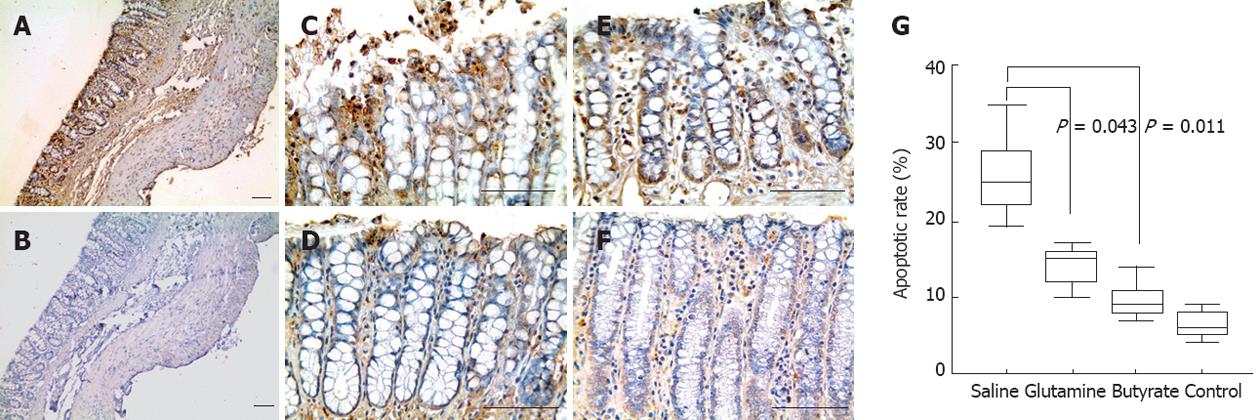
To analyze apoptosis within the colon, fragmented DNA was stained by the terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) assay, with the Apoptag® Plus Peroxidase In Situ Apoptosis Kit (Millipore, Billerica, MA, United States). Paraffin sections were first deparaffinized, incubated with proteinase K solution for 15 min at room temperature, and then immersed in hydrogen peroxide to block endogenous peroxidase activity. After rinsing with phosphate buffered saline (PBS), slides were incubated with equilibration buffer for at least 1 min, followed by incubation with a solution containing TdT enzyme. Sections were incubated at 37 °C for 1 h. A second section from each sample, incubated without TdT enzyme, constituted the negative controls. Positive controls were prepared by treating samples with DNase I (Sigma, Deisenhofen, Germany). The reaction was terminated by washing the slides with pre-warmed stop/wash buffer solution for 10 min. Specimens were subsequently incubated with non-immune horse serum for 20 min and subsequently with an anti-digoxigenin peroxidase conjugate for 30 min at room temperature. After being rinsed in PBS, all sections were developed with a solution containing hydrogen peroxide and diaminobenzidine. Preparations were lightly counterstained in Harris’s hematoxylin, dehydrated, and mounted in Permount (Fisher Scientific, Pittsburgh, PA, United States). Morphologically preserved TUNEL-positive cells and apoptotic bodies were referred to as apoptotic cells and identified by using pre-defined measurements in the computer-assisted image analyzer in conjunction with careful evaluation of morphologic criteria. Percentages of apoptotic cells were defined by the number of immunoreactive cells in relation to total cells (immunoreactive and nonimmunoreactive cells; ×400 magnification), counted among at least 500 epithelial cells in the crypts and in the surface epithelium of longitudinally sectioned colonic crypts. Two independent observers who were unaware of the experimental data examined all tissue sections and captured images.
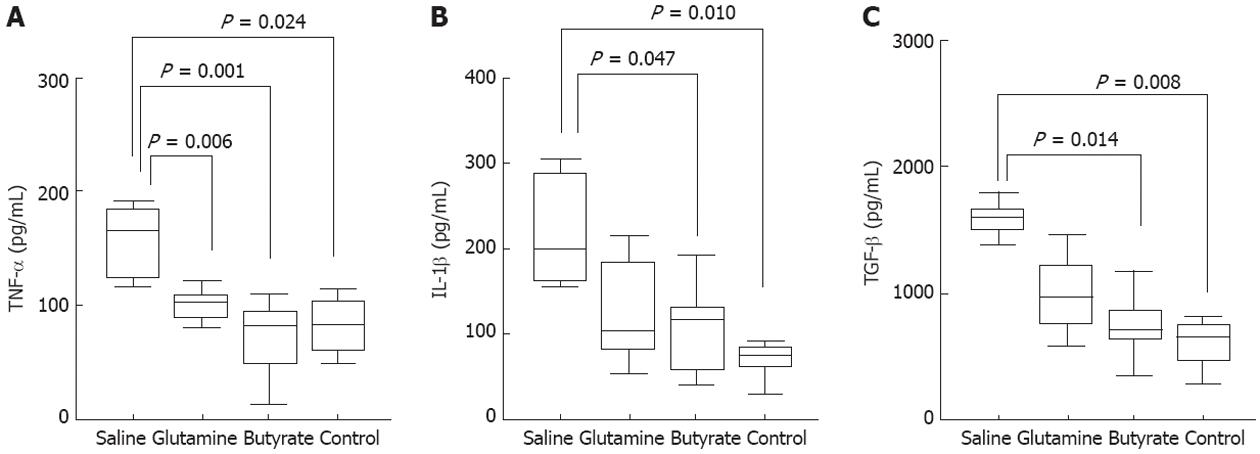
Colonic mucosal explants were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Life Technologies, United States), 10 mmol HEPES (Promega, United States), penicillin (100 kU/L) and streptomycin (100 mg/L) (Sigma-Aldrich, St. Louis, MO, United States) for 24 h at 37 °C in a 50 mL/L CO2 humidified incubator. After incubation for 24 h, the supernatant was collected and stocked at -20 °C. Samples were centrifuged and the supernatants used to measure the concentrations of the cytokines tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β and transforming growth factor beta (TGF-β) by enzyme-linked immunosorbent assay (ELISA) (R and D Systems, MN, United States). The total protein content of the biopsy specimens was estimated by the bicinchoninic acid method, and values were used to normalize ELISA results. The minimum detectable concentrations of rat TNF-α, IL-1β, and TGF-β were typically less than 5.0 ng/L.
Statistical analyses were performed using the statistical software SPSS for Windows (version 10.0.1, SPSS Inc., 1989-1999, United States). Significant differences among the experimental groups were evaluated by one-way ANOVA followed by pairwise multiple comparisons using Dunnett’s T3 test. Colonoscopic score changes before and after treatment were compared by the Wilcoxon matched-pair signed-rank test. Values are expressed as medians (1st quartile, 3rd quartile). The level of significance was set at P < 0.05.
An inflammatory process similar to human diversion colitis was well reproduced in the experimental model, and the peak inflammation was observed at 8 wk after induction.
Diversion colitis manifested predominantly as patchy inflammatory lesions in the excluded distal colon. Colonoscopic images of the diverted segment with colitis displayed a fragile mucosa, with hyperemia, increased number of vessels, spontaneous bleeding, and increased mucous secretion (Figure 2). Animals treated with either butyrate or glutamine showed a significant reduction in the endoscopic scores compared to saline-treated animals at both 8 and 12 wk (Figure 2).
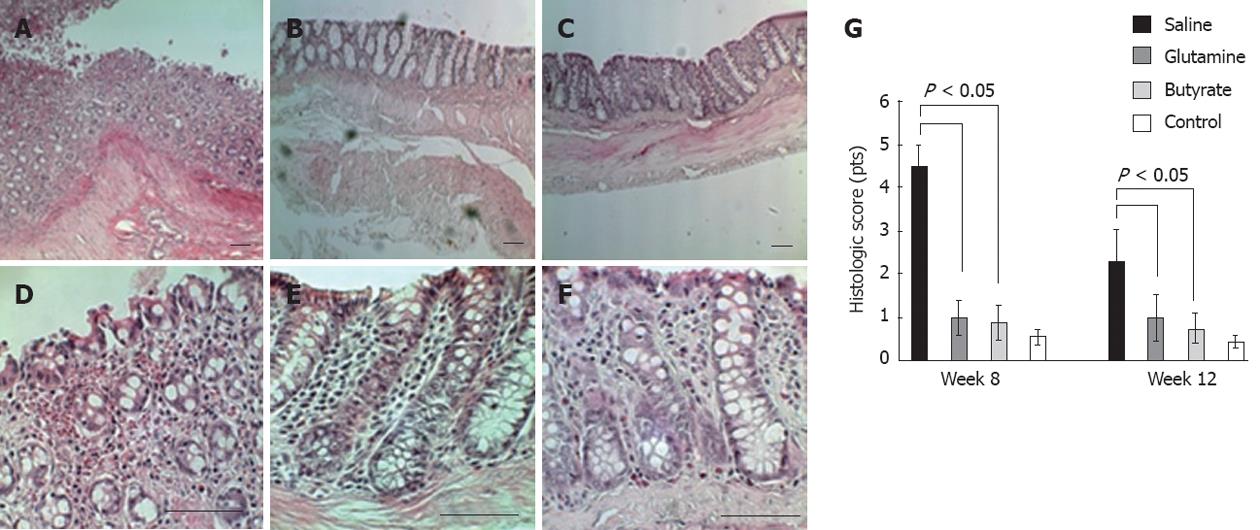
Four weeks after surgery, the histological evaluation of the involved areas of the colon showed mucosal edema, inflammatory infiltration with follicular lymphoid hyperplasia, vascular congestion, decreased number and depth of crypts, expansion of lamina propria cellular elements, and evidence of transmural inflammation (Figure 3A-F). Significantly higher histologic inflammatory scores were found in the intestinal mucosa of all animals submitted to colitis induction through colostomy compared to the normal mucosa of controls. Butyrate- or glutamine-treated animals presented significantly reduced histologic scores of the colon compared to those of the saline-treated group at 8 and 12 wk (Figure 3G).
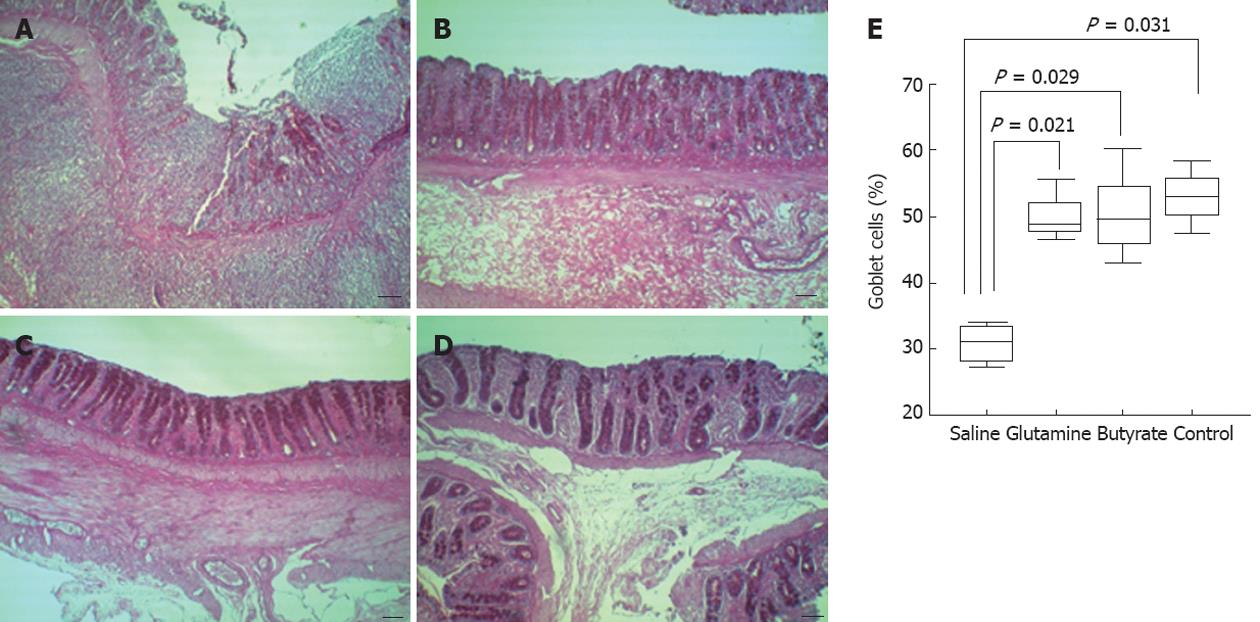
The number of goblet cells in the colonic mucosa was significantly lower in saline-treated rats, compared with the higher numbers found in the therapeutic glutamine or butyrate groups, which were similar to those of the normal control group (Figure 4).
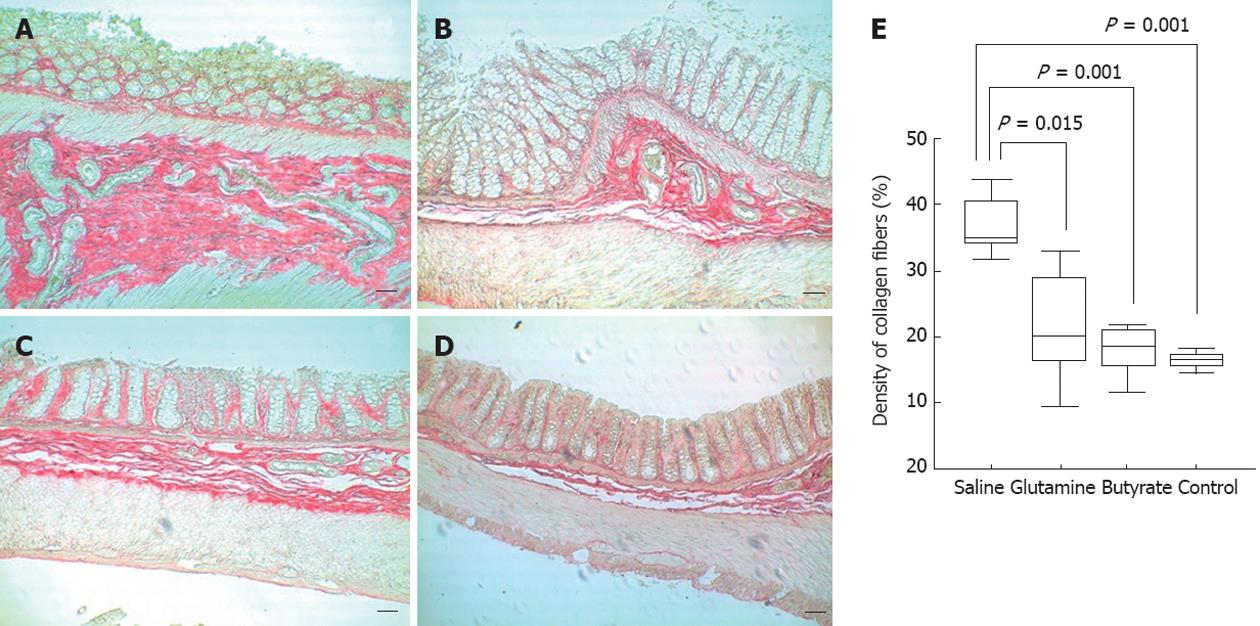
Increased densities of collagen fibers were found in saline-treated animals, with a diffuse distribution throughout the colon wall. In contrast, rats treated with glutamine or butyrate enemas showed a significant reduction in collagen deposition, with levels similar to those observed in control animals (Figure 5).
In this diversion colitis model, significantly reduced rates of apoptotic TUNEL-positive cells were observed in the epithelium of glutamine- (P = 0.042) and butyrate-treated colitic animals (P = 0.011) compared to saline-treated animals. No significant difference was detected between glutamine- and butyrate-treated animals (Figure 6).
TNF-α, IL-1β, and TGF-β production by the colonic mucosa of the diverted segment obtained at 8 wk was evaluated by ELISA in the supernatants of 24 h organ cultures. High levels of TNF-α (Figure 7A), IL-1β (Figure 7B), and TGF-β (Figure 7C) were detected in the supernatants of saline-treated colitis, and significantly lowered to normal values following treatment with glutamine (Figure 7A) or butyrate (Figure 7A-C).
The experimental model presented in this study demonstrates that the simple deviation of the fecal stream through a colostomy is capable of inducing inflammation similar to human diversion colitis, even in specific pathogen-free animals. Both glutamine and butyrate treatments significantly attenuated the inflammatory process, supporting a crucial role for specific luminal nutrients in intestinal homeostasis. We show that the inflammatory process is characterized by inflammatory cell infiltration, collagen fiber deposition, and atrophic changes in the colon, with loss of terminally differentiated goblet cells and increased local apoptotic rates, resulting in colonic remodeling. In addition, we show that a Th1-type of immune response is likely to be responsible for the inflammatory process and that the simple feeding of glutamine or butyrate can downregulate pro-inflammatory cytokines, reducing colonic inflammation.
Diversion colitis constitutes an inflammatory process that develops within a colon segment following surgical deviation of the bowel traffic, and it usually includes the whole excluded segment, with findings common to other inflammatory conditions of the colon[1,2]. Hence, it would be expected that the human disease could be reproducible in animal models[30,31]. In accordance with our results, another study in rats demonstrated that inflammatory abnormalities can be detected after the fourth week following colostomy, reaching their peak between week 8 and 12[32]. In the present study, we aimed to establish an inflammatory curve for diversion colitis. To accomplish this, we performed serial colonoscopies every two weeks from week 4 to week 20 in a pilot study, testing loop colostomy vs Hartmann’s surgery. We confirmed that the inflammatory process appears after week four, peaking at week 8, and that the best results were obtained with Hartmann’s colostomy. In addition, we showed that at sixteen week, most animals displayed a spontaneous attenuation of the inflammation. Because of these findings, we determined the best period of time for the study of inflammation and treatment outcomes in the experimental model.
In humans, diversion colitis typically occurs between three and thirty-six months after surgery, and once developed, the pathologic process and clinical manifestations can persist for decades[10,33]. Nevertheless, it seems important to note that the biologic cycle of rats is faster compared with humans, and as a consequence, the timing of the establishment of colitis and the response to treatment are also expected to be different. Regarding the severity of diversion colitis, there appears to be considerable variation among individuals submitted to colostomy[7,10]. This fact can be explained, at least in part, by the type of surgical procedure. For example, the loop colostomy technique allows the passage of some fecal residues to the excluded segment, sufficient to nourish colonocytes and most likely preserving their viability and function.
Although the exact mechanism underlying the pathogenesis of diversion colitis is yet to be determined, the deficiency of SCFAs is thought to play an important role in the inflammatory process[34,35]. Because SCFAs constitute the major source of energy for human colonocytes[36,37], SCFA depletion in the defunctionalized colon segment would most likely result in a homeostatic imbalance of a rapid-turnover tissue. Energy deprivation can limit cell restoration or induce colonocyte apoptosis, consequently disrupting the epithelial barrier. In this work, the reduction of goblet cells together with increased apoptotic rates within the colonic mucosa reflects the characteristic atrophic changes of the inflammatory process. Once the mucosal barrier is disrupted, the lamina propria is overexposed to luminal antigens, thus exacerbating the inflammatory response. Of note, some studies suggest that SCFA deprivation may constitute an additional factor for the development or the exacerbation of IBD[35,38,39]. In addition, enemas containing SCFAs have been utilized with relative success in subsets of patients with IBD[40,41].
Among the SCFAs, butyrate is the preferential nutrient for colonocytes. In vitro in both rat and human colonocytes, most oxygen consumption is due to butyrate oxidation[14,15]. Therefore, in this work we chose to work with butyrate in its isolated form, devoid of other SCFAs, in contrast to previous works[35,42,43].
Although the etiopathogenesis of IBD is complex and multifactorial, the chronic inflammatory process involves the excessive production of proinflammatory cytokines by the intestinal mucosa[44]. In particular, in Crohn’s disease, the disruption of the intestinal epithelial barrier and increased apoptosis are paralleled by a predominant local Th1/Th17-type of immune response[18,45,46]. In our model, we observed a remarkable mononuclear cell accumulation within the lamina propria and atrophic changes in the epithelial layer, together with overproduction of IL-1β and TNF-α. Most interesting, butyrate and to a lesser extent glutamine significantly reduced the production of these Th1-type pro-inflammatory cytokines. In addition, the high levels of cytokines were correlated with the other macroscopic and histologic parameters used to analyze diversion colitis.
The highest levels of TGF-β were also detected in samples from animals with diversion colitis, in conjunction with the increased densities of collagen fibers. TGF-β is a multifunctional cytokine with potentially high anti-inflammatory activity, but it is also critical in extra-cellular remodeling in chronic inflammatory bowel diseases[47,48]. Here, we showed that treatment with topical butyrate significantly reduced the levels of TGF-β and collagen deposition in the colon. Although the mechanism responsible for the reduction of TGF-β is not clear, it is likely that the immunomodulatory actions of butyrate, including suppression of pro-inflammatory cytokines, could indirectly downregulate TGF-β[49,50]. Independently of the exact mechanism responsible for the downregulation of TGF-β, it is relevant to highlight the anti-fibrotic effect of butyrate in this model. This finding seems to indicate a potential influence of butyrate counteracting fibrogenesis, an end-point consequence of many different diseases in which chronic inflammation is the dominant pathological process.
In conclusion, the successful treatment of this experimental model with either glutamine or butyrate supports the hypothesis of luminal nutrient deprivation as a major etiopathogenic mechanism underlying diversion colitis. In particular, butyrate, with its remarkable anti-inflammatory and regenerative effects, appears as a potential new alternative to topical therapy for human diversion colitis.
The lack of luminal nutrients has been suggested to play a crucial role in the pathogenesis of diversion colitis. The aim of this study was to test whether butyrate or glutamine enemas could diminish inflammation in a model of diversion colitis.
Investigating the effects of topical administration of specific nutrients in an experimental model of diversion colitis may be relevant to mechanisms of chronicity and new therapeutic approaches in human diversion colitis.
The novelty of the work is not exclusively related to the use of topical butyrate or glutamine to treat experimental diversion colitis or other inflammatory conditions but rather to the new mechanistic observation of the anti-inflammatory action and intestinal mucosal repair of specific luminal nutrients. Furthermore, authors clearly show that the therapeutic agents proposed, in particular butyrate, actively regenerate the mucosa and influence colonic remodeling, resulting in colitis amelioration. Another fundamental and unique point of this work is the successful real-time demonstration of the anti-inflammatory effect of butyrate or glutamine in diversion colitis by a video-colonoscopy imaging system. To the knowledge, this in vivo observation of the benefit of topical therapy has never been presented in the field of intestinal inflammatory disorders.
The findings in experimental diversion colitis appear to be shared with other inflammatory disorders and offer a conceptually new approach to the treatment of chronic inflammatory diseases. In particular, butyrate, with its remarkable anti-inflammatory and regenerative effects, appears as a potential new alternative to topical therapy for human diversion colitis.
This experimental study shows the improvement of experimental diversion colitis following glutamine or butyrate enemas. The results obtained in this study clearly highlight the importance of specific luminal nutrients in the homeostasis of the colonic mucosa and support the utilization of these nutrients for the treatment of human diversion colitis.
Peer reviewer: Ana Cristina Simões e Silva, MD, PhD, Professor, Department of Pediatrics, Faculty of Medicine of Federal, University of Minas Gerais, Avenida Bernardo Monteiro, 1300 apt 1104, Bairro Funcionários, Belo Horizonte, Minas Gerais 30150-281, Brazil
S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Glotzer DJ, Glick ME, Goldman H. Proctitis and colitis following diversion of the fecal stream. Gastroenterology. 1981;80:438-441. [PubMed] |
| 2. | Ma CK, Gottlieb C, Haas PA. Diversion colitis: a clinicopathologic study of 21 cases. Hum Pathol. 1990;21:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Ona FV, Boger JN. Rectal bleeding due to diversion colitis. Am J Gastroenterol. 1985;80:40-41. [PubMed] |
| 4. | Lusk LB, Reichen J, Levine JS. Aphthous ulceration in diversion colitis. Clinical implications. Gastroenterology. 1984;87:1171-1173. [PubMed] |
| 5. | Whelan RL, Abramson D, Kim DS, Hashmi HF. Diversion colitis. A prospective study. Surg Endosc. 1994;8:19-24. [PubMed] |
| 6. | Longatti TS, Acedo SC, de Oliveira CC, Miranda DD, Priolli DG, Ribeiro ML, Gambero A, Martinez CA. Inflammatory alterations in excluded colon in rats: a comparison with chemically induced colitis. Scand J Gastroenterol. 2010;45:315-324. [PubMed] |
| 7. | Castro LS, Schanaider A, Bettina WC. Colitis following fecal diversion: still a challenge. Acta Cir Bras. 2000;15:3-6. [DOI] [Full Text] |
| 8. | Haas PA, Fox TA, Szilagy EJ. Endoscopic examination of the colon and rectum distal to a colostomy. Am J Gastroenterol. 1990;85:850-854. [PubMed] |
| 9. | Martinez CA, Nonose R, Spadari AP, Máximo FR, Priolli DG, Pereira JA, Margarido NF. Quantification by computerized morphometry of tissue levels of sulfomucins and sialomucins in diversion colitis in rats. Acta Cir Bras. 2010;25:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Geraghty JM, Talbot IC. Diversion colitis: histological features in the colon and rectum after defunctioning colostomy. Gut. 1991;32:1020-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 79] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Neut C, Colombel JF, Guillemot F, Cortot A, Gower P, Quandalle P, Ribet M, Romond C, Paris JC. Impaired bacterial flora in human excluded colon. Gut. 1989;30:1094-1098. [PubMed] |
| 12. | Edwards CM, George B, Warren B. Diversion colitis--new light through old windows. Histopathology. 1999;34:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Martinez CA, Ribeiro ML, Gambero A, Miranda DD, Pereira JA, Nadal SR. The importance of oxygen free radicals in the etiopathogenesis of diversion colitis in rats. Acta Cir Bras. 2010;25:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Jørgensen JR, Clausen MR, Mortensen PB. Oxidation of short and medium chain C2-C8 fatty acids in Sprague-Dawley rat colonocytes. Gut. 1997;40:400-405. [PubMed] |
| 15. | Hamer HM, Jonkers DM, Bast A, Vanhoutvin SA, Fischer MA, Kodde A, Troost FJ, Venema K, Brummer RJ. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin Nutr. 2009;28:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 279] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain JP. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: a transport deficiency. Inflamm Bowel Dis. 2010;16:684-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 17. | Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ. Butyrate-induced transcriptional changes in human colonic mucosa. PLoS One. 2009;4:e6759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1348] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 19. | Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514-521. [PubMed] |
| 20. | Oliveira AJ, Pinto Júnior FE, Formiga MC, Melo SP, Brandao-Neto J, Ramos AM. Comparison of prophylactic and therapeutic use of short-chain fatty acid enemas in diversion colitis: a study in Wistar rats. Clinics (Sao Paulo). 2010;65:1351-1356. [PubMed] [DOI] [Full Text] |
| 21. | Scheppach W, Müller JG, Boxberger F, Dusel G, Richter F, Bartram HP, Christl SU, Dempfle CE, Kasper H. Histological changes in the colonic mucosa following irrigation with short-chain fatty acids. Eur J Gastroenterol Hepatol. 1997;9:163-168. [PubMed] |
| 22. | Bloemen JG, Schreinemacher MH, de Bruine AP, Buurman WA, Bouvy ND, Dejong CH. Butyrate enemas improve intestinal anastomotic strength in a rat model. Dis Colon Rectum. 2010;53:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Wilmore DW, Shabert JK. Role of glutamine in immunologic responses. Nutrition. 1998;14:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 115] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Coëffier M, Marion-Letellier R, Déchelotte P. Potential for amino acids supplementation during inflammatory bowel diseases. Inflamm Bowel Dis. 2010;16:518-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Feng D, Xu W, Chen G, Hang C, Gao H, Yin H. Influence of glutamine on intestinal inflammatory response, mucosa structure alterations and apoptosis following traumatic brain injury in rats. J Int Med Res. 2007;35:644-656. [PubMed] |
| 26. | Israeli E, Berenshtein E, Wengrower D, Aptekar L, Kohen R, Zajicek G, Goldin E. Prophylactic administration of topical glutamine enhances the capability of the rat colon to resist inflammatory damage. Dig Dis Sci. 2004;49:1705-1712. [PubMed] |
| 27. | Kaya E, Gür ES, Ozgüç H, Bayer A, Tokyay R. L-glutamine enemas attenuate mucosal injury in experimental colitis. Dis Colon Rectum. 1999;42:1209-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | San-Miguel B, Crespo I, Kretzmann NA, Mauriz JL, Marroni N, Tuñón MJ, González-Gallego J. Glutamine prevents fibrosis development in rats with colitis induced by 2,4,6-trinitrobenzene sulfonic acid. J Nutr. 2010;140:1065-1071. [PubMed] |
| 29. | Roda A, Simoni P, Magliulo M, Nanni P, Baraldini M, Roda G, Roda E. A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J Gastroenterol. 2007;13:1079-1084. [PubMed] |
| 30. | Keli E, Bouchoucha M, Devroede G, Carnot F, Ohrant T, Cugnenc PH. Diversion-related experimental colitis in rats. Dis Colon Rectum. 1997;40:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Neut C, Guillemot F, Gower-Rousseau C, Biron N, Cortot A, Colombel JF. [Treatment of diversion colitis with short-chain fatty acids. Bacteriological study]. Gastroenterol Clin Biol. 1995;19:871-875. [PubMed] |
| 32. | Pinto-Jnior FEL, Oliveira AJF, Medeiros KF, Ramos AM, Ramos CC. Histopathological consequences of colostomy in the defunctional intestinal segment: an experimental study in rats. Col Bras Cir. 1999;26:327-333. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Deruyter L, Delvaux G, Willems G. Restoration of colorectal continuity reverses atrophy in human rectal mucosa. Dig Dis Sci. 1990;35:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1742] [Cited by in RCA: 1856] [Article Influence: 97.7] [Reference Citation Analysis (0)] |
| 35. | Scheppach W, Christl SU, Bartram HP, Richter F, Kasper H. Effects of short-chain fatty acids on the inflamed colonic mucosa. Scand J Gastroenterol Suppl. 1997;222:53-57. [PubMed] |
| 36. | Ahmad MS, Krishnan S, Ramakrishna BS, Mathan M, Pulimood AB, Murthy SN. Butyrate and glucose metabolism by colonocytes in experimental colitis in mice. Gut. 2000;46:493-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 137] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 37. | Velázquez OC, Lederer HM, Rombeau JL. Butyrate and the colonocyte. Production, absorption, metabolism, and therapeutic implications. Adv Exp Med Biol. 1997;427:123-134. [PubMed] |
| 38. | Galvez J, Rodríguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005;49:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Hamer HM, Jonkers DM, Vanhoutvin SA, Troost FJ, Rijkers G, de Bruïne A, Bast A, Venema K, Brummer RJ. Effect of butyrate enemas on inflammation and antioxidant status in the colonic mucosa of patients with ulcerative colitis in remission. Clin Nutr. 2010;29:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Assisi RF. Combined butyric acid/mesalazine treatment in ulcerative colitis with mild-moderate activity. Results of a multicentre pilot study. Minerva Gastroenterol Dietol. 2008;54:231-238. [PubMed] |
| 41. | Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1657] [Cited by in RCA: 1842] [Article Influence: 108.4] [Reference Citation Analysis (0)] |
| 42. | Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med. 1989;320:23-28. [PubMed] |
| 43. | de Oliveira-Neto JP, de Aguilar-Nascimento JE. Intraluminal irrigation with fibers improves mucosal inflammation and atrophy in diversion colitis. Nutrition. 2004;20:197-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 44. | Podolsky DK. Inflammatory bowel disease (1). N Engl J Med. 1991;325:928-937. [PubMed] |
| 45. | Schreiber S, Nikolaus S, Hampe J, Hämling J, Koop I, Groessner B, Lochs H, Raedler A. Tumour necrosis factor alpha and interleukin 1beta in relapse of Crohn's disease. Lancet. 1999;353:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 199] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 46. | Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77-97. [PubMed] |
| 47. | Dignass AU, Stow JL, Babyatsky MW. Acute epithelial injury in the rat small intestine in vivo is associated with expanded expression of transforming growth factor alpha and beta. Gut. 1996;38:687-693. [PubMed] |
| 48. | Kiliç ZM, Ayaz S, Ozin Y, Nadir I, Cakal B, Ulker A. Plasma transforming growth factor-beta1 level in inflammatory bowel disease. Turk J Gastroenterol. 2009;20:165-170. [PubMed] |
| 49. | Nancey S, Moussata D, Graber I, Claudel S, Saurin JC, Flourié B. Tumor necrosis factor alpha reduces butyrate oxidation in vitro in human colonic mucosa: a link from inflammatory process to mucosal damage? Inflamm Bowel Dis. 2005;11:559-566. [PubMed] [DOI] [Full Text] |
| 50. | Theiss AL, Simmons JG, Jobin C, Lund PK. Tumor necrosis factor (TNF) alpha increases collagen accumulation and proliferation in intestinal myofibroblasts via TNF receptor 2. J Biol Chem. 2005;280:36099-36109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |